Abstract
Seasonal reproduction represents a naturally occurring example of functional plasticity in the adult brain since it reflects changes in neuroendocrine pathways controlling GnRH secretion and, in particular, the responsiveness of GnRH neurons to estradiol negative feedback. Structural plasticity within this neural circuitry may, in part, be responsible for seasonal switches in the negative feedback control of GnRH secretion that underlies annual reproductive transitions. In this paper, we review evidence for structural changes in the circuitry responsible for seasonal inhibition of GnRH secretion in sheep. These include changes in synaptic inputs onto GnRH neurons, as well as onto dopamine neurons in the A15 cell group, a nucleus that play a key role in estradiol negative feedback. We also present preliminary data suggesting a role for neurotrophins and neurotrophin receptors as an early mechanistic step in the plasticity that accompanies seasonal reproductive transitions in the sheep. Finally, we review recent evidence suggesting that kisspeptin cells of the arcuate nucleus constitute a critical intermediary in the control of seasonal reproduction. While a majority of the data for a role of neuronal plasticity in seasonal reproduction has come from the sheep model, the players and principles are likely to have relevance for reproduction in a wide variety of vertebrates, including humans, and in both health and disease.
Keywords: GnRH, neuroendocrine, dopamine, seasonality, thyroid hormone
1. Introduction
Morphological plasticity is key to the ability of the adult nervous system to adapt readily and predictably to changes in the internal milieu of the body, as well as the changing world outside. In the hypothalamus, alterations in the size and shapes of neurons, their inputs and outputs dramatically change their responsiveness to endogenous and environmental signals during development and adult life (Plant & Shahab, 2002; Tasker et al., 2002; Theodosis et al., 2004; Naftolin et al., 2007; Prevot et al., 2010). The reproductive neuroendocrine system is one such example – reproductive activity can be reversibly turned on and off during key points in adult life, such as during lactation and pregnancy (Smith, 1978; McNeilly et al., 1994b), under conditions of nutritional restriction (Warren et al., 1999; Meczekalski et al., 2008), and, in many animals, in response to the time of year (Goodman et al., 1982; Malpaux et al., 1988; Karsch et al., 1993; Lehman et al., 1997). Seasonal reproduction is a common and key adaptation to a changing environment that allows for offspring to be born at the most advantageous time of year to survive and grow (Malpaux et al., 1988; Lehman et al., 1997; Anderson et al., 2003). The mechanistic changes responsible for seasonal reproduction in mammals occur primarily at the level of the brain, and, as such, this type of seasonality represents a conspicuous example of functional plasticity in the adult nervous system (Karsch et al., 1984; Barrell et al., 1992). In sheep, the neural circuitry mediating seasonal reproduction is comprised of a multi-synaptic pathway in the preoptic area and hypothalamus that ultimately impinges on gonadotropin-releasing hormone (GnRH) neurons (Lehman et al., 2002; Goodman et al., 2010), the final common neuroendocrine component for the control of reproduction. Despite a detailed knowledge of a large part of this circuitry, the changes which enable it to be functional at one time of year and not another are still unknown.
Seasonal reproductive transitions in sheep occur over weeks and months, and in view of this time frame, it has been interesting to speculate that long-term morphological changes may contribute to the functional switch in reproduction. Indeed, recent work has revealed evidence of neuronal plasticity at several levels in this circuitry, and these alterations closely parallel seasonal changes in reproduction. In this paper we will review the evidence for plasticity in this neuroendocrine circuitry, focusing on data obtained using the female sheep (ewe) as a model and, in particular, on plasticity at the level of the dopaminergic A15 nucleus and its connections, direct and indirect, to GnRH neurons (Lehman et al., 1997; Xiong et al., 1997; Jansen et al., 2003; Pompolo et al., 2003; Adams et al., 2006; Sergeeva & Jansen, 2009). While this work has identified several components of the pathway at which plasticity occurs, experimental work to test the functional significance of these morphological changes has just begun; thus, at present, most of the data we will discuss provide a framework for hypothesis testing rather than a definitive explanation for seasonal reproduction. In addition to morphological plasticity, there are likely to be functional changes in this circuitry, in the expression of either neurotransmitters, receptors, and/or other signalling molecules, which contribute to the seasonal switch in reproduction (Goodman et al., 2010).
Female sheep, like other seasonally breeding mammals, show distinct annual cycles of ovarian function, with ovulatory cycles occurring during the autumn and winter, and anovulation in the spring and summer (Karsch, 1980). The key environmental signal that regulates seasonal reproduction in sheep and other mammals is changing daylength (photoperiod); sheep use changes in daylength as a signal to distinguish time of year, and synchronize what appears to be an endogenous rhythm of reproduction to the external world (Lincoln & Short, 1980). Photoperiodic information is conveyed to the reproductive neuroendocrine system through a retinohypothalamic pathway that ultimately regulates a nocturnal rhythm in pineal melatonin secretion which serves as a hormonal signal for daylength (Bittman et al., 1983; Karsch et al., 1984; Malpaux et al., 1997). Melatonin appears to act in the premammillary region (PMR) of the caudal hypothalamus (Malpaux et al., 1995; Sliwowska et al., 2004), but how daylength information is conveyed to the efferent neuroendocrine circuitry responsible for seasonal reproduction (see below) still remains a mystery. While this review focuses on changes in the efferent component of this circuitry, the possibility also exists that there is plasticity in upstream transmission of the photoperiodic signal to this circuitry at the level of melatonin targets in the PMR or elsewhere.
The primary neuroendocrine change responsible for seasonal reproductive transitions in the ewe is a striking change in the ability of estradiol (E2) to inhibit GnRH and LH pulse frequency (Legan & Karsch, 1979; Malpaux et al., 1988; Karsch et al., 1993); this negative feedback loop is common to all mammals, whether they exhibit seasonal patterns of reproduction or not, and an increase in responsiveness to negative feedback often accompanies periods of anovulation (Goodman & Inskeep, 2006; McNeilly, 2006; Plant & Witchel, 2006). The change in the responsiveness of the GnRH system to E2 negative feedback is seen most dramatically in ovariectomized (OVX) animals bearing implants that maintain a constant low, physiological level of E2 throughout the year. In such animals (OVX+E), GnRH/LH pulse frequencies are high during the breeding season, but slow markedly with the onset of the anestrous or non-breeding season. These changes reflect an increased responsiveness of the GnRH pulse generator to the inhibitory influence of E2; inhibition of GnRH pulses, in turn, directly precludes the rise in circulating LH and E2 necessary for the mid-cycle GnRH/LH surge that triggers ovulation. Consequently, during anestrus, ovarian cycles are blocked. Nevertheless, it is important to note that the positive feedback influence of E2 to induce a GnRH/LH surge is not impaired during anestrous, and a GnRH surge can be artificially produced by high levels of E2 at any time of year (Karsch et al., 1980). Thus, the seasonal interruption of ovarian cycles is directly due to the increased ability of E2 to inhibit episodic GnRH secretion during anestrous, and much of the research into the neuroendocrine mechanisms underlying seasonal reproduction has focused on how this negative feedback influence is conveyed to the GnRH system.
2. Neural circuitry mediating estradiol negative feedback during anestrus
GnRH cell bodies in sheep and other mammals appear to lack detectable estrogen receptor-α protein (ER-α) (Lehman et al., 1993; Lehman & Karsch, 1993a), the isoform critical for the negative feedback influence of E2 during anestrus (Hardy et al., 2003). Hence, much work has focused on identifying the afferent circuitry by which E2 exerts its inhibitory effects on GnRH neurons. A key set of neurons implicated in the control of seasonal breeding in the ewe, and in conveying the negative feedback influence of E2, is a collection of dopaminergic cells located in the retrochiasmatic area (RCh) of the sheep hypothalamus, termed the A15 cell group (Meyer & Goodman, 1985; Thiery et al., 1989; Gayrard et al., 1994; Havern et al., 1994; Lehman et al., 1996; Goodman et al., 2000). Evidence that dopaminergic neurons might be involved in this circuitry stemmed from earlier observations that dopamine D2 receptor antagonists block the ability of E2 to inhibit GnRH/LH pulses during anestrus when delivered either peripherally or intracerebrally (Meyer & Goodman, 1985). The role of the A15 dopamine cell group as the origin of this influence has been supported by a variety of experimental evidence, including: (1) the ability of A15 lesions to block E2 negative feedback in anestrous ewes (Havern et al., 1994); (2) the ability of E2 to induce the immediate early gene product, Fos, specifically during anestrus, but not the breeding season (Lehman et al., 1996); (3) the ability of E2 to increase multi-unit activity in the A15 during anestrus (Goodman et al., 2000); and electrophysiological data showing that (4) A15 multi-unit activity decreases before GnRH/LH pulses (Martin & Thiery, 1987), and that (5) stimulation of the A15 inhibits pulses (Martin & Thiery, 1987).
It is notable, however, that A15 dopamine cells, just like GnRH neurons, do not contain ER-a (Lehman et al., 1993; Skinner & Herbison, 1997); thus, estradiol’s influence must again be indirect. More recent anatomical and hormone implant studies have provided strong evidence for two sites of ER-α containing cells in the sheep brain which project to the A15, and convey the E2 signal to this nucleus: the ventromedial preoptic area (vmPOA) and RCh (Lehman & Karsch, 1993b; Anderson et al., 2001). As we currently understand the circuitry mediating E2 negative feedback during anestrus it consists of E2-responsive neurons in the vmPOA and RCh, their connections to dopaminergic A15 neurons, and A15 projections, either indirectly (see discussion below) or directly to GnRH cell bodies. In sheep, GnRH cell bodies are located in both the mediobasal hypothalamus (MBH) and preoptic area. Since dopaminergic terminals have been shown to be in axo-axonic contact with GnRH terminals in the sheep median eminence (Havern et al., 1991), this is another potential site of influence at which A15 neurons might influence GnRH secretion.
3. Thyroid hormones are required for seasonal reproductive transitions
In addition to melatonin and its role as a tranducer of photoperiod, thyroid hormone is another critical endogenous signal necessary for seasonal reproductive transitions in sheep (Karsch et al., 1995), as well as other mammals and birds (Bechtold & Loudon, 2007). In ewes, the presence of thyroid hormone is an essential signal for the increase in E2 negative feedback that underlies the transition from the breeding season to anestrus; thyroidectomy (THX) blocks this transition resulting in animals that remain in a persistent “breeding season”-like state (Dahl et al., 1994). However, THX in anestrus does not alter the animal’s reproductive status or her transition into the breeding season (Karsch et al., 1995) so thyroid hormone is only necessary for the events underlying the onset of anestrus. It is also noteworthy that THX affects seasonal rhythms in reproduction, but not other endpoints. For example, THX ewes still show annual rhythms in prolactin secretion (Dahl et al., 1994).
One important unresolved issue is whether thyroid hormones are permissive or a long day-induced increase drives important reproductive changes. Systemic replacement studies in ewes indicate that thyroid hormone was permissive since constant low levels of T4 are as effective as high ones in promoting the seasonal transition (Dahl et al., 1995). However, more recent data primarily from birds and hamsters (reviewed by (Nakao et al., 2008b; Revel et al., 2009)), has raised the possibility that an increase in the local conversion of T4 to T3 is a critical first step in the reproductive effects of long days. Circulating T4 must be converted to T3 by the enzyme type 2 deiodinase (Dio2) before producing effects in the brain. In rodents and birds, Dio2 expression is concentrated in tancytes, specialized cells in the ependymal layer lining the lower portion of the third ventricle, and its expression increases rapidly after exposure to long days. This increase is melatonin-dependent and there is increasing evidence that it reflects an action of melatonin in the pars tuberalis (where the highest concentration of melatonin receptors are found in all species), and more specifically in thyrotropes found in this region (Nakao et al., 2008a; Hanon et al., 2010). According to this intriguing hypothesis, a switch from short to long days induces TSH synthesis and secretion from these thyrotropes, which acts on the tanycytes to induce synthesis of Dio2. The resulting local increase in T3 then stimulates the reproductive axis. While there is strong evidence for this sequence of events, there are also two key questions as to its applicability to seasonal changes in the hamster. First, in contrast to sheep and birds, thyroidectomized hamsters appear to respond relatively normally to photoperiodic manipulations (Champney, 1988). Second, in the natural environment, testicular growth occurs several months before male hamsters experience long days, a condition known as photorefractoriness (Turek & Campbell, 1979), so it is unclear how a long-day induced increase in Dio2 can be physiologically important.
