Abstract
Oxidative stress in photodynamic therapy (PDT)-treated tumor cells is known to instigate a strong upregulation of the expression of heat shock proteins. However, the treatment of mouse Lewis lung carcinoma (LLC) cells with Photofrin™ PDT resulted in the upregulation of heat shock protein 70 (Hsp70) gene not only in these cells but also in co-incubated untreated Hepa 1-6 cells. To investigate whether this phenomenon extends in vivo, LLC tumors growing in C57BL/6 mice were treated with Photofrin™ PDT. The tumors and the livers from the mice were collected at 4, 8, or 24 h after therapy for quantitative reverse transcriptase polymerase chain reaction-based analysis of Hsp70 gene expression. Increased Hsp70 gene expression was detected in both the tumor and liver tissues and was most pronounced at 4 h after PDT. This effect was inhibited by treatment of host mice with glucocorticoid synthesis inhibitor metyrapone. Hsp70 protein levels in the livers of mice bearing PDT-treated tumors gradually decreased after therapy while serum levels increased at 4 h after therapy and then continually decreased. The exposure of in vitro PDT-treated LLC cells to Hsp70 and subsequent flow cytometry analysis revealed binding of this protein to cells that was dependent on PDT dose and more pronounced with dying than viable cells. Thus, following the induction of tumor injury by PDT, Hsp70 can be produced in the liver and spleen as acute phase reactant and released into circulation, from where it can be rapidly sequestered to damaged tumor tissue to facilitate the disposal of dying cells.
Keywords: Heat shock proteins, Acute phase response, Photodynamic therapy, Dead tumor cell disposal, Glucocorticoid hormones
Introduction
Since their initial identification as intracellular proteins specialized in chaperoning nascent proteins and degradation of aberrantly folded/damaged polypeptides, heat shock proteins (HSPs) have been characterized as important mediators of an expanding array of key physiological functions (Hartl and Hayer-Hartl 2002; Asea 2003; Pockley 2003). These proteins are now recognized as participants in signal transduction pathways and important regulators of inflammatory and immune response upon their translocation to the cell surface or release from the cells (Asea 2003; Pockley 2003; Calderwood et al. 2007; Gehrmann et al. 2008). It is now established that soluble heat shock protein 70 (Hsp70), the major stress-inducible member of HSPs, is present in the peripheral circulation of normal individuals and that its levels change with pregnancy, aging, and various pathophysiological conditions (Terry et al. 2006; Molvarec et al. 2006). The ability of Hsp70 to efficiently shuttle a broad spectrum of antigenic peptides to antigen-presenting cells was recently shown to involve a high affinity binding to LOX-1 endocytic receptors (Theriault et al. 2005). Another important recent observation is that Hsp70 interacts with cellular death signal membrane-exposed phosphatidylserine (Arispe et al. 2004).
Photodynamic therapy (PDT) is a regulatory approved modality for treating a variety of malignant and non-oncological lesions with continuing development for a number of other applications (Dougherty et al. 1998; Huang 2005). The destruction of targeted lesions by PDT is achieved by localized generation of reactive oxygen species mediated by the transfer of energy absorbed by light-activated drugs (photosensitizers) to molecular oxygen (Dougherty et al. 1998; Henderson and Dougherty 1992). The patient is first given a photosensitizing drug (by systemic or lesion-localized administration) and this is followed by lesion-targeted illumination using wavelengths compatible with the photosensitizer’s absorption characteristics. Phototoxic lesions inflicted in PDT-treated tumors elicit a complex multifactorial response producing both direct and indirect tumor cell death (Dougherty et al. 1998; Henderson and Dougherty 1992; Korbelik 2006). The initial injurious impact (oxidative stress caused predominantly by singlet oxygen-mediated damage to cellular proteins and lipids) gives rise to lethal photooxidative lesions with both apoptotic and necrotic cell death (Dougherty et al. 1998). The indirect cancer cell death comes either from oxygen/nutrient starvation caused by the destruction of tumor vasculature or from cytotoxic action of inflammatory/immune effectors of PDT-induced host reaction (Dougherty et al. 1998; Korbelik 2006; Castano et al. 2006).
