Abstract
CCT is a member of the chaperonin family of molecular chaperones and consists of eight distinct subunit species which occupy fixed positions within the chaperonin rings. The activity of CCT is closely linked to the integrity of the cytoskeleton as newly synthesized actin and tubulin monomers are dependent upon CCT to reach their native conformations. Furthermore, an additional role for CCT involving interactions with assembling/assembled microfilaments and microtubules is emerging. CCT is also known to interact with other proteins, only some of which will be genuine folding substrates. Here, we identify the actin filament remodeling protein gelsolin as a CCT-binding partner, and although it does not behave as a classical folding substrate, gelsolin binds to CCT with a degree of specificity. In cultured cells, the levels of CCT monomers affect levels of gelsolin, suggesting an additional link between CCT and the actin cytoskeleton that is mediated via the actin filament severing and capping protein gelsolin.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-010-0230-x) contains supplementary material, which is available to authorized users.
Keywords: Molecular chaperone, CCT, Actin cytoskeleton, Gelsolin
Introduction
CCT, a member of the chaperonin subfamily of molecular chaperones, is an oligomer assembled from eight distinct subunit species (Cct1-8p in yeast and CCTα-θ in mammals) that form a double-barreled ring structure (reviewed by Grantham 2010). The major folding substrates of CCT are the abundant cytoskeletal proteins actin and tubulin (Sternlicht et al. 1993), while, in addition, less abundant proteins, such as the cell cycle regulators Cdh1 and Cdc20, are known to require interactions with CCT in order to reach their native states (reviewed by Brackley and Grantham 2009). The range of CCT substrates appears to be somewhat limited, for example, using proteomic and genomic approaches, 136 proteins and genes were identified within the CCT interactome in yeast (Dekker et al. 2008). A further study in yeast using genomic approaches estimated that 7% of proteins may interact with CCT (Yam et al. 2008). These estimates will include both obligate and nonobligate folding substrates, regulatory proteins, and proteins that utilize CCT as a platform for oligomerization. However, it is probable that the majority of CCT oligomers will be involved in folding the highly abundant substrate proteins actin and tubulin. In the case of actin, it appears that CCT is required to overcome a particular kinetic barrier in the later stages of its folding pathway (Altschuler and Willison 2008). This is consistent with the observation that interactions between CCT and actin are charged/polar in nature (Hynes and Willison 2000) rather than binding, being mediated via nonspecific hydrophobic sites. Furthermore, specific CCT subunits interact with actin molecules that are already partially folded (Llorca et al. 1999, 2001). The folding requirements of actin are dependent upon CCT, and this need cannot be compensated for by the bacterial chaperonin GroEL (Pappenberger et al. 2006). This reliance upon CCT for folding intrinsically links CCT activity to the formation of a functional actin cytoskeleton. Indeed, when CCT levels are reduced in mammalian cells by small interfering ribonucleic acid (siRNA) not only is there an arrest in cell cycle progression but also disorganization of the actin cytoskeleton and a reduction in levels of native monomeric actin (Grantham et al. 2006).
In addition to the well-documented role of the CCT oligomer in the folding of newly synthesized actin molecules, an additional role for CCT is emerging involving the polymerization/organization of actin filaments. In vitro, CCT reduces the rate but not final yields of polymerized actin, with CCT subunits selectively remaining associated with actin filaments (Grantham et al. 2002). It is probable that the CCT subunits, when monomeric, act as functional units: CCTε has been shown to colocalize with F-actin in vivo, and changes to the levels of this subunit as a monomer influence cell shape (Brackley and Grantham 2010). This additional role for CCT monomers is not unique to influencing the actin cytoskeleton. CCTα, γ, ζ, and θ have been shown to bind to microtubules in vitro (Roobol et al. 1999), and increased levels of CCT subunits as monomers increase the rate of microtubule recovery following nocodazole treatment in mammalian cells (Brackley and Grantham 2010). Therefore, the role of CCT in the microfilament/microtubule cytoskeletal systems appears to extend from the folding of actin and tubulin monomers to influencing the assembly or organization of microfilaments and microtubules.