There is also some evidence for this model in sheep (Hanon et al., 2008), but there are similar caveats. In particular, thyroid hormones are critical for the transition to anestrus during the shortest days of the year (December through February; (Thrun et al., 1996)) so it is again difficult to see the relevance of long day-induced changes. Moreover, microimplant studies point to the PMR (Malpaux et al., 1998b), not the pars tuberalis (Malpaux et al., 1995), as the important site of melatonin action to time reproductive seasons in ewes. Thus the physiological relevance of the photoperiodic control of this TSH-Dio2 axis in ewes remains to be determined. It is interesting to note that there is a restricted window of time during which T4 replacement is able to reinstate the transition to anestrus; T4 replacement is effective when initiated during the period that extends from the normal late breeding season to mid-anestrus, but not at other times of year (Wayne et al., 1990; Karsch et al., 1995; Thrun et al., 1996; Billings et al., 2002). It is tempting to speculate that changes in Dio2 expression underlie this window of response to T4, but this awaits experimental confirmation. Finally, the permissive influence of thyroid hormones is due to action in the CNS since intraventricular or intracerebral delivery of T4 is sufficient to reverse the effect of THX. Interestingly, two of the targets of T4 action in the sheep brain are the PMR and vmPOA (Anderson et al., 2003). The PMR may be a common site of action where the influences of both thyroid hormone and photoperiod (via melatonin) converge to control seasonal reproduction, whereas the vmPOA, as mentioned above, is a key target for estradiol’s negative feedback action. While T4 implants in the RCh alone are not effective in reversing the effects of THX (Anderson et al., 2003), thyroid hormone receptor-alpha is present within A15 dopamine neurons (Jansen et al., 1997), suggestive that T4 may possibly have direct effects on this nucleus as well.
Of particular interest in the context of seasonal plasticity is a large body of evidence implicating thyroid hormones as promoters of morphological rearrangements that occur in the developing brain (Slotkin & Slepetis, 1984; Thompson & Potter, 2000; Horn & Heuer, 2010). T4 deficiency results in multiple morphological alterations in the neonatal rat brain, leading to significantly decreased axonal density and reduced number and altered distribution of dendritic spines in the cerebral cortex (Bradley et al., 1960). In addition, hypothyroid neonatal rats also demonstrate reduced dendritic branching of neurons, a deficiency in axonal myelin formation, and a reduction in synapse formation (Thompson & Potter, 2000; Stoica et al., 2007). Since thyroid hormones are required for the transition from the breeding season to anestrus, but not for the subsequent transition back to the breeding season, their role has been seen as analogous to the organizational effects of steroid hormones during development in enabling structural changes that are responsible for the “anestrous” state. Given the dependence of the seasonal change in E2 negative feedback upon thyroid hormones, one strong prediction is that morphological changes causally related to seasonal reproduction should be blocked by thyroidectomy, and reinstated by T4 replacement. As described below, this criterion has proven especially useful in identifying functionally relevant morphological changes in the circuitry controlling seasonal reproduction.
4. Anatomical sites of seasonal morphological plasticity
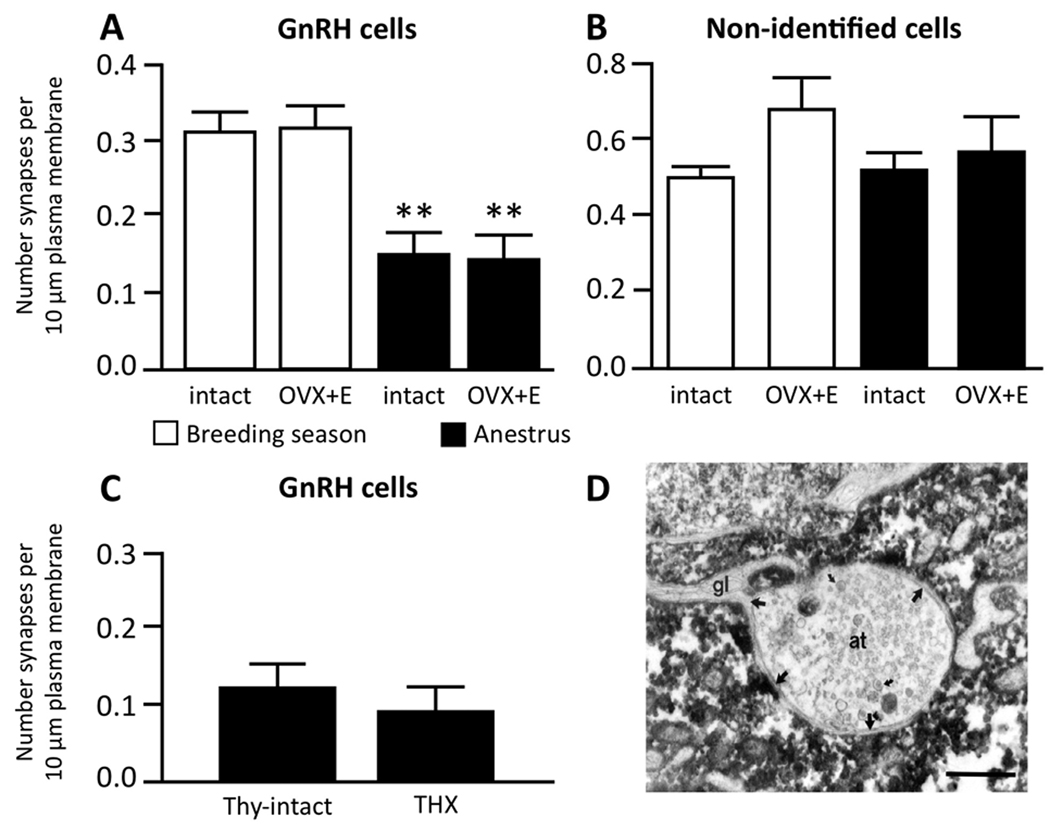
The possibility that morphological changes are responsible for the functional switch in E2 negative feedback between the breeding season and anestrous has been investigated at several levels of this neural circuit. Initial studies focused on the possibility of morphological plasticity at the level of GnRH neurons and their inputs (Lehman et al., 1997; Xiong et al., 1997). No seasonal differences in the number of detectable GnRH neurons, their location, or number of dendrites were observed in the sheep brain (Lehman et al., 1986). However, electron microscopic (EM) analysis revealed a significant increase in the number of synaptic inputs onto GnRH neurons in the POA (Fig. 1A), with these particular GnRH cells shown to receive almost twice the density of synaptic inputs during the breeding season compared to anestrus (Fig. 1A) (Xiong et al., 1997). In contrast, no seasonal change in the amount of synaptic input to neighboring, non-identified (non-GnRH) neurons was observed (Fig. 1B). In addition to the increase in the synaptic input to GnRH neurons, a seasonal rearrangement of glial elements in association with GnRH cell bodies was also observed. Specifically, an inverse relationship was seen between the degree of glial ensheathment of GnRH somas and dendrites, and the density of synapses onto those postsynaptic elements (Xiong et al., 1997). These morphological observations of glial-neuronal interactions at the level of GnRH cell bodies (e.g., Fig. 1D), and their seasonal variation, complemented evidence in non-seasonal rodents of functional plasticity in glial-GnRH associations at the level of terminals in the median eminence (Prevot, 2002; Ojeda et al., 2008). Studies in the Japanese quail have shown seasonal morphological alterations in the associations between GnRH terminals and glial elements in the median eminence (Yamamura et al., 2004), but this possibility has yet to be explored in the sheep or other seasonal mammals.
Figure 1.
Seasonal plasticity in synaptic inputs onto GnRH neurons in the sheep. A: GnRH neurons in the preoptic area receive about half the density of synaptic inputs (synapses/10 µm plasma membrane) during anestrus compared to the breeding season. This change was seen in both ovary-intact (intact) and ovariectomized ewes bearing 1 cm sc E2 implants (OVX+E). B: Adjacent non-identified neurons in the preoptic area showed no significant changes in their synapses; n=8/group for A and B. C: Thyroidectomy (THX) failed to block the decrease in synaptic density onto GnRH neurons observed in thyroid (Thy)-intact ewes during anestrus; n=8/group. D: Glial-neuronal interactions are important for plasticity of GnRH inputs. Electron micrograph of a GnRH cell from an anestrous ewe demonstrating an axon terminal (at) almost entirely surrounded by a GnRH cell but separated from it by extensions of a thin glial process (gl). Bar = 2 µm. (A, B modified from (Xiong et al., 1997); C: modified from (Adams et al., 2006)).
Since the direction of these seasonal changes in synaptic input were opposite of what we might have predicted (anestrous > breeding season), we speculated that they may reflect seasonal alterations in the proportions of excitatory and inhibitory inputs. Thus the increased sensitivity to E2 negative feedback during anestrus may not simply be the result of increased inhibitory dopaminergic input, but rather be due to a decrease in excitatory input. In fact, there is evidence that the number of dopaminergic synapses onto GnRH neurons of sheep does not change with season (Pompolo et al., 2003). However, this study examined only GnRH neurons in the POA, while the inhibitory effects of dopamine on pulsatile GnRH release appear to be exerted at the level of the MBH (Havern et al., 1991). Furthermore, the observed changes in synaptic input to POA GnRH neurons may not be causally related to increased sensitivity to E2 negative feedback during anestrus. Although THX prevents the transition from the breeding season to anestrus, it fails to block the decrease in synaptic input to GnRH neurons observed in thyroid intact anestrous ewes (Adams et al., 2006). However, definitive interpretation of these results is made difficult by the fact that the observations are based on the total number of synaptic inputs, rather than specific inputs. There are numerous specific neurotransmitter/neuropeptide systems which show seasonal variation in the inputs to GnRH neurons (Smith et al., 2008a; Sergeeva & Jansen, 2009), and one or more of these may be important for the seasonal reproductive changes. One of these (discussed in more detail below) is the seasonal change recently observed in kisspeptin inputs to GnRH neurons, specifically to GnRH cells located in the MBH (Smith et al., 2008a).