We have identified the actin filament severing and capping protein gelsolin as a CCT interacting partner using an immunoprecipitation/proteomic approach. Gelsolin binds to the CCT oligomer with distinctly different kinetics to that of actin, suggesting that it does not behave as a classical folding substrate, although binding to CCT occurs specifically via gelsolin domain 4. To extend our previous study on the effects of CCT subunit monomer levels upon cell shape, we analyzed levels of gelsolin following the reduction of individual CCT subunits levels by siRNA and found that in some, but not all, knockdowns, gelsolin levels were in fact elevated, consistent with gelsolin not requiring interactions with CCT to become folded. The data presented here indicate that CCT may have an additional level of influence upon the actin cytoskeleton via interactions with an actin capping and severing protein.
Methods
Immunoprecipitation and protein identification
Balb 3T3 cells were synchronized with regard to cell cycle progression by contact inhibition and serum starvation. Protein-G sepharose beads (Sigma Aldrich) covalently coupled to the anti-CCT εAD1 antibody (according to Harlow and Lane 1999) were incubated with cell lysate in breaking buffer (50 mM HEPES pH 7.2, 90 mM KCl) with a final concentration of 0.5% Igepal for 2 h at 4°C. Immunoprecipitated proteins were separated by two-dimensional polyacrylamide gel electrophoresis [PAGE; isoelectric focusing (IEF) PAGE pH 3.5–10 1st dimension and 9% polyacrylamide gel electrophoresis (SDS) PAGE 2nd dimension], and those visible by silver staining were analyzed by liquid chromatography–mass spectrometry/mass spectrometry and protein identities confirmed using Mascot (Perkins et al. 1999).
Proximity ligation assays
T47D cells were plated 24 h prior to staining and prepared by fixation in 4% formaldehyde/phosphate buffered saline (PBS) and permeablization in 0.2% TX100/PBS. Following blocking, proximity ligation assays (Olink Bioscience) were performed according to manufacturer’s instructions using anti-rabbit plus and anti-mouse minus PLA probes and PLA signals were detected using the Duolink detection kit 563. Images were taken using an AxioCamHR camera with Axiovision acquisition software.
In vitro transcription/translation
In vitro translations using the TNT® coupled rabbit reticulocyte lysate system (Promega) were performed at 30°C in the presence of 2 μg deoxyribonucleic acid (DNA) and 22 μCi l-[35S] methionine (Perkin-Elmer) per 50 μl reaction. Plasmids were pcDNA3.1+ for gelsolin and pET11d for human β-actin. During time course experiments, 2 μl samples were stored on ice prior to native PAGE (Liou and Willison 1997). For antibody binding to sharpen product bands or to shift the CCT oligomer, antibody was added for up to 30 min on ice.
Plasmids
Constructs were designed based upon the domain assignments of Kwiatkowski et al. (1986) and the numbering of mouse plasma gelsolin (uniprot entry P13020), individual domains were designed in pcDNA3.1+ encoding:
M50-K160
S161-E283
D284-E416
R417-G538
G539-P653
R654-A780
In order to increase the number of methionine residues for radioisotope incorporation and reduce the pI of individual domains to allow separation using this native PAGE system (pH 8.8), a 26 amino acid C-terminal epitope tag encoding two copies of the myc epitope and two additional methionine resides (LMEEQKLISEEDLLMEEQKLISEEDL) was added.
Small interfering RNA
BE cells were transfected with siRNA duplexes targeting individual CCT subunits as described previously (Brackley and Grantham 2010; Grantham et al. 2006) and harvested 4 days posttransfection. Cell lysates were separated by SDS PAGE and transferred to nitrocellulose for Western blot analysis.
Antibodies
Antibodies to CCT subunits were the rat monoclonals anti-CCTα 23c, anti-CCTη 81a, anti-CCTε εAD1, and rabbit polyclonal antibody CCTε-c recognizing the C-terminus of CCTε. Mouse monoclonal antibodies were anti-gelsolin clone GS-2C4, anti-β-actin clone AC15 (Sigma Aldrich), and anti-γ-catenin clone H-1 (Santa Cruz).