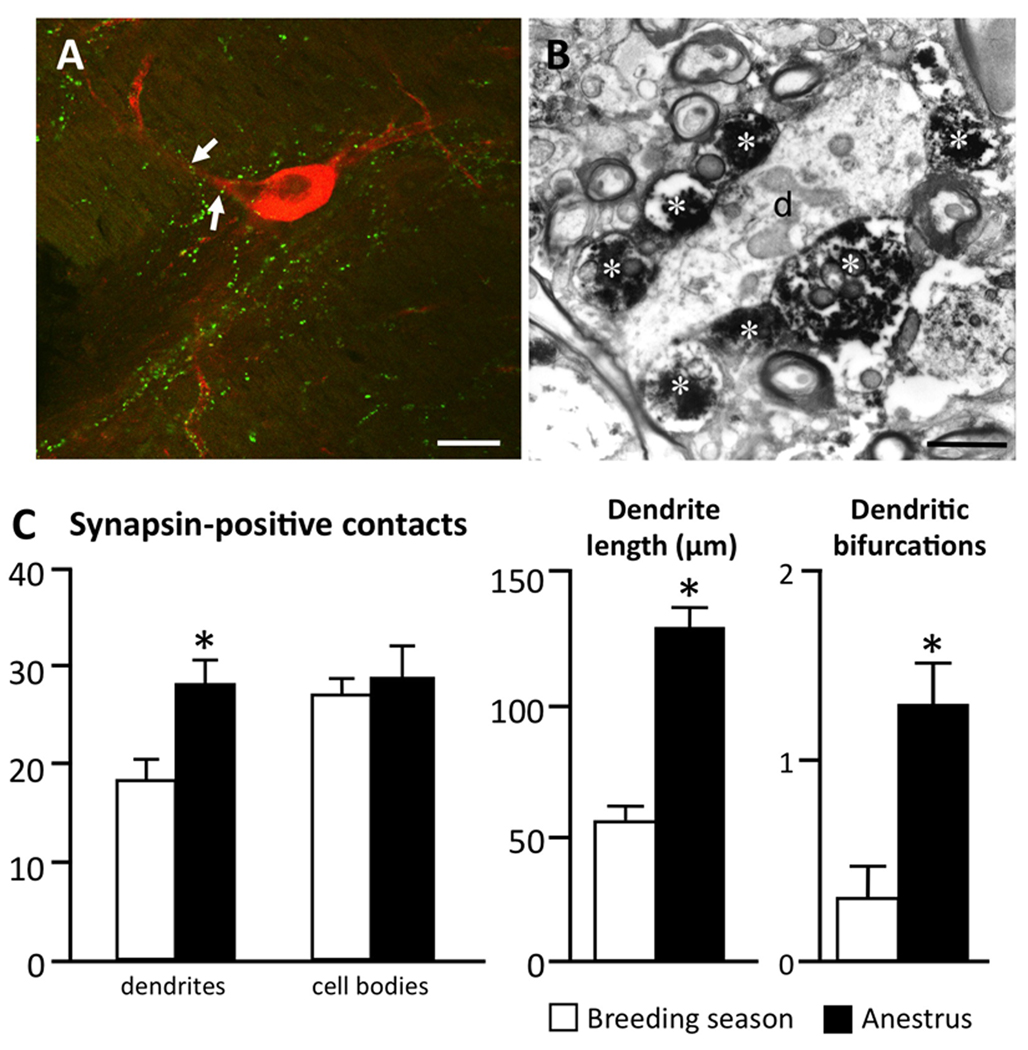
The possibility that seasonal morphological plasticity at the level of the A15 dopamine neurons or their inputs may contribute to this seasonal transition to anestrus has also been examined. It appears that both the dendritic morphology of the A15 neurons and their synaptic input vary (Adams et al., 2006; Singh et al., 2009). For analysis of A15 inputs, as an alternative for EM level analyses, confocal microscopic observations using markers of presynaptic terminals (e.g., synapsin protein) were used in these studies. To validate this approach, correlative light and EM analysis was performed in which close contacts between synapsin-positive terminals and tyrosine hydroxylase (TH)-positive A15 neurons observed in 1 µm thick confocal optical sections (Fig. 2A) were later shown to be bona fide presynaptic terminals at an EM level (Fig. 2B). With this approach, we found an increase in the number of synapsin-positive close contacts onto A15 dendrites during anestrus compared to the breeding season (Fig. 2C). In addition, A15 neurons showed increased dendritic length and bifurcations during the anestrus season (Fig. 2D), and an effect abolished when the thyroid gland was removed (Adams et al., 2006). These results provided initial evidence that plasticity at the level of inputs onto the A15 dopaminergic neurons may contribute to the seasonal transition to anestrus.
Figure 2.
A: Confocal image (1µm thick) of synapsin-positive contacts (e.g., white arrows) onto a tyrosine hydroxylase (TH)-positive cell in the A15 of an anestrous ewe. Bar = 20 µm. B: Electron micrograph demonstrating synapsin-positive axon terminals (asterisks) contacting an unidentified dendrite (d) in the A15 of an anestrus ewe. Bar = 2 µm. C: Seasonal change in mean number (±SEM) of synapsin-positive terminals onto TH-immunoreactive dendrite and cell bodies, TH-immunoreactive dendrite length, and mean number of dendritic bifurcations of A15 neurons. n=6/anestrous group, n=8/breeding season group. * p<0.05. (modified from (Adams et al., 2006)).
More recently, the neurochemical phenotype of afferents controlling A15 neural activity in anestrus has been investigated. Pharmacological and anatomical studies have implicated both GABA and glutamate as key regulators of dopaminergic control of GnRH secretion in the ewe (Singh et al., 2009). Virtually all A15 neurons receive synaptic input from glutamatergic and GABAergic afferents and the results of local administration of receptor agonists and antagonists indicate that E2 stimulates glutamate, and inhibits GABA, release (Singh et al., 2009). Interestingly, there are seasonal changes in glutamatergic, but not GABAergic inputs, with A15 neurons receiving more close contacts with terminals containing vesicular glutamate transporter-2 (vGlut2) during anestrus than the breeding season (Singh et al., 2009). While the source of this glutamatergic input has yet to be determined, the evidence thus far suggests that seasonal plasticity in this stimulatory input to the A15 may account, at least in part, for the increased activity of A15 neurons during anestrus (Gayrard et al., 1994; Lehman et al., 1996), perhaps leading to the inhibition of GnRH pulses.
As noted above, a key prediction is that plasticity which plays a functional role in the circuitry underlying seasonal reproduction should be thyroid hormone dependent. With this rationale, we asked whether seasonal changes in glutamatergic input to A15 neurons are thyroid-dependent. To determine this, we compared A15 neurons between three groups of OVX+E ewes: 1) thyroid-intact animals perfused during the breeding season; 2) thyroid-intact animals perfused during anestrus, and thyroidectomized (THX) animals perfused during anestrus, which should remain in a “breeding season” state. Thyroidectomies were performed in late November or early December, before the end of the breeding season, and confirmed as complete by undetectable (<1.6ng/ml) serum T4 concentrations. Serum LH levels in thyroid-intact, anestrus ewes were undetectable as expected due to the heightened level of E2 negative feedback seen at that time of year, whereas THX ewes maintained high LH levels in anestrus similar to those seen in thyroid-intact, breeding season ewes. Sections through the A15 region of these animals were processed for dual-immunofluorescent detection of either vGLUT-1 or vGLUT-2 and TH, and A15 neurons analysed by confocal microscopy for close contacts between vGLUT-positive terminals and TH neuronal somas and dendrites (for details see Supporting Information).
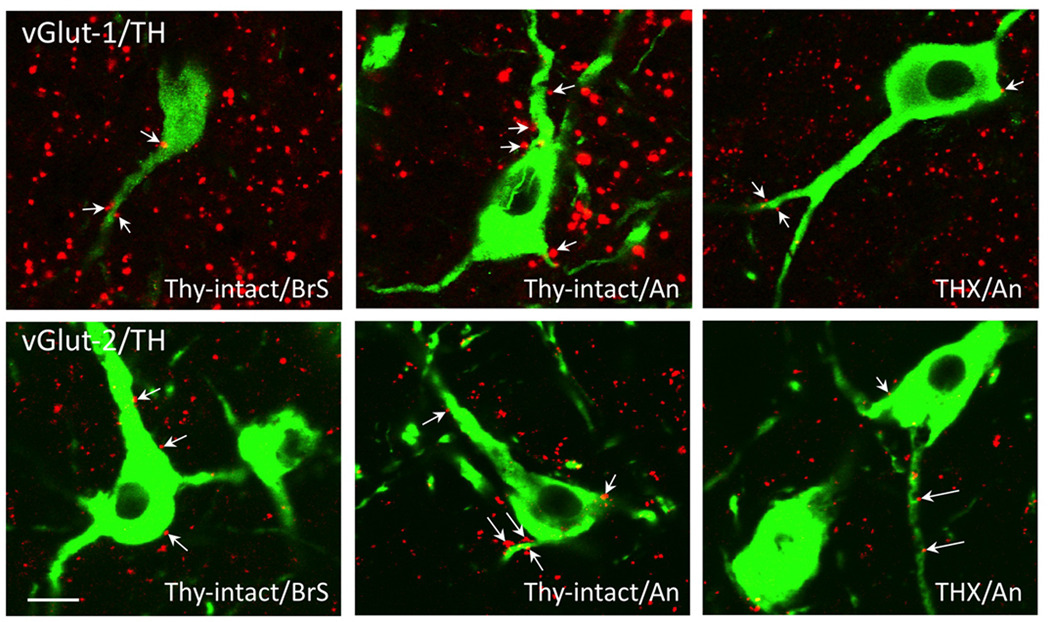
Numerous vGLUT-1 and −2 positive terminals were seen in contact with TH-positive A15 neurons on both their somas and dendrites (Fig. 3). Since dual-label experiments have not shown vGLUT-1 and vGLUT-2-immunoreactivity to be colocalized in the sheep hypothalamus (Merkley, 2009), the terminals observed in contact with A15 neurons (Fig. 3) likely represent different sets of inputs. In the case of both vGLUT-1 and vGLUT-2, we found a significant increase in the mean density of inputs to A15 neurons in anestrous ewes compared to the breeding season (Fig. 4). Significant differences were seen both at the level of axosomatic and axodendritic inputs; this result differs from the earlier analysis of seasonal changes in total inputs using synapsin-1 as a marker (Fig. 2), where seasonal differences were seen at the level of dendrites only. The fact that the methods of analysis varied between these studies (absolute numbers of contacts vs. contacts per unit cell surface) may account for this difference.
Figure 3.
Representative confocal images (1µm thick) of vGlut1 (red, top row) and vGlut2 (red, bottom row) close contacts (e.g., arrows) onto TH-immunoreactive cells (green) in thyroid-intact ewes sacrificed during the breeding season (Thy-intact/BrS) or anestrus (Thy-intact/An), and thyroidectomized ewes sacrificed during anestrus (THX/An). Bar = 20 µm.
Figure 4.
Number of close contacts/100µm cell surface (mean ± SEM) of vGlut1-positive (A) and vGlut2-positive (B) terminals onto TH-immunoreactive A15 cells (soma, dendrite, combined total) in thyroid-intact ewes sacrificed during the breeding season (Thy-intact/BrS; black bars; n=6) or anestrus (Thy-intact/An; white bars; n=6), and in thyroidectomized ewes sacrificed during anestrus (THX/An; grey bars, n=6). Mean density of inputs were compared between groups using a one-way ANOVA, followed by post hoc analyses using the Fisher LSD Method. *indicates significant difference from Thy-intact/BrS (p<0.05); # indicates significant difference from THX/An (p<0.05).
The seasonal change in somal and dendritic vGlut1 and vGLUT-2 inputs to A15 neurons during the anestrus season were completely blocked in THX ewes, and the density of inputs seen in these animals was similar to that seen in the brain of thyroid-intact animals during the breeding season (Fig. 4). Thus, the seasonal change in glutamatergic inputs to A15 appears to be dependent on the presence of thyroid hormones, paralleling the seasonal alteration in E2 negative feedback. In earlier work we have also shown that seasonal changes in dendrite length, like those in glutamate inputs, are blocked by THX thyroidectomy, and are rescued by T4 replacement in THX animals (Adams et al., 2006). However, since the analyses of both A15 inputs and dendritic morphology were based on TH-immunoreactivity, a caveat to these observations is the possibility that these changes reflect seasonal differences in the expression of the markers used, vGLUT-1, −2 and TH, rather than morphological plasticity. For glutamatergic inputs, this possibility could be addressed by including a third marker for synaptic terminals (e.g., synapsin); for dendritic changes, it may be possible to use techniques such as diolistics (O’Brien & Lummis, 2006) to completely label the dendritic processes of individual TH-positive neurons and perform seasonal comparisons of A15 morphology using this material.
5. Neurotrophins as possible mediators of seasonal plasticity
Neurotrophins are growth factors that have been defined as trophic factors required for the survival of specific types of neurons (Poo, 2001). The neurotrophins comprise a family of at least four structurally related proteins: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (Poo, 2001). These factors exert their effects through two classes of receptors—high-affinity tyrosine kinase receptors (TrkA, TrkB, and TrkC) and a lower-affinity receptor, p75 (Kaplan & Miller, 1997; Poo, 2001). Binding of a neurotrophin initiates Trk dimerization, phosphorylation of tyrosine residues, and kinase activation (Kaplan & Miller, 1997; Poo, 2001). Trk receptors, in turn, recruit specific signaling molecules that eventually modify gene expression and protein synthesis and result in neuronal differentiation, survival, and morphological changes (Kaplan & Miller, 1997). These neurotrophic factors are necessary for peripheral and central nervous system development, maintenance and response to injury (Lewin & Barde, 1996) but also often mediate morphological plasticity as it occurs normally in the adult brain (McAllister et al., 1999).
The possible role of neurotrophins as mediators of seasonal plasticity arises in part from evidence that many of the organizational actions of T4 during brain development appear to be mediated by the neurotrophins (Alvarez-Dolado et al., 1994; Lindholm et al., 1994; Koibuchi et al., 2003) as well as by evidence in the songbird that neurotrophins are involved in adult neuroplasticity (Bentley et al., 1997; Dittrich et al., 1999; Wissman & Brenowitz, 2009). The latter includes demonstration that NGF infusions produce gonadal regression in thyroidectomized male starlings, as well as the ability of anti-NGF antibody treatment to increase LH levels in photorefractory starlings held on long days (Bentley et al., 1997). In addition, in the male white-crowned sparrow, BDNF mRNA levels increase in response to breeding season photoperiods and BDNF infusion is sufficient for breeding season-like changes in the area and neuronal density of song nuclei (Wissman & Brenowitz, 2009). In mammals, NGF activity appears to be more important than other neurotrophins for T4 actions on the development of a septohippocampal cholinergic system (Hashimoto et al., 1994), whereas the actions of T4 in the hypothalamus are probably mediated by BDNF (Camboni et al., 2003). Interestingly, in the rat BDNF stimulates neurite growth of A14 dopamine neurons (Berg-von der Emde et al., 1995; Loudes et al., 1999) (rodents do not have an A15), producing structural changes similar to those seen in ovine A15 neurons during the transition to anestrus. In contrast, BDNF has no effect on A12 DA cells in the arcuate nucleus (ARC), which respond to NT-3 (Loudes et al., 1999). Moreover, BDNF can be transported by axonal anterograde transport (Spalding et al., 2002), which could explain how T4 actions in the PMR and/or vmPOA might affect functional changes in the A15.
Based on this background, we hypothesized that thyroid hormones influence seasonal plasticity in the A15, and perhaps other sites in the circuit controlling seasonal reproduction, by actions of the neurotrophins, NGF and BDNF, and their receptors TrkA and TrkB. As a first step in testing the role of these neurotrophin signaling pathways in the transition to anestrus, we examined the effects of short-term thyroidectomy on expression of NGF, TrkA, BDNF and TrkB in the A15, vmPOA, and arcuate nucleus (ARC), using quantitative RT-PCR (QPCR) (for details see Supporting Information). OVX+E ewes were either thyroidectomized (THX) in the late breeding season (n=6), or left thyroid-intact (n=5), and sacrificed one month later, prior to the transition to anestrus. Fresh, frozen hypothalami were obtained from these animals, bilateral micropunches of the A15, POA and ARC collected from frozen brain sections, and RNA extracted from individual punches. QRT-PCR was performed for NGF and BDNF, and their receptors, TrkA and TrkB, with fold changes in gene expression calculated using the comparative Ct method (to GAPDH) (Schmittgen & Livak, 2008); data was normalized to the average of all controls animals, and compared between groups.
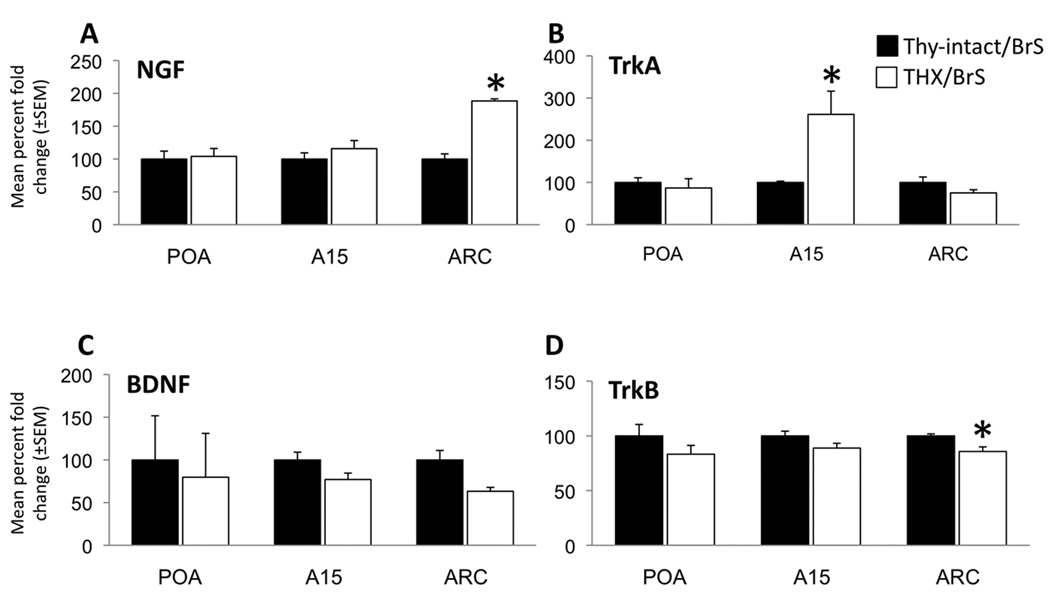
While there were no significant differences between intact and THX ewes in neurotrophin or Trk receptor expression in the POA, we found that NGF mRNA in the ARC was elevated by approximately two-fold in THX ewes, while TrkA in A15 was elevated nearly three-fold in the same animals (Fig. 5). In addition, a smaller but significant decrease in TrkB was seen in the ARC of THX animals compared to intact controls (Fig. 5). There was a trend toward decreased BDNF in the ARC of THX animals as well but this did not reach significance. In interpreting these data, it should be kept in mind that these changes in mRNA levels may not result in similar changes in protein concentrations. Nevertheless, the thyroid-dependent changes in NGF and TrkA suggests that one of the early effects of THX is to increase signaling via NGF/TrkA in a retrograde manner from the ARC to the A15, perhaps contributing to the plasticity observed at the level of A15 neurons and their morphology. It may also be that these changes in both NGF/TrkA, and in TrkB, in the ARC reflect plasticity at the level of either A15 and other inputs to neurons located in that region (see below), or their connections to GnRH cells.
Figure 5.
Percentage fold change ( mean ± SEM) of neurotrophin and receptor mRNA expression by real-time qPCR in the preoptic area (POA), A15, and arcuate nucleus (ARC) of the hypothalamus for NGF (A), TrkA (B), BDNF (C), and TrkB (D) in thyroid-intact (Thy-intact/BrS, black bars, n=5) and thyroidectomized (THX/BrS, white bars, n=6) ewes; * indicate significant difference from Thy-intact/BrS, p<0.05.
It should be noted that the direction of the changes observed in NGF and TrkA mRNA were opposite to that expected if these neurotrophins are required for the plasticity normally seen during the transition from the breeding season to anestrus; i.e., an increase rather than a decrease. However, there is evidence that, in some instances, increases in neurotrophin receptor expression may restrict corresponding neurotrophin availability to the extent that it prevents plasticity (Scott & Ramer, 2010). Another possibility is that increased TrKA in the A15 after THX acts to promote the growth of inhibitory inputs (e.g., GABAergic) with the net result being increased inhibition of A15 neurons in THX ewes, thus preventing their activation in response to E2 during anestrous. In addition, although thyroid hormones can act as transcriptional co-activators or co-repressors (Shibata et al., 1997), the increased NGF/TrkA gene expression we observed after THX is consistent with evidence that a major target for T3 action in the brain is a transcriptional co-repressor gene (Potter et al., 2002). Finally, the modest decreases in BDNF and TrkB might reflect physiologically important changes in a subset of neurons in the ARC and A15 regions. Regardless of the possible mechanisms, confirmation of a functional role for any thyroid-dependent changes in neurotrophin signalling awaits demonstration that manipulating levels of NGF, BDNF, or the activity of their intracellular signalling pathways in the A15 or ARC has functional effects on seasonal reproduction.
6. How do A15 inhibit GnRH release in anestrus? Possible role of arcuate kisspeptin neurons
The projections by which A15 neurons inhibit GnRH pulse frequency in anestrus remain largely unknown. This system appears to act in the MBH, since local disruption of DA action or synthesis in the MBH (Havern et al., 1991; Viguie et al., 1998), but not the POA (Havern et al., 1991), increases LH pulse frequency during anestrus. Tract tracing studies have confirmed that the primary projections of A15 neurons are caudal toward the MBH, not rostral toward the POA (Gayrard et al., 1995; Goubillon et al., 1999; Goodman et al., 2010). Many of these projections are to the internal zone of the median eminence (ME), but TH-positive varicosities containing an anterograde tracer injected into the A15 region were observed in the external zone of the ME and the MBH (Gayrard et al., 1995). As noted above, projections to the ME are consistent with direct inhibition of GnRH via axo-axonal connections, and a few DA synapses on GnRH terminals have been identified in the ME of breeding season ewes (Kuljis & Advis, 1989). However, DA levels (Thiery, 1991) and TH bioactivity (Viguié et al., 1996; Viguie et al., 1997) in the ME are not increased by E2 in anestrous ewes; thus a mechanism for axo-axonal inhibition of GnRH pulse frequency is not readily apparent.
One alternative to direct monosynaptic inhibition of GnRH cells by the A15 is that these neurons act via a multisynaptic pathway. The relatively long delay before increased episodic LH secretion following blockade of DA action (Havern et al., 1991) or inhibition of A15 neurons (Bogusz et al., 2008) is suggestive, but not conclusive, of a multisynaptic rather than a monosynaptic connection. Moreover, a subpopulation in the ARC that is an ideal candidate for mediating DA actions on GnRH secretion are kisspeptin neurons. Kisspeptin is a member of the RFamide peptide family that is well recognized as a key stimulator of the GnRH system (Oakley et al., 2009). Kisspeptin neurons of the ARC in sheep and other mammals also colocalize neurokinin B (Goodman et al., 2004), another neuropeptide that is stimulatory to GnRH secretion (Billings et al., 2010), as well as dynorphin, an endogenous opioid peptide implicated in inhibitory control of GnRH pulses by progesterone (Goodman et al., 2004). Emerging evidence suggests that the ARC population of kisspeptin cells may serve as an important common conduit for the regulation of pulsatile GnRH secretion by endogenous gonadal steroids (Lehman et al., 2010a) as well as stress (Castellano et al., 2010) and nutritional status (Backholer et al., 2010; Corander et al., 2010).