Results
Identification of a CCT-binding protein that displays nonclassical binding kinetics
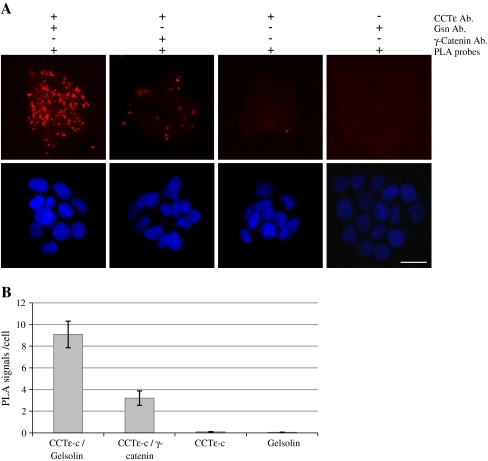
In order to identify novel CCT-binding partners, we immunoprecipitated the CCT oligomer from mouse 3T3 fibroblasts which had been synchronized with regard to cell cycle progression by contact inhibition and serum starvation. In addition to the well-characterized, abundant CCT-folding substrates actin and tubulin, we identified gelsolin as a coprecipitating protein during G1 and S phase. In each case, one unique peptide was identified; DGGQTAPASIR, mascot score 49 and DSQEEEKTEALTSAK, mascot score 44. We then utilized a proximity ligation assay to visualize potential interactions between gelsolin and CCT in the human breast epithelial cell line T47D. A positive signal was obtained between antibodies recognizing CCTε and gelsolin (Fig. 1a). In order to address the issue of nonspecific signals occurring due to the random proximity of any two proteins, we performed a control assay using the CCTε antibody and an antibody against γ-catenin (which gives a diffuse cytoplasmic stain by immunofluorescence), where a much reduced signal was observed (Fig. 1a). Quantification of the number of PLA signals/cell, including additional controls omitting one antibody, is shown in Fig. 1b.
Fig. 1.
Detection of potential CCT–gelsolin interactions in T47D cells using a proximity ligation assay. Proximity ligation assays were performed using primary antibody pairs against CCTε/gelsolin and CCTε/γ-catenin, both with anti-rabbit plus and anti-mouse minus PLA probes. Controls with one primary antibody and both PLA probes are indicated (a). The scale bar represents 20 μm. PLA signals/cell were counted from five fields of view (b). Error bars represent the standard error where n = 5
In order to verify that gelsolin binds to CCT, we performed an in vitro translation assay in rabbit reticulocyte lysate as this system has been used extensively to study proteins binding to endogenous rabbit CCT (e.g., Grantham et al. 2000; McCormack et al. 2001). Full-length mouse gelsolin was translated in the presence of 35S methionine, followed by native PAGE and autoradiography, confirming that gelsolin binds to CCT (Fig. 2a). In order to perform quantification of gelsolin production, in vitro translation samples were incubated with an anti-gelsolin antibody prior to resolution by native PAGE, as it has been shown previously that when proteins are bound by antibody, they migrate as sharper bands during native PAGE (Grantham et al. 2000). To this end, we translated full-length human gelsolin, as the mouse protein is not recognized by the anti-gelsolin antibody, and found that the presence of the antibody results in better resolution of the gelsolin product (Fig. 2b). We then compared the binding of gelsolin to CCT with the well-characterized binding of actin to CCT. SDS PAGE indicated that the majority of the gelsolin and actin produced in this system was full length (Fig. 2c). Time course samples were analyzed by native PAGE and quantification performed as described by Grantham et al. (2000), where counts associated with CCT are expressed as a percentage of total counts/lane (Fig. 2d and e). To identify the position of the CCT oligomer, autorads were aligned with the Coomassie-stained gel and samples also incubated with anti-CCT antibodies to induce a shift in the CCT oligomer position, where counts associated with CCT were also shifted (Fig. S1). The time course analysis shown here indicates that gelsolin and actin appear to bind to CCT with different kinetics. Actin binds quickly to CCT as does the other well-characterized obligate substrate tubulin (see Llorca et al. 2000), whereas the gelsolin binds much more slowly (see, in particular, levels of binding between 6 and 20 min). Furthermore, the percentage of total gelsolin bound to CCT at any time during the reaction is much less than observed for actin. The binding of gelsolin to CCT is reminiscent of a fragment of actin that binds to, but is not released by, CCT (Llorca et al. 1999). This indicates that gelsolin–CCT interactions are different to those of the obligate folding substrates actin and tubulin, which bind rapidly and are then released from CCT. An example of a nonclassical substrate interaction is observed with cyclin E–CCT binding. This protein was shown to interact with CCT (Won et al. 1998) but was found not to require this interaction in order to form a complex with its native binding partner Cdk2 (Grantham et al. 2006). Furthermore, the binding of cyclin E to CCT in rabbit reticulocyte lysate was a low-level accumulation distinct from that of actin binding to CCT (Grantham et al. 2006).
Fig. 2.
Cytoplasmic gelsolin binds the chaperonin CCT, but not as a classical folding substrate. Gelsolin was translated in the presence of 35S methionine at 30°C in a rabbit reticulocyte lysate in vitro translation system. Samples (2 μl) were separated by native PAGE, the CCT oligomer is indicated, and a negative control with water instead of DNA was included in all experiments (a, c, and d). Samples were collected at the time points shown (in minutes) during translation of mouse cytoplasmic gelsolin and separated by nondenaturing electrophoresis and visualized by autoradiography (a). A 45-min translation of human cytoplasmic gelsolin was performed in (b) with and without a further incubation with an anti-gelsolin antibody for 20 min on ice. The positions of unshifted (u) and shifted (s) gelsolin are indicated. 2 μl of 45-min time in vitro translations of human gelsolin and β-actin were separated by SDS PAGE (c). Time courses of human cytoplasmic gelsolin (with subsequent antibody incubation as in b) and β-actin in vitro translations were compared on the same native gel (d), where an arrowhead and bracket denote gelsolin and native actin products, respectively. An asterisk indicates actin bound to endogenous CAP (McCormack et al. 2001). The counts on CCT were calculated as a percentage of total counts per lane by phosphor imaging using a Molecular Imager FX (BioRad) (e) ▲ represents gelsolin and ■ β-actin
Gelsolin interacts with CCT in a domain-specific manner
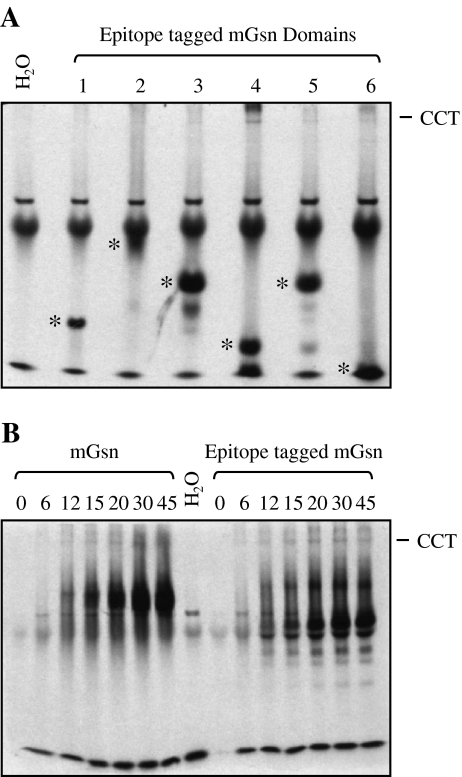
We then addressed the question of which domains of gelsolin interact with the CCT oligomer. To this end, each of the six gelsolin domains including a 2 × LM-myc tag at the extreme C-terminus, to increase incorporation of 35S methionine and to reduce the pI, were translated in rabbit reticulocyte lysate. All domains were well expressed and migrated on native PAGE as distinct bands, suggesting that they are able to fold. Gelsolin domain 4 bound strongly to CCT with very weak CCT binding displayed by domains 3, 5, and 6, indicating the domain specificity of the gelsolin–CCT interaction (Fig. 3a). The gelsolin domain 4:CCT interaction was confirmed using anti-CCT antibodies to shift counts associated with CCT on native PAGE (Fig. S1).
Fig. 3.