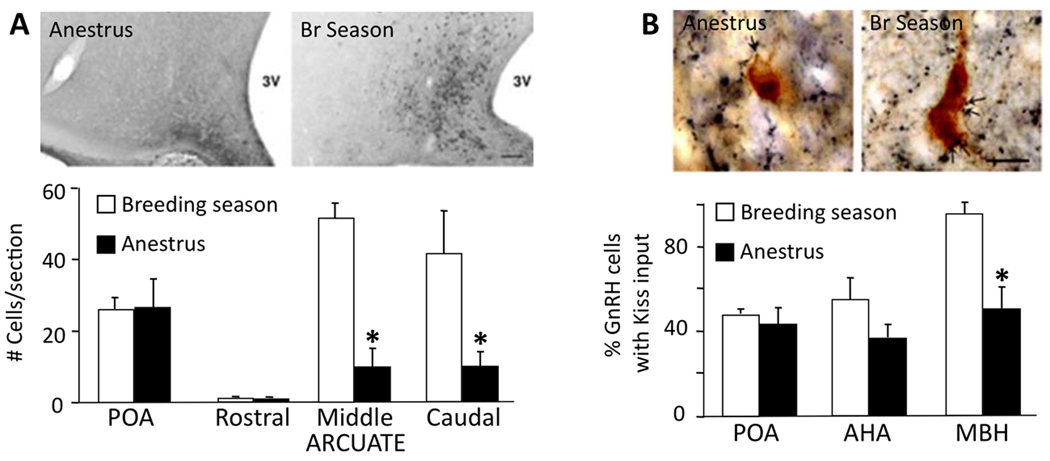
There are several pieces of evidence suggesting that ARC kisspeptin neurons may play an important functional role in the circuitry controlling seasonal changes in E2 negative feedback. First, kisspeptin protein and mRNA expression in the ARC varies seasonally in the ewe, with numbers of immunoreactive cells and mRNA levels being markedly lower during anestrus (Fig. 6A) (Smith et al., 2008b). Importantly, this seasonal difference was observed in OVX+E ewes so that changes in kisspeptin expression were not simply a response to changing E2 levels. Similar seasonal changes have been reported in Kiss-1 mRNA expression in the hamster ARC (Simonneaux et al., 2009). Second, in addition to changes in ARC kisspeptin expression, there are also seasonal changes in kisspeptin-containing inputs onto GnRH cells in the sheep, with a decrease during anestrus in the percentage of GnRH cells receiving such inputs specifically in the MBH (Fig. 6B) (Smith et al., 2008a). Whether this seasonal change reflects a decrease in synaptic input or simply decreased expression of kisspeptin remains to be determined. Thus, a decrease in stimulatory inputs to GnRH neurons during anestrus may allow for increased inhibition of GnRH pulses via either direct input from A15 neurons (at the level of GnRH terminals) and/or other inhibitory afferents. One such candidate for the latter may be GnIH neurons in the dorsomedial hypothalamic nucleus of the sheep, since there are seasonal changes in GnIH expression that are the opposite of those seen for kisspeptin (Smith et al., 2008a). Third, we have preliminary evidence that ARC kisspeptin cells coexpress the D2 dopamine receptor (D2R), the subtype responsible for the seasonal inhibition of GnRH pulses (Meyer & Goodman, 1985; 1986); furthermore, this coexpression changes with season, with about 80% of kisspeptin cells colocalizing D2R in anestrus compared to 45% in the breeding season (Maltby et al., 2008). Fourth, and perhaps most importantly, pilot experiments show that kisspeptin antagonists delivered i.c.v. block the ability of D2R antagonists to stimulate GnRH pulses during anestrous (Lehman et al., 2010b). Thus, during anestrus, A15 dopamine neurons may act to inhibit arcuate kisspeptin neurons, with the net result being the inhibition of GnRH pulses.
Figure 6.
Seasonal changes in kisspeptin cells (A) and their inputs to GnRH neurons (B). A: The number of kisspeptin-immunoreactive neurons in the middle and caudal ARC is decreased during anestrous (* p<.001). Images show examples of sections through the middle ARC immunostained for kisspeptin in OVX+E ewes perfused during either the breeding season or anestrus. Bar = 100 µm. B: The percentage of GnRH cells in the MBH that receive one or more kisspeptin close contact was less in anestrous than in breeding season ewes (* p<.001); GnRH cells in the POA or anterior hypothalamic area (AHA) showed no significant seasonal differences. Images show examples of dual immunoperoxidase stained sections in which close contacts (e.g., arrows) are seen between kisspeptin terminals (blue-black) and GnRH somas (brown). Bar = 15 µm. (A and B modified from (Smith et al., 2008b))
Taken together, these results suggest that kisspeptin cells of the ARC nucleus may be a target for A15 dopamine efferents, and a critical link in the seasonal inhibition of GnRH pulse frequency. This role of kisspeptin neurons is also consistent with results showing that kisspeptin administration induces ovulation in anestrous ewes (Caraty et al., 2007) and reverses the effects of inhibitory photoperiod in male hamsters [Revel, 2006 #237], and raises the possibility that kisspeptin analogues may represent a useful tool for overcoming seasonal anestrus in sheep and other seasonally breeding species.
7. Conclusions and Relevance
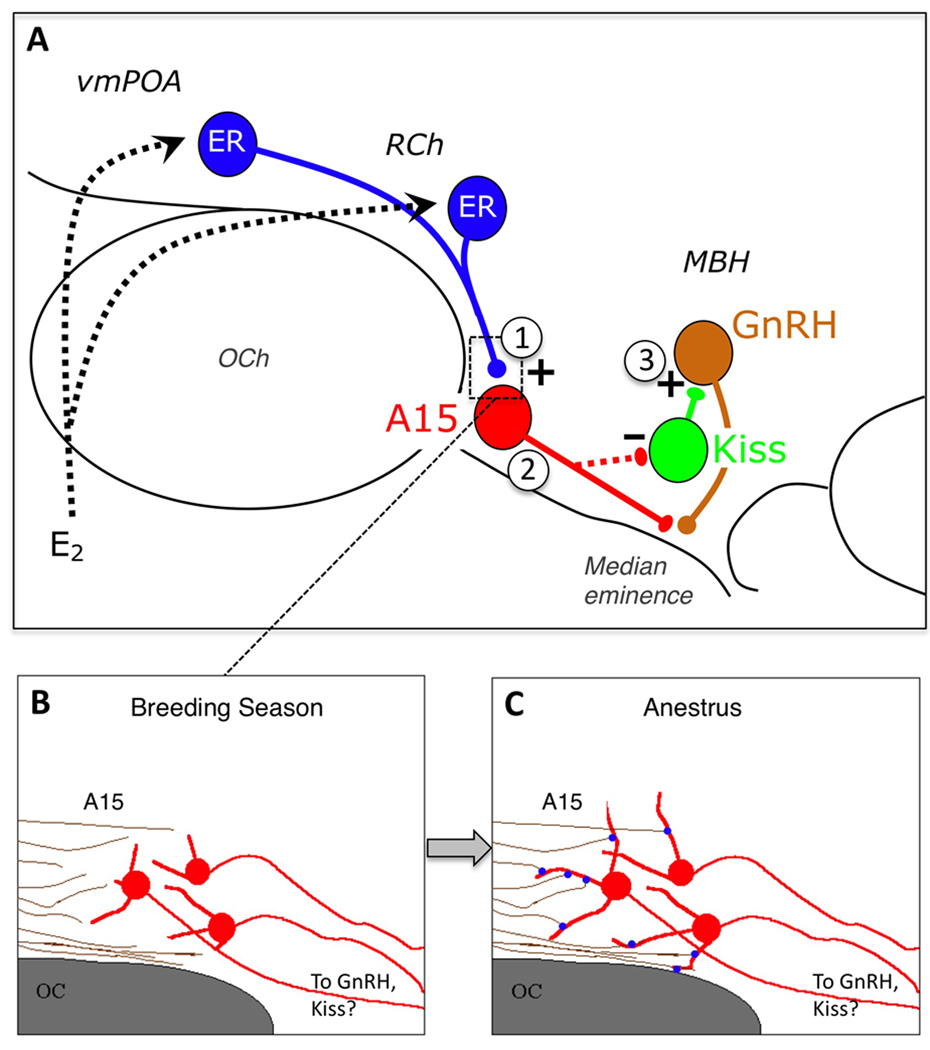
At present, there is evidence for potential neuronal plasticity at three sites in the hypothalamic circuitry mediating seasonal reproduction in the ewe (Fig. 7). Specifically, there are seasonal changes 1) at the level of synaptic inputs to A15 dopamine neurons, specifically glutamatergic terminals; 2) in the dendritic morphology of A15 neurons; and 3) in kisspeptin-containing inputs to GnRH neurons in the MBH. Seasonal changes in both glutamatergic inputs to A15 neurons, and the dendritic morphology of A15 neurons, are thyroid-dependent and parallel the effects of thyroidectomy on the reproductive axis that result in a failure of ewes to enter anestrus (Karsch et al., 1995). The direction of seasonal change in synaptic input, at the level of A15, and for kisspeptin inputs onto GnRH neurons, is consistent with a functional interruption of the circuit whose activity is required for E2 negative feedback. Thus, these morphological changes may well underlie the changing responsiveness of the GnRH pulse generator to the inhibitory influence of E2 that underlies seasonal reproductive transitions in the ewe.
Figure 7.
A: Schematic depiction of the neural circuitry in the hypothalamus regulating seasonal control of estradiol (E2) negative feedback, and sites at which morphological plasticity may contribute to seasonal reproductive transitions. E2 acts upon ER-a (ER) containing cells in the ventromedial preoptic area (vmPOA) and retrochiasmatic area (RCh); these neurons, in turn, contact A15 dopamine cells, which project caudally to the mediobasal hypothalamus (MBH), containing GnRH cells directly at the level of their terminals in the median eminence, and/or via kisspeptin (Kiss) cells in the arcuate nucleus. Thyroid hormone-dependent seasonal plasticity in this circuit has been demonstrated at the level of (1) synaptic contacts onto A15 neurons, specifically those which are glutamatergic, and (2) the dendritic morphology of dopaminergic A15 neurons. In addition, there are seasonal changes in the number of kisspeptin inputs to GnRH neurons in the MBH (3), and plasticity may exist at the level of kisspeptin cell bodies and their inputs as well. B, C: An example of how seasonal plasticity may underlie reproductive transitions in the ewe. Changes in the length of A15 dendrites (red) and the number of glutamatergic synaptic contacts (blue) onto them, that occur between the breeding season and anestrus, may result in a functional “reconnection” of this circuitry, and underlie the ability of E2 to inhibit GnRH secretion at this time of year. The latter may be mediated either directly by A15 projections to GnRH neurons or indirectly via arcuate kisspeptin cells. OCh = optic chiasm.
A putative mechanism for seasonal plasticity in this circuitry involves the potential role of neurotrophins and their receptors, focusing on NGF, BDNF, TrkA and TrkB. In preliminary work, we have found that one of the early events associated with thyroidectomy, occurring before the reproductive transition to anestrus, is an increase in gene expression for NGF and TrkA in the ARC and A15, respectively. Whether alterations in NGF and/or TrkA are maintained for longer periods after thyroidectomy, and whether they can be reversed by T4 replacement, remain to be determined. More importantly, future experiments will need to determine whether intervening in these or other early events is able to functionally reinstate the seasonal shift in responsiveness to E2 negative feedback and the normal transition from the breeding season to anestrus.
Finally, there is growing evidence that kisspeptin neurons in the ARC may be an essential component of the pathway by which A15 dopamine neurons act to inhibit GnRH pulses. Although ARC kisspeptin cells coexpress D2R, we do not yet know whether they receive direct synaptic input from A15 dopamine cells (Fig. 7), or whether there is plasticity in the A15 connection to ARC kisspeptin cells. Given the substantial evidence for the ARC as a site of neuronal plasticity associated with reproductive function and the estrous cycle (Garcia-Segura et al., 1994; Mong & McCarthy, 1999; Naftolin et al., 2007) it is likely that this is also a site at which synaptic and dendritic plasticity play an important role in the seasonal control of reproduction.
Although the findings described here are based on work in the sheep, they are likely to have relevance for reproduction in other vertebrates, including other seasonal breeders. Dopamine has been implicated in the control of seasonal reproduction in teleost fish (Zohar et al., 2010), frogs (Sotowska-Brochocka et al., 1994), birds (El Halawani et al., 2009), deer (Anderson & Barrell, 1998), horses (Besognet et al., 1997; Aurich et al., 2002) and hamsters (Krajnak et al., 1995). For example, in most fish, dopamine neurons in the preoptic-hypothalamic region act directly on gonadotrophs to inhibit LH release (Trudeau, 1997; Zohar et al., 2010) but also act to inhibit GnRH release by direct contacts onto GnRH cells and their terminals onto gonadotrophs (Trudeau, 1997). Interestingly, the inhibitory effects of dopamine on gonadotrophs are mediated by D2 dopamine receptors (Chang et al., 1990), while the direct effects on GnRH neurons are mediated by D2 and D1 receptors at the level of GnRH terminals and cell bodies respectively (Yu et al., 1991). In contrast to the situation in sheep, dopamine cells in fish are thought to be direct targets for E2 and are probably the principal mediators of negative feedback in fish (Trudeau, 1997). However, similar to sheep and other mammals, kisspeptin cells in fish seem to be key stimulatory regulators of both GnRH and gonadotrophs, although it remains to be determined whether they are integrators of photoperiod information or how they interact with dopaminergic circuits (Zohar et al., 2010).