Domain-specific binding of gelsolin to CCT. Individual epitope (2 × LM-myc) tagged domains of cytoplasmic gelsolin were translated for 45 min in the presence of l-[35S] methionine, separated by native PAGE (a) and visualized by autoradiography. Asterisks denote individual domain products and the position of the CCT oligomer is indicated. In vitro translations of full-length cytoplasmic gelsolin with and without the epitope tag were compared by resolving time course samples by native page followed by autoradiography (b)
Translation of full-length mouse gelsolin with and without the epitope tag indicated that there was no change in gelsolin binding to CCT in the presence of this tag (Fig. 3b). The domain-specific binding of gelsolin to CCT is similar to that of actin in that distinct binding sites on actin have been implicated in binding to CCT (Hynes and Willison 2000; Neirynck et al. 2006).
siRNA targeting of CCT subunits has varying effects on gelsolin levels
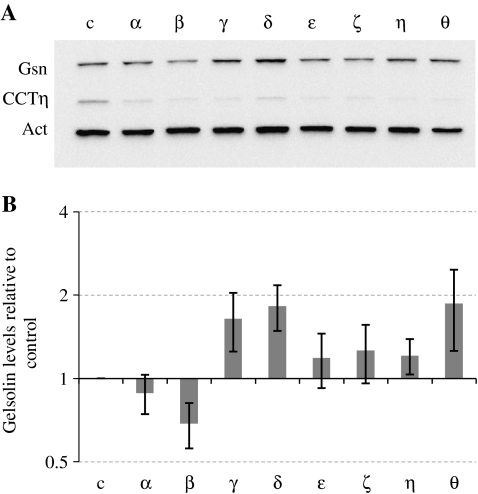
Gelsolin influences the dynamics of the microfilament system by severing actin filaments and capping the fast-growing filament ends (e.g., reviewed by Kwiatkowski 1999). We therefore investigated the levels of gelsolin following the knockdown of individual CCT subunits by siRNA as this has been shown previously to affect cell shape, probably via altered levels of the CCTε subunit, which has been shown to bind to actin filaments in vivo (Brackley and Grantham 2010). Intriguingly, Western blot analysis showed that the levels of gelsolin were altered, depending on which CCT subunit was targeted by siRNA, and were decreased in the CCTβ knockdown, yet increased in the CCTγ, δ, and θ knockdowns (Fig. 4a). Quantification of gelsolin levels in relation to the control sample was performed (from three individual experiments) using the β-actin signal to standardize gel loadings (Fig. 4b), as it has been shown previously that total actin levels do not change when targeting CCT subunits by siRNA (Brackley and Grantham 2010; Grantham et al. 2006).
Fig. 4.
Altering levels of CCT monomers affects gelsolin expression. BE cells were transfected with either a control nontargeting duplex (c) or siRNA duplexes to each of the CCT subunits (α-θ) and harvested four days after transfection. Western blot analysis (a) was performed probing for gelsolin (Gsn), CCTη, and actin (Act). Quantification of the gelsolin signals in each transfection was performed from three individual experiments, using the actin signal to standardize for gel loading. Gelsolin levels in transfected samples were compared to those of the control and the standard error indicated (b)
Discussion
CCT is an essential chaperone that has a well-established role in the folding of newly synthesized actin and tubulin molecules and is known to interact with a range of other proteins (Dekker et al. 2008; Yam et al. 2008). A role for CCT subunits when monomeric is also emerging that links CCT to polymerized actin (Brackley and Grantham 2010; Grantham et al. 2002) and also to the microtubule system (Brackley and Grantham 2010; Roobol et al. 1999). Here, we identify the actin filament severing and capping protein, gelsolin as a novel CCT-interacting protein providing evidence for an additional way in which CCT may influence the actin cytoskeleton.
We find that although gelsolin does not display the classical binding kinetics of the major folding substrates of CCT, actin and tubulin, it does appear to bind to CCT with a degree of specificity via domain 4. This could be due to the presence of a CCT-specific binding site residing in gelsolin domain 4 or could be indicative of this domain having a particular folding requirement. In favor of the former possibility, gelsolin consists of six domains, each of which have a similar fold (Burtnick et al. 1997), yet domain 4 appears to be the major CCT-binding domain.