While it is controversial as to whether humans show seasonal rhythms of reproduction (Bronson, 2004), similar reversible episodes of infertility occur before puberty (Terasawa & Fernandez, 2001), post-partum (Yen, 1999), and during lactation (Smith, 1978; McNeilly, 1994). All of these involve, at least in part, a change in response to E2 negative feedback (Kulin et al., 1969; Kelch et al., 1973; McNeilly et al., 1994a), but the underlying alterations in the hypothalamic-pituitary-gonadal axis remain largely unknown. However, there are some interesting pieces of evidence that suggest the underlying circuitry as described above in sheep may be conserved in primates. This includes: 1) evidence implicating GABA and glutamate in menarchy in monkeys (Mitsushima et al., 1994; Kasuya et al., 1999), 2) the analogous role of the PMR in controlling changes in response to E2 in sheep (Malpaux et al., 1998a), monkeys (Terasawa et al., 1984), and humans (Miller & Styne, 1999), and 3) reports that acquired hypothyroidism can lead to precocious puberty (LaFranchi, 2000; Chattopadhyay et al., 2003). In addition, there are a number of clinical reproductive disorders characterized by altered E2 negative feedback (Reame et al., 1985; Couzinet et al., 1999); one of these, hypothalamic amenorrhea, appears to involve increased DA inhibition of LH (Quigley et al., 1980). Thus information gleaned from studies in seasonally breeding mammals may have relevance not only for understanding normal physiological processes that control human fertility, but also for our understanding of diseases where there is an interruption of ovarian function due to changes in responsiveness to E2 at the level of the brain. The ability in the sheep to directly sample the output of GnRH neurons in portal blood (Caraty et al., 1982; Clarke & Cummins, 1982; Moenter et al., 1991) performed in conjunction with experimental manipulations to functionally dissect neural pathways, should continue to make it a valuable model for investigating these issues, as well as the physiological role of neuronal plasticity in the adult brain.
Supplementary Material
Acknowledgements
The work described in this paper was supported by NIH grant R01 HD017864 to M.N.L. and R.L.G. We would like to thank the many trainees, collaborators, and friends who contributed to this work and the development of the sheep as a model for seasonal reproduction.
References
- Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147:4843–4851. doi: 10.1210/en.2006-0408. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Iglesias T, Rodriguez-Pena A, Bernal J, Munoz A. Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Brain Res Mol Brain Res. 1994;27:249–257. doi: 10.1016/0169-328x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Barrell GK. Pulsatile luteinizing hormone secretion in the ovariectomized, thyroidectomized red deer hind following treatment with dopaminergic and opioidergic agonists and antagonists. Biol Reprod. 1998;59:960–968. doi: 10.1095/biolreprod59.4.960. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL. Oestradiol microimplants in the ventromedial preoptic area inhibit secretion of luteinizing hormone via dopamine neurones in anoestrous ewes. J Neuroendocrinol. 2001;13:1051–1058. doi: 10.1046/j.1365-2826.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Hardy SL, Valent M, Billings HJ, Connors JM, Goodman RL. Evidence that thyroid hormones act in the ventromedial preoptic area and the premammillary region of the brain to allow the termination of the breeding season in the ewe. Endocrinology. 2003;144:2892–2901. doi: 10.1210/en.2003-0322. [DOI] [PubMed] [Google Scholar]
- Aurich C, Gerlach T, Aurich JE, Hoppen HO, Lange J, Parvizi N. Dopaminergic and opioidergic regulation of gonadotropin and prolactin release in stallions. Reprod Domest Anim. 2002;37:335–340. doi: 10.1046/j.1439-0531.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- Barrell GK, Moenter SM, Caraty A, Karsch FJ. Seasonal changes of gonadotropin-releasing hormone secretion in the ewe. Biol Reprod. 1992;46:1130–1135. doi: 10.1095/biolreprod46.6.1130. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Loudon ASI. Hypothalamic thyroid hormones: Mediators of seasonal physiology. Endocrinology. 2007;148:3605–3607. doi: 10.1210/en.2007-0596. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Goldsmith AR, Juss TS, Dawson A. The effects of nerve growth factor and anti-nerve growth factor antibody on the neuroendocrine reproductive system in the european starling sturnus vulgaris. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1997;181:133–141. doi: 10.1007/s003590050100. [DOI] [PubMed] [Google Scholar]
- Berg-von der Emde K, Dees WL, Hiney JK, Hill DF, Dissen GA, Costa ME, Moholt-Siebert M, Ojeda SR. Neurotrophins and the neuroendocrine brain: Different neurotrophins sustain anatomically and functionally segregated subsets of hypothalamic dopaminergic neurons. J Neurosci. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besognet B, Hansen BS, Daels PF. Induction of reproductive function in anestrous mares using a dopamine antagonist. Theriogenology. 1997;47:467–480. doi: 10.1016/s0093-691x(97)00005-8. [DOI] [PubMed] [Google Scholar]
- Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin b acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151:3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings HJ, Viguie C, Karsch FJ, Goodman RL, Connors JM, Anderson GM. Temporal requirements of thyroid hormones for seasonal changes in lh secretion. Endocrinology. 2002;143:2618–2625. doi: 10.1210/endo.143.7.8924. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Karsch FJ, Hopkins JW. Role of the pineal gland in ovine photoperiodism: Regulation of seasonal breeding and negative feedback effects of estradiol upon luteinizing hormone secretion. Endocrinology. 1983;113:329–336. doi: 10.1210/endo-113-1-329. [DOI] [PubMed] [Google Scholar]
- Bogusz AL, Hardy SL, Lehman MN, Connors JM, Hileman SM, Sliwowska JH, Billings HJ, McManus CJ, Valent M, Singh SR, Nestor CC, Coolen LM, Goodman RL. Evidence that {gamma}-aminobutyric acid is part of the neural circuit mediating estradiol negative feedback in anestrous ewes. Endocrinology. 2008;149:2762–2772. doi: 10.1210/en.2007-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PB, Eayrs JT, Schmalbach K. The electroencephalogram of normal and hypothyroid rats. Electroencephalogr Clin Neurophysiol. 1960;12:467–477. doi: 10.1016/0013-4694(60)90022-5. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Are humans seasonally photoperiodic? J Biol Rhythms. 2004;19:180–192. doi: 10.1177/0748730404264658. [DOI] [PubMed] [Google Scholar]
- Camboni D, Roskoden T, Schwegler H. Effect of early thyroxine treatment on brain-derived neurotrophic factor mrna expression and protein amount in the rat medial septum/diagonal band of broca. Neuroscience Letters. 2003;350:141–144. doi: 10.1016/s0304-3940(03)00880-2. [DOI] [PubMed] [Google Scholar]
- Caraty A, Orgeur P, Thiery JC. [demonstration of the pulsatile secretion of lh-rh into hypophysial portal blood of ewes using an original technic for multiple samples] C R Seances Acad Sci III. 1982;295:103–106. [PubMed] [Google Scholar]
- Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Romero M, Pineda R, Ruiz-Pino F, Garcia-Galiano D, Sanchez-Garrido MA, Pinilla L, Mikkelsen JD, Tena-Sempere M. Acute inflammation reduces kisspeptin immunoreactivity at the arcuate nucleus and decreases responsiveness to kisspeptin independently of its anorectic effects. Am J Physiol Endocrinol Metab. 2010;299:E54–E61. doi: 10.1152/ajpendo.00081.2010. [DOI] [PubMed] [Google Scholar]
- Champney TH. Hormonal modulation of pineal-mediated seasonal events: Effects of thyroid or gonadal manipulations. J Pineal Res. 1988;5:219–227. doi: 10.1111/j.1600-079x.1988.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Chang JP, Yu KL, Wong AO, Peter RE. Differential actions of dopamine receptor subtypes on gonadotropin and growth hormone release in vitro in goldfish. Neuroendocrinology. 1990;51:664–674. doi: 10.1159/000125408. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Kumar V, Marulaiah M. Polycystic ovaries, precocious puberty and acquired hypothyroids: The van wyk and grunbach syndrome. J Pediatric Surg. 2003;38:1390–1392. doi: 10.1016/s0022-3468(03)00403-2. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (gnrh) and luteinizing hormone (lh) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP. The effects of neurokinin b upon gonadotrophin release in male rodents. J Neuroendocrinol. 2010;22:181–187. doi: 10.1111/j.1365-2826.2009.01951.x. [DOI] [PubMed] [Google Scholar]
- Couzinet B, Young J, Brailly S, Bouc YL, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: A partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol. 1999;50 doi: 10.1046/j.1365-2265.1999.00649.x. [DOI] [PubMed] [Google Scholar]
- Dahl GE, Evans NP, Moenter SM, Karsch FJ. The thyroid gland is required for reproductive neuroendocrine responses to photoperiod in the ewe. Endocrinology. 1994;135:10–15. doi: 10.1210/endo.135.1.8013340. [DOI] [PubMed] [Google Scholar]
- Dahl GE, Evans NP, Thrun LA, Karsch FJ. Thyroxine is permissive to seasonal transitions in reproductive neuroendocrine activity in the ewe. Biol Reprod. 1995;52:690–696. doi: 10.1095/biolreprod52.3.690. [DOI] [PubMed] [Google Scholar]
- Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mrna in a forebrain song control nucleus of the juvenile zebra finch. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8241–8246. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Halawani ME, Kang SW, Leclerc B, Kosonsiriluk S, Chaiseha Y. Dopamine-melatonin neurons in the avian hypothalamus and their role as photoperiodic clocks. Gen Comp Endocrinol. 2009;163:123–127. doi: 10.1016/j.ygcen.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Duenas M, Torres-Aleman I, Naftolin F. Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology. 1994;19:445–453. doi: 10.1016/0306-4530(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Gayrard V, Malpaux B, Tillet Y, Thiery JC. Estradiol increases tyrosine hydroxylase activity of the a15 nucleus dopaminergic neurons during long days in the ewe. Biology of Reproduction. 1994;50:1168–1177. doi: 10.1095/biolreprod50.5.1168. [DOI] [PubMed] [Google Scholar]
- Gayrard V, Thiery JC, Thibault J, Tillet Y. Efferent projections from the retrochiasmatic area to the median eminence and to the pars nervosa of the hypophysis with special reference to the a15 dopaminergic cell group in the sheep. Cell Tissue Res. 1995;281:561–567. doi: 10.1007/BF00417874. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Bittman EL, Foster DL, Karsch FJ. Alterations in the control of luteinizing hormone pulse frequency underlie the seasonal variation in estradiol negative feedback in the ewe. Biol Reprod. 1982;27:580–589. doi: 10.1095/biolreprod27.3.580. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Inskeep EI. Neuroendocrine control of the ovarian cycle in sheep. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 2006. pp. 2389–2447. [Google Scholar]
- Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. Neural systems mediating seasonal breeding in the ewe. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Thiery J-C, Delaleu B, Malpaux B. Estradiol increases multiunit electrical activity in the a15 area of ewes exposed to inhibitory photoperiods. Biol Reprod. 2000;63:1352–1357. doi: 10.1095/biolreprod63.5.1352. [DOI] [PubMed] [Google Scholar]
- Goubillon M, Delaleu B, Tillet Y, Caraty A, Herbison AE. Localization of estrogen-receptive neurons projecting to the gnrh neuron-containing rostral preoptic area of the ewe. Neuroendocrinology. 1999;70:228–236. doi: 10.1159/000054481. [DOI] [PubMed] [Google Scholar]
- Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral tsh mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- Hanon EA, Routledge K, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Effect of photoperiod on the thyroid-stimulating hormone neuroendocrine system in the european hamster (cricetus cricetus) J Neuroendocrinol. 2010;22:51–55. doi: 10.1111/j.1365-2826.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- Hardy SL, Anderson GM, Valent M, Connors JM, Goodman RL. Evidence that estrogen receptor alpha, but not beta, mediates seasonal changes in the response of the ovine retrochiasmatic area to estradiol. Biol Reprod. 2003;68:846–852. doi: 10.1095/biolreprod.102.010215. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Furukawa S, Omae F, Miyama Y, Hayashi K. Correlative regulation of nerve growth factor level and choline acetyltransferase activity by thyroxine in particular regions of infant rat brain. J Neurochem. 1994;63:326–332. doi: 10.1046/j.1471-4159.1994.63010326.x. [DOI] [PubMed] [Google Scholar]
- Havern RL, Whisnant CS, Goodman RL. Hypothalamic sites of catecholamine inhibition of luteinizing hormone in the anestrous ewe. Biol Reprod. 1991;44:476–482. doi: 10.1095/biolreprod44.3.476. [DOI] [PubMed] [Google Scholar]
- Havern RL, Whisnant CS, Goodman RL. Dopaminergic structures in the ovine hypothalamus mediating estradiol negative feedback in anestrous ewes. Endocrinology. 1994;134:1905–1914. doi: 10.1210/endo.134.4.7907976. [DOI] [PubMed] [Google Scholar]
- Horn S, Heuer H. Thyroid hormone action during brain development: More questions than answers. Mol Cell Endocrinol. 2010;315:19–26. doi: 10.1016/j.mce.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (gnrh) system of the ewe: Changes in identified gnrh inputs and glial association. Endocrinology. 2003;144:3663–3676. doi: 10.1210/en.2002-0188. [DOI] [PubMed] [Google Scholar]
- Jansen HT, Lubbers LS, Macchia E, DeGroot LJ, Lehman MN. Thyroid hormone receptor ({alpha}) distribution in hamster and sheep brain: Colocalization in gonadotropin-releasing hormone and other identified neurons. Endocrinology. 1997;138:5039–5047. doi: 10.1210/endo.138.11.5481. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- Karsch FJ. Twenty-fifth annual bowditch lecture. Seasonal reproduction: A sage of reversible fertility. Physiologist. 1980;23:29–38. [PubMed] [Google Scholar]
- Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res. 1984;40:185–232. doi: 10.1016/b978-0-12-571140-1.50010-4. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dahl GE, Evans NP, Manning JM, Mayfield KP, Moenter SM, Foster DL. Seasonal changes in gonadotropin-releasing hormone secretion in the ewe: Alteration in response to the negative feedback action of estradiol. Biol Reprod. 1993;49:1377–1383. doi: 10.1095/biolreprod49.6.1377. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dahl GE, Hachigian TM, Thrun LA. Involvement of thyroid hormones in seasonal reproduction. J Reprod Fertil Suppl. 1995;49:409–422. [PubMed] [Google Scholar]
- Karsch FJ, Goodman RL, Legan SJ. Feedback basis of seasonal breeding: Test of an hypothesis. J Reprod Fertil. 1980;58:521–535. doi: 10.1530/jrf.0.0580521. [DOI] [PubMed] [Google Scholar]
- Kasuya E, Nyberg CL, Mogi K, Terasawa E. A role of γ-aminobutyric acid (gaba) and glutamate in control of puberty in female rhesus monkey: Effects of an antisense oligodeoxynucleotide for gad67 messenger ribonucleic acid and mk801 on luteinizing hormone-releasing hormone release. Endocrinology. 1999;140:705–712. doi: 10.1210/endo.140.2.6574. [DOI] [PubMed] [Google Scholar]
- Kelch RP, Kaplan SL, Grumbach MM. Suppression of urinary and plasma follicle-stimulating hormone by exogenous estrogens in prepubertal and pubertal children. J Clin Invest. 1973;52:1122–1128. doi: 10.1172/JCI107278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koibuchi N, Jingu H, Iwasaki T, Chin WW. Current perspectives on the role of thyroid hormone in growth and development of cerebellum. Cerebellum. 2003;2:279–289. doi: 10.1080/14734220310011920. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Lookingland KJ, Nunez AA. Seasonal changes in median eminence dopamine in male syrian hamsters: Role of the gonads and duration of exposure to short days. Brain Res Bull. 1995;37:617–622. doi: 10.1016/0361-9230(95)00053-h. [DOI] [PubMed] [Google Scholar]
- Kulin HE, Grumbach MM, Kaplan SL. Changing sensitivity of the pubertal gonadal hypothalamic feedback mechanism in man. Science. 1969;166:1012–1016. doi: 10.1126/science.166.3908.1012. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Advis JP. Immunocytochemical and physiological evidence of a synapse between dopamine- and luteinizing hormone releasing hormone-containing neurons in the ewe median eminence. Endocrinology. 1989;124:1579–1581. doi: 10.1210/endo-124-3-1579. [DOI] [PubMed] [Google Scholar]
- LaFranchi S. Hypothyroidism. In: Behrman RE, Kleigman RM, Jenson HB, editors. Nelson textbook of pediatrics. Philadelphia: W.B. Saunders Company; 2000. pp. 1698–1794. [Google Scholar]
- Legan SJ, Karsch FJ. Neuroendocrine regulation of the estrous cycle and seasonal breeding in the ewe. Biol Reprod. 1979;20:74–85. doi: 10.1093/biolreprod/20.1.74. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/neurokinin b/dynorphin (kndy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010a doi: 10.1210/en.2010-0022. en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL, Viguié C, Billings HJ, Karsch FJ. Seasonal plasticity in the brain: The use of large animal models for neuroanatomical research. Reprod Suppl. 2002;59:149–165. [PubMed] [Google Scholar]
- Lehman MN, Durham DM, Jansen HT, Adrian B, Goodman RL. Dopaminergic a14/a15 neurons are activated during estradiol negative feedback in anestrous, but not breeding season, ewes. Endocrinology. 1996;137:4443–4450. doi: 10.1210/endo.137.10.8828506. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Ebling FJ, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology. 1993;133:876–886. doi: 10.1210/endo.133.2.8344223. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Goodman RL, Karsch FJ, Jackson GL, Berriman SJ, Jansen HT. The gnrh system of seasonal breeders: Anatomy and plasticity. Brain Research Bulletin. 1997;44:445–457. doi: 10.1016/s0361-9230(97)00225-6. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta- endorphin-immunoreactive neurons contain estrogen receptors? A double- label immunocytochemical study in the suffolk ewe. Endocrinology. 1993a;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the suffolk ewe. Endocrinology. 1993b;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (lhrh) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;244:19–35. doi: 10.1002/cne.902440103. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Tseng AS, Whited B, Nestor CC, Millar RP, Hileman SM, Coolen LM, Goodman RL. Evidence that dopamine acts via kisspeptin to hold gnrh pulse frequency in check in anestrous ewes; Annual Meeting of the Society for Neuroscience. City; (Year) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Short RV. Seasonal breeding: Nature's contraceptive. Recent Prog Horm Res. 1980;36:1–52. doi: 10.1016/b978-0-12-571136-4.50007-3. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Castren E, Berzaghi M, Blochl A, Thoenen H. Activity-dependent and hormonal regulation of neurotrophin mrna levels in the brain--implications for neuronal plasticity. J Neurobiol. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- Loudes C, Petit F, Kordon C, Faivre-Bauman A. Distinct populations of hypothalamic dopaminergic neurons exhibit differential responses to brain-derived neurotrophic factor (bdnf) and neurotrophin-3 (nt3) Eur J Neurosci. 1999;11:617–624. doi: 10.1046/j.1460-9568.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Daveau A, Maurice-Mandon F, Duarte G, Chemineau P. Evidence that melatonin acts in the premammillary hypothalamic area to control reproduction in the ewe: Presence of binding sites and stimulation of luteinizing hormone secretion by in situ microimplant delivery. Endocrinology. 1998a;139:1508–1516. doi: 10.1210/endo.139.4.5879. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Daveau A, Maurice-Mandon F, Duarte G, Chemineau P. Evidence that melatonin acts in the premammillary hypothalamic area to control reproduction in the ewe: Presence of binding sites and stimulation of luteinizing hormone secretion by in situ microimplant delivery. Endocrinology. 1998b;139:1508–1516. doi: 10.1210/endo.139.4.5879. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Skinner DC, Maurice F. The ovine pars tuberalis does not appear to be targeted by melatonin to modulate luteinizing hormone secretion, but may be important for prolactin release. J Neuroendocrinol. 1995;7:199–206. doi: 10.1111/j.1365-2826.1995.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Malpaux B, ViguiÉ C, Skinner DC, Thiéry JC, Chemineau P. Control of the circannual rhythm of reproduction by melatonin in the ewe. Brain Research Bulletin. 1997;44:431–438. doi: 10.1016/s0361-9230(97)00223-2. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Wayne NL, Karsch FJ. Termination of the breeding season in the suffolk ewe: Involvement of an endogenous rhythm of reproduction. Biol Reprod. 1988;39:254–263. doi: 10.1095/biolreprod39.2.254. [DOI] [PubMed] [Google Scholar]
- Maltby ML, Brown E, Coolen LM, Cheng G, Goodman RL, Lehman MN. Dopamine d2 receptors are colocalized in gonadotropin-releasing hormone (gnrh) and dynorphin neurons in the mediobasal hypothalamus of the anestrous ewe; Annual Meeting of the Society for Neuroscience. City; (Year) [Google Scholar]
- Martin GB, Thiery JC. Hypothalamic multiunit activity and lh secretion in conscious sheep. Exp Brain Res. 1987;67:469–478. doi: 10.1007/BF00247280. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annual Review of Neuroscience. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McNeilly A. Suckling and the control of gonadotropin secretion. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press; 1994. pp. 1179–1212. [Google Scholar]
- McNeilly A. Suckling and the control of gonadotropin secretion. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 2006. pp. 2511–2552. [Google Scholar]
- McNeilly AS, Tay CC, Glasier A. Physiological mechanisms underlying lactational amenorrhea. Ann N Y Acad Sci. 1994a;709:145–155. doi: 10.1111/j.1749-6632.1994.tb30394.x. [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Tay CCK, Glasier A. Physiological mechanisms underlying lactational amenorrhea. Annals of the New York Academy of Sciences. 1994b;709:145–155. doi: 10.1111/j.1749-6632.1994.tb30394.x. [DOI] [PubMed] [Google Scholar]
- Meczekalski B, Podfigurna-Stopa A, Warenik-Szymankiewicz A, Genazzani AR. Functional hypothalamic amenorrhea: Current view on neuroendocrine aberrations. Gynecological Endocrinology. 2008;24:4–11. doi: 10.1080/09513590701807381. [DOI] [PubMed] [Google Scholar]
- Merkley C, Jackson L, Goodman RL, Lehman MN. Evidence for transcriptional activation of arcuate kisspeptin neurons, and glutamatergic input to kisspeptin during the preovulatory gnrh surge of the sheep. Endocrine Society Abstr 2009. 2009:P3–P220. [Google Scholar]
- Meyer SL, Goodman RL. Neurotransmitters involved in mediating the steroid-dependent suppression of pulsatile luteinizing hormone secretion in anestrous ewes: Effects of receptor antagonists. Endocrinology. 1985;116:2054–2061. doi: 10.1210/endo-116-5-2054. [DOI] [PubMed] [Google Scholar]
- Meyer SL, Goodman RL. Separate neural systems mediate the steroid-dependent and steroid-independent suppression of tonic luteinizing hormone secretion in the anestrous ewe. Biol Reprod. 1986;35:562–571. doi: 10.1095/biolreprod35.3.562. [DOI] [PubMed] [Google Scholar]