Several studies have used functional gelsolin that was expressed in Escherichia coli (Azuma et al. 2000), suggesting that gelsolin is not dependent upon CCT to reach its native state. This is consistent with the findings in this study where it is shown that gelsolin binds CCT with kinetics distinct from actin, an obligate folding substrate, and that siRNA, targeting individual CCT subunits (thereby reducing CCT oligomer levels), does not universally reduce cellular levels of gelsolin.
This raises the question of why gelsolin binds to CCT. It is possible that CCT could act as a sequesterer of gelsolin or that a percentage of gelsolin molecules require interactions with CCT, possibly to enable a particular function in mammalian cells. These findings demonstrate that not all CCT-binding proteins interact with the CCT oligomer in the same way as the major folding substrates actin and tubulin. Therefore, rates/levels of binding should be considered when analyzing genomic screens for CCT-binding partners, and this study highlights that not all CCT-binding proteins will be obligate folding substrates.
When CCTβ is targeted by siRNA, gelsolin levels decrease, whereas targeting CCTγ, δ, or θ results in increased levels of gelsolin. As the CCT oligomer would be depleted regardless of which CCT subunit is targeted, this provides further evidence that gelsolin does not require interactions with the CCT oligomer to reach its native state. Therefore, the changes in gelsolin levels may be due to a response to changes in CCT monomer levels and/or changes to the actin cytoskeleton. We have shown previously that when targeting all CCT subunits except CCTε by siRNA, cells adopt a flattened shape, while when targeting CCTε, cells become narrow (Brackley and Grantham 2010). These findings link CCT monomer levels to cell shape, which is supported by the observation that some CCT monomers interact with the actin cytoskeleton. As gelsolin is involved in influencing the dynamic nature of the actin cytoskeleton via its activity as an actin filament severing/capping protein, then it is possible that when cells become flattened due to changes in CCT monomer levels, the cell will attempt to counteract this by raising levels of gelsolin.
It is intriguing that the gelsolin levels respond differently depending upon which CCT subunit is the siRNA target. This could reflect a cross-talk between CCT subunits/F-actin levels/gelsolin, where CCT monomers have distinct roles. In the case of targeting CCTβ, although cells adopt a flattened phenotype, gelsolin levels actually decrease, indicating that this CCT subunit may in some way be involved in stabilizing gelsolin. It is possible that binding of gelsolin to the CCT oligomer and the effects of altered CCT monomer levels on gelsolin levels are not connected or, alternatively, may indicate a complex feedback mechanism between CCT subunits/F-actin/gelsolin that also includes the assembled CCT oligomer.
During carcinogenesis, gelsolin is downregulated epigenetically in many breast tumors (reviewed by Mielnicki et al. 2001), while the growth-suppressing and apoptosis-inducing drugs radicol and trichostatin A increase gelsolin expression (as reviewed by Kwiatkowski 1999). It was found that reductions in gelsolin levels reduced the invasive and motile nature of some cell lines (Van den Abbeele et al. 2007), while it has also been demonstrated that in epithelial cell lines, increased levels of gelsolin promoted invasion (De Corte et al. 2002). The role of gelsolin in cancer cell invasion therefore remains unclear, but it has been suggested that in urothelial carcinomas, gelsolin may be involved in the transition of tumors from being noninvasive to invasive (Rao et al. 2002). It is therefore of great importance to gain a greater understanding of how gelsolin levels are linked to cell shape and the actin cytoskeleton. Here, gelsolin has been identified as a CCT-binding protein indicating another possible role for CCT in cytoskeletal organization.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Band shift analysis of gelsolin binding to the CCT oligomer. Full-length human gelsolin (hGsn), mouse gelsolin domain 4 (mGsn domain 4), and full-length human β-actin were translated in the presence of 35S methionine at 30°C for 45 min in a rabbit reticulocyte lysate in vitro translation system. Samples (2 μl) were incubated for 30 min on ice in the presence and absence of 2.5 μg anti-CCT antibodies made up to a total volume of 10 μl with PBS. Proteins were separated by native PAGE and visualized by autoradiography. Arrow heads indicate the unshifted CCT oligomer and asterisks the antibody shifted CCT oligomer (PPT 170 kb)
Acknowledgements
The authors would like to thank Drs. Anne Roobol and Martin Carden for the rabbit anti-CCTε antibody, Prof. Keith Willison for the monoclonal antibodies to CCT subunits, and Prof. Shigeomi Shimizu for the provision of gelsolin plasmids. Protein identification was carried out by the Proteomics Core Facility at the University of Gothenburg. We acknowledge grants from The Swedish Research Council, Assar Gabrielsson’s Fond, Carl Tryggers Stiftelse, and The Royal Society of Arts and Sciences in Gothenburg.