- Miller WL, Styne DM. Female puberty and its disorders. In: Yen SSC, Jaffe RB, Barbiere RL, editors. Reproductive endocrinology. Philadelphia: W.B. Saunders Company; 1999. pp. 388–412. [Google Scholar]
- Mitsushima D, Hel DL, Terasawa E. Γ-aminobutyric acid is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (gnrh) secretion leading up to ovulation in the ewe: Existence of a preovulatory gnrh surge. Endocrinology. 1991;129:1175–1182. doi: 10.1210/endo-129-3-1175. [DOI] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: Implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, Leranth C, Vondracek-Klepper S, Lewis C, Chang A, Parducz A. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008a;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- Nakao N, Ono H, Yoshimura T. Thyroid hormones and seasonal reproductive neuroendocrine interactions. Reproduction. 2008b;136:1–8. doi: 10.1530/REP-08-0041. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lummis SCR. Diolistic labeling of neuronal cultures and intact tissue using a hand-held gene gun. Nat. Protocols. 2006;1:1517–1521. doi: 10.1038/nprot.2006.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau US. Glial-gonadotrophin hormone (gnrh) neurone interactions in the median eminence and the control of gnrh secretion. J Neuroendocrinol. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- Plant TM, Shahab M. Neuroendocrine mechanisms that delay and initiate puberty in higher primates. Physiol Behav. 2002;77:717–722. doi: 10.1016/s0031-9384(02)00924-1. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Knobil E, Neill JD, editors. Physiology of reproduction. New York: Academic Press; 2006. pp. 2177–2232. [Google Scholar]
- Pompolo S, Pereira A, Kaneko T, Clarke IJ. Seasonal changes in the inputs to gonadotropin-releasing hormone neurones in the ewe brain: An assessment by conventional fluorescence and confocal microscopy. Journal of Neuroendocrinology. 2003;15:538–545. doi: 10.1046/j.1365-2826.2003.01030.x. [DOI] [PubMed] [Google Scholar]
- Poo M-m. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Potter GB, Zarach JM, Sisk JM, Thompson CC. The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol. 2002;16:2547–2560. doi: 10.1210/me.2002-0115. [DOI] [PubMed] [Google Scholar]
- Prevot V. Glial-neuronal-endothelial interactions are involved in the control of gnrh secretion. J Neuroendocrinol. 2002;14:247–255. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- Prevot V, Bellefontaine N, Baroncini M, Sharif A, Hanchate NK, Parkash J, Campagne C, de Seranno S. Gonadotrophin-releasing hormone nerve terminals, tanycytes and neurohaemal junction remodelling in the adult median eminence: Functional consequences for reproduction and dynamic role of vascular endothelial cells. J Neuroendocrinol. 2010;22:639–649. doi: 10.1111/j.1365-2826.2010.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley ME, Sheehan KL, Casper RF, Yen SSC. Evidence for increased dopaminergic and opioid activity in patients with hypothalamic hypogonadotropic amenorrhea. J Clin Endocrinol Metab. 1980;50:949–954. doi: 10.1210/jcem-50-5-949. [DOI] [PubMed] [Google Scholar]
- Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic ammenorrhea: Evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61:851–858. doi: 10.1210/jcem-61-5-851. [DOI] [PubMed] [Google Scholar]
- Revel FG, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Melatonin controls seasonal breeding by a network of hypothalamic targets. Neuroendocrinology. 2009;90:1–14. doi: 10.1159/000219588. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time pcr data by the comparative ct method. Nat. Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Scott ALM, Ramer MS. Schwann cell p75ntr prevents spontaneous sensory reinnervation of the adult spinal cord. Brain. 2010;133:421–432. doi: 10.1093/brain/awp316. [DOI] [PubMed] [Google Scholar]
- Sergeeva A, Jansen HT. Neuroanatomical plasticity in the gonadotropin-releasing hormone system of the ewe: Seasonal variation in glutamatergic and gamma-aminobutyric acidergic afferents. The Journal of Comparative Neurology. 2009;515:615–628. doi: 10.1002/cne.22087. [DOI] [PubMed] [Google Scholar]
- Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. discussion 164–145. [PubMed] [Google Scholar]
- Simonneaux V, Ansel L, Revel FG, Klosen P, Pevet P, Mikkelsen JD. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides. 2009;30:146–153. doi: 10.1016/j.peptides.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Singh SR, Hileman SM, Connors JM, McManus CJ, Coolen LM, Lehman MN, Goodman RL. Estradiol negative feedback regulation by glutamatergic afferents to a15 dopaminergic neurons: Variation with season. Endocrinology. 2009;150:4663–4671. doi: 10.1210/en.2009-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide y, and {beta}-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology. 1997;138:2585–2595. doi: 10.1210/endo.138.6.5208. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Billings HJ, Goodman RL, Coolen LM, Lehman MN. The premammillary hypothalamic area of the ewe: Anatomical characterization of a melatonin target area mediating seasonal reproduction. Biology of Reproduction. 2004;70:1768–1775. doi: 10.1095/biolreprod.103.024182. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Slepetis RJ. Obligatory role of thyroid hormones in development of peripheral sympathetic and central nervous system catecholaminergic neurons: Effects of propylthiouracil-induced hypothyroidism on transmitter levels, turnover and release. J Pharmacol Exp Ther. 1984;230:53–61. [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and rfamide-related peptide (rfrp) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: A novel medium for seasonal breeding in the sheep. Endocrinology. 2008a;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and rfamide-related peptide (rfrp) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: A novel medium for seasonal breeding in the sheep. Endocrinology. 2008b;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS. The relative contribution of suckling and prolactin to the inhibition of gonadotropin secretion during lactation in the rat. Biol Reprod. 1978;19:77–83. doi: 10.1095/biolreprod19.1.77. [DOI] [PubMed] [Google Scholar]
- Sotowska-Brochocka J, Martynska L, Licht P. Dopaminergic inhibition of gonadotropic release in hibernating frogs, rana temporaria. Gen Comp Endocrinol. 1994;93:192–196. doi: 10.1006/gcen.1994.1022. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Tan MML, Hendry IA, Harvey AR. Anterograde transport and trophic actions of bdnf and nt-4/5 in the developing rat visual system. Molecular and Cellular Neuroscience. 2002;19:485–500. doi: 10.1006/mcne.2001.1097. [DOI] [PubMed] [Google Scholar]
- Stoica G, Lungu G, Xie X, Abbott LC, Stoica HM, Jaques JT. Inherited tertiary hypothyroidism in sprague-dawley rats. Brain Research. 2007;1148:205–216. doi: 10.1016/j.brainres.2007.02.042. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Boudaba C. Functional synaptic plasticity in hypothalamic magnocellular neurons. Prog Brain Res. 2002;139:113–119. doi: 10.1016/s0079-6123(02)39011-3. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Noonan JJ, Nass TE, Loose MD. Posterior hypothalamic lesions advance the onset of puberty in the female rhesus monkey. Endocrinology. 1984;115:2241–2250. doi: 10.1210/endo-115-6-2241. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Piet R, Poulain DA, Oliet SH. Neuronal, glial and synaptic remodeling in the adult hypothalamus: Functional consequences and role of cell surface and extracellular matrix adhesion molecules. Neurochem Int. 2004;45:491–501. doi: 10.1016/j.neuint.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Thiery JC. Monoamine content of the stalk-median eminence and hypothalamus in adult female sheep as affected by daylength. Journal of Neuroendocrinology. 1991;3:407–411. doi: 10.1111/j.1365-2826.1991.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Thiery JC, Martin GB, Tillet Y, Caldani M, Quentin M, Jamain C, Ravault JP. Role of hypothalamic catecholamines in the regulation of luteinizing hormone and prolactin secretion in the ewe during seasonal anestrus. Neuroendocrinology. 1989;49:80–87. doi: 10.1159/000125094. [DOI] [PubMed] [Google Scholar]
- Thompson CC, Potter GB. Thyroid hormone action in neural development. Cereb. Cortex. 2000;10:939–945. doi: 10.1093/cercor/10.10.939. [DOI] [PubMed] [Google Scholar]
- Thrun LA, Dahl GE, Evans NP, Karsch FJ. Time-course of thyroid hormone involvement in the development of anestrus in the ewe. Biol Reprod. 1996;55:833–837. doi: 10.1095/biolreprod55.4.833. [DOI] [PubMed] [Google Scholar]
- Trudeau VL. Neuroendocrine regulation of gonadotrophin ii release and gonadal growth in the goldfish, carassius auratus. Rev Reprod. 1997;2:55–68. doi: 10.1530/ror.0.0020055. [DOI] [PubMed] [Google Scholar]
- Turek FW, Campbell CS. Photoperiodic regulation of neuroendocrine-gonadal activity. Biol Reprod. 1979;20:32–50. doi: 10.1093/biolreprod/20.1.32. [DOI] [PubMed] [Google Scholar]
- Viguie C, Picard S, Thiery JC, Malpaux B. Blockade of tyrosine hydroxylase activity in the median eminence partially reverses the long day-induced inhibition of pulsatile lh secretion in the ewe. J Neuroendocrinol. 1998;10:551–558. doi: 10.1046/j.1365-2826.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- Viguié C, Thibault J, Thiéry J-C, Tillet Y, Malpaux B. Photoperiodic modulation of monoamines and amino-acids involved in the control of prolactin and lh secretion in the ewe: Evidence for a regulation of tyrosine hydroxylase activity. Journal of Neuroendocrinology. 1996;8:465–474. doi: 10.1046/j.1365-2826.1996.04758.x. [DOI] [PubMed] [Google Scholar]
- Viguie C, Thibault J, Thiery JC, Tillet Y, Malpaux B. Characterization of the short day-induced decrease in median eminence tyrosine hydroxylase activity in the ewe: Temporal relationship to the changes in luteinizing hormone and prolactin secretion and short day-like effect of melatonin. Endocrinology. 1997;138:499–506. doi: 10.1210/endo.138.1.4865. [DOI] [PubMed] [Google Scholar]
- Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothalamic amenorrhea: Hypoleptinemia and disordered eating. J Clin Endocrinol Metab. 1999;84:873–877. doi: 10.1210/jcem.84.3.5551. [DOI] [PubMed] [Google Scholar]
- Wayne NL, Malpaux B, Karsch FJ. Photoperiodic requirements for timing onset and duration of the breeding season of the ewe: Synchronization of an endogenous rhythm of reproduction. J Comp Physiol A. 1990;166:835–842. doi: 10.1007/BF00187330. [DOI] [PubMed] [Google Scholar]
- Wissman AM, Brenowitz EA. The role of neurotrophins in the seasonal-like growth of the avian song control system. J. Neurosci. 2009;29:6461–6471. doi: 10.1523/JNEUROSCI.0638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J-J, Karsch FJ, Lehman MN. Evidence for seasonal plasticity in the gonadotropin-releasing hormone (gnrh) system of the ewe: Changes in synaptic inputs onto gnrh neurons. Endocrinology. 1997;138:1240–1250. doi: 10.1210/endo.138.3.5000. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in japanese quail. Endocrinology. 2004;145:4264–4267. doi: 10.1210/en.2004-0366. [DOI] [PubMed] [Google Scholar]
- Yen SSC. Chronic anovulation due to cns-hypothalamic-pituitary dysfunction. In: Yen SSC, Jaffe RB, Barbiere RL, editors. Reproductive endocrinology. Philadelphia: W.B. Saunders Company; 1999. pp. 516–561. [Google Scholar]
- Yu KL, Rosenblum PM, Peter RE. In vitro release of gonadotropin-releasing hormone from the brain preoptic-anterior hypothalamic region and pituitary of female goldfish. Gen Comp Endocrinol. 1991;81:256–267. doi: 10.1016/0016-6480(91)90010-4. [DOI] [PubMed] [Google Scholar]
- Zohar Y, Munoz-Cueto JA, Elizur A, Kah O. Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocrinol. 2010;165:438–455. doi: 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.