References
- Altschuler GM, Willison KR. Development of free-energy-based models for chaperonin containing TCP-1 mediated folding of actin. J R Soc Interface. 2008;5:1391–1408. doi: 10.1098/rsif.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma TK, Koths K, Flanagan L, Kwiatkowski D. Gelsolin in complex with phosphatidylinositol4, 5-bisphosphate inhibits caspase-3 and -9 to retard apoptotic progression. J Biol Chem. 2000;275:3761–3766. doi: 10.1074/jbc.275.6.3761. [DOI] [PubMed] [Google Scholar]
- Brackley KI, Grantham J. Activities of the chaperonin containing TCP-1 (CCT): implications for cell cycle progression and cytoskeletal organization. Cell Stress Chaperones. 2009;14:23–31. doi: 10.1007/s12192-008-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackley KI, Grantham J. Subunits of the chaperonin CCT interact with F-actin and influence cell shape and cytoskeletal assembly. Exp Cell Res. 2010;316:543–553. doi: 10.1016/j.yexcr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC. The crystal structure of plasma gelsolin: implications for actin severing, capping and nucleation. Cell. 1997;90:661–670. doi: 10.1016/S0092-8674(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Corte V, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J. Gelsolin-induced epithelial cell invasion is dependent on Ras–Rac signaling. EMBO J. 2002;21:6781–6790. doi: 10.1093/emboj/cdf680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker C, Stirling PC, McCormack EA, Filmore H, Paul A, Brost RL, Costanzo M, Boone C, Leroux MR, Willison KR. The interaction network of the chaperonin CCT. EMBO J. 2008;27:1827–1839. doi: 10.1038/emboj.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. The eukaryotic chaperonin CCT (TRiC): structure, mechanisms of actin and substrate diversity. In: Durante P, Colucci L, editors. Molecular chaperones: roles structures and mechanisms. New York: Nova Science; 2010. [Google Scholar]
- Grantham J, Llorca O, Valpuesta JM, Willison KR. Partial occlusion of both cavities of the eukaryotic chaperonin with antibody has no effect upon the rates of beta-actin or alpha-tubulin folding. J Biol Chem. 2000;275:4587–4591. doi: 10.1074/jbc.275.7.4587. [DOI] [PubMed] [Google Scholar]
- Grantham J, Ruddock LW, Roobol A, Carden MJ. Eukaryotic chaperonin containing T-complex polypeptide 1 interacts with filamentous actin and reduces the initial rate of actin polymerization in vitro. Cell Stress Chaperones. 2002;7:235–242. doi: 10.1379/1466-1268(2002)007<0235:ECCTCP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J, Brackley KI, Willison KR. Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp Cell Res. 2006;312:2309–2324. doi: 10.1016/j.yexcr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Using antibodies: a laboratory manual. New York: Cold Spring Harbor Laboratory; 1999. [Google Scholar]
- Hynes GM, Willison KR. Individual subunits of the eukaryotic cytosolic chaperonin mediate interactions with binding sites located on subdomains of beta-actin. J Biol Chem. 2000;275:18985–18994. doi: 10.1074/jbc.M910297199. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ. Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol. 1999;11:103–108. doi: 10.1016/S0955-0674(99)80012-X. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Stossel TPO, Orkin SH, Mole JE, Colten HR, Yin HL. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicating actin-binding domain. Nature. 1986;323:455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Liou AK, Willison KR. Elucidation of the subunit orientation in CCT (chaperonin containing TCP1) from the subunit composition of CCT micro-complexes. EMBO J. 1997;16:4311–4316. doi: 10.1093/emboj/16.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O, McCormack EA, Hynes G, Grantham J, Cordell J, Carrascosa JL, Willison KR, Fernandez JJ, Valpuesta JM. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature. 1999;402:693–696. doi: 10.1038/45294. [DOI] [PubMed] [Google Scholar]
- Llorca O, Martin-Benito J, Ritco-Vonsovici M, Grantham J, Hynes GM, Willison KR, Carrascosa JL, Valpuesta JM (2000) Eukaryotic chaperonin CCT stabilizes actin and tubulin folding intermediates in open quasi-native conformations. EMBO J 19:5971–5979 [DOI] [PMC free article] [PubMed]
- Llorca O, Martin-Benito J, Grantham J, Ritco-Vonsovici M, Willison KR, Carrascosa JL, Valpuesta JM. The ‘sequential allosteric ring’ mechanism in the eukaryotic chaperonin-assisted folding of actin and tubulin. EMBO J. 2001;20:4065–4075. doi: 10.1093/emboj/20.15.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack EA, Rohman MJ, Willison KR. Mutational screen identifies critical amino acid residues of beta-actin mediating interaction between its folding intermediates and eukaryotic cytosolic chaperonin CCT. J Struct Biol. 2001;135:185–197. doi: 10.1006/jsbi.2001.4389. [DOI] [PubMed] [Google Scholar]
- Mielnicki LM, Asch HL, Asch BB. Genes, chromatin, and breast cancer: an epigenetic tale. J Mammary Gland Biol Neoplasia. 2001;6:169–182. doi: 10.1023/A:1011356623442. [DOI] [PubMed] [Google Scholar]
- Neirynck K, Waterschoot D, Vandekerckhove J, Ampe C, Rommelaere H. Actin interacts with CCT via discrete binding sites: a binding transition-release model for CCT-mediated actin folding. J Mol Biol. 2006;355:124–138. doi: 10.1016/j.jmb.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Pappenberger G, McCormack EA, Willison KR. Quantitative actin folding reactions using yeast CCT purified via an internal tag in the CCT3/γ subunit. J Mol Biol. 2006;360:484–496. doi: 10.1016/j.jmb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rao J, Seligson D, Visapaa H, Horvath S, Eeva M, Michel K, Pantuck A, Belldegrun A, Palotie A. Tissue microarray analysis of cytoskeletal actin-associated biomarkers gelsolin and E-cadherin in urothelial carcinoma. Cancer. 2002;95:1247–1257. doi: 10.1002/cncr.10823. [DOI] [PubMed] [Google Scholar]
- Roobol A, Sahyoun ZP, Carden MJ. Selected subunits of the cytosolic chaperonin associate with microtubules assembled in vitro. J Biol Chem. 1999;274:2408–2415. doi: 10.1074/jbc.274.4.2408. [DOI] [PubMed] [Google Scholar]
- Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffee MB. The t-complex polypeptide 1 is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci. 1993;90:9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeele AV, Corte V, Impe K, Bruyneel E, Boucherie C, Bracke M, Vandekerckhove J, Gettemans J. Down regulation of gelsolin family proteins counteracts cancer cell invasion in vitro. Cancer Lett. 2007;255:57–70. doi: 10.1016/j.canlet.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Won KA, Schumacher RJ, Farr GW, Horwich AL, Reed SI (1998) Maturation of human cyclin E requires the function of eukaryotic chaperonin CCT. Mol Cell Biol 18:7584–7589. [DOI] [PMC free article] [PubMed]
- Yam AY, Xia Y, Lin H-TJ, Burlingame A, Gerstein M, Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. J Nat Struct Mol Biol. 2008;15:1255–1262. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Band shift analysis of gelsolin binding to the CCT oligomer. Full-length human gelsolin (hGsn), mouse gelsolin domain 4 (mGsn domain 4), and full-length human β-actin were translated in the presence of 35S methionine at 30°C for 45 min in a rabbit reticulocyte lysate in vitro translation system. Samples (2 μl) were incubated for 30 min on ice in the presence and absence of 2.5 μg anti-CCT antibodies made up to a total volume of 10 μl with PBS. Proteins were separated by native PAGE and visualized by autoradiography. Arrow heads indicate the unshifted CCT oligomer and asterisks the antibody shifted CCT oligomer (PPT 170 kb)