Clinical cancer therapies including PDT were shown to induce changes in the expression of Hsp70 protein in tumor cells (Gehrmann et al. 2008). It has long been known that tumor treatment by PDT triggers in the targeted cancerous tissue the upregulation of genes encoding various HSPs and the accumulation of protein products of these genes (Gomer et al. 1996). Our recent study showed that PDT induces the expression of HSPs including Hsp70 on the surface of treated cells and the release of Hsp70 from these cells (Korbelik et al. 2005). This work also suggested that Hsp70 functions as a danger signal alerting the host of PDT-inflicted injury and that its action involves the activation of Toll-like receptor signaling (Korbelik 2006, 2009; Korbelik et al. 2005; Zhou et al. 2009).
In the present study, we used PDT as a tool to test the hypothesis that Hsp70 is not produced only locally at the stress-sustaining site but also systemically as an acute phase reactant. We show that PDT treatment induces the upregulation of Hsp70 production not only in tumor tissue sustaining direct PDT-mediated oxidative insult but also at distant organs (liver and spleen). The presented investigation indicates that following tumor PDT there is an induction of Hsp70 production in the host liver and its release into the circulation with subsequent rapid sequestration to damaged tumor tissue.
Materials and methods
Cell culture and mouse model
Lewis lung carcinoma (LLC) cells (ATCC CRL-164) and hepatoma Hepa 1-6 cells (ATCC CRL-1830) both originate from tumors that arose spontaneously in C57BL/6 mice. These cells were routinely cultured in alpha minimal essential medium, supplemented with 10% fetal bovine serum, both from GIBCO Invitrogen (Carlsbad, CA, USA). For implanting subcutaneous LLC tumors, 1 × 106 cells were injected into the lower dorsal region of 7–9-week-old female syngeneic mice. The tumors were treated when they reached around 8 mm in the largest diameter. All mouse procedures were performed in compliance with the Animal Care Committee of the University of British Columbia.
Photodynamic therapy
Photofrin™ (provided by Axcan Pharma Inc., Mont Saint-Hilaire, Quebec, Canada) was administered to C57BL/6 mice bearing LLC tumors by i.v. injection at 10 mg/kg. The tumors were treated 24 h later with light produced by a FB-QTH high throughput illuminator (Sciencetech Inc., London, Ontario, Canada) as described in detail elsewhere (Merchant et al. 2007). Briefly, light delivered through a 630 ± 10-nm interference filter for superficial tumor illumination had a fluence rate of 80–90 mW/cm2, and the light dose used was 150 J/cm2. During this procedure, the mice were restrained unanesthetized in metal holders exposing their backs. For PDT treatment in vitro, LLC cells growing in 35-mm Petri dishes (0.8 × 106 per dish) were exposed to Photofrin™ (20 μg/ml in full growth medium) for 24 h, then washed with phosphate-buffered saline and exposed to the light dose of 1 J/cm2. The growth medium was then returned and the cells were kept in culture in protein- and serum-free medium (S8284, Sigma Chemical Co., St. Louis, MO, USA) for further 4 or 8 h at 37°C before they were collected using RNeasy® Plus Mini kit (Qiagen Inc., Mississauga, Ontario, Canada) and stored for reverse transcriptase polymerase chain reaction (RT-PCR) analysis. In additional experiments, LLC cells were plated in Millicell culture inserts (PIHP03050, Millipore Corporation, Billerica, MA, USA) with porous polycarbonate membrane bottom and were transferred after PDT treatment to Petri dishes with PDT-untreated Hepa 1-6 cells for 4 or 8 h co-incubation before collection of Hepa 1-6 cells for quantitative RT-PCR. In all cases, the number of cells per dish and insert was kept around 0.8 × 106 (1:1 ratio for LLC/Hepa 1-6).
Hsp70 gene expression analysis
Mice with PDT-treated tumors were sacrificed at various time points after therapy and their tumors, livers, and spleens excised. Homogenates of these tissues, or cells harvested after in vitro experiments, were processed for total RNA isolation that served for assessing Hsp70 gene expression levels based on quantitative real-time RT-PCR as described previously in detail (Korbelik et al. 2008). In brief, 1-μg samples of total RNA were processed employing SuperScript III Platinum Two-Step qRT-PCR kit with SYBR green (Invitrogen Canada Inc., Burlington, Ontario, Canada). The mouse Hsp70 gene-specific primers used were ATCACCATCACCAACGACAAG (forward) and GAGATGACCTCCTGGCACTT (reverse), designed to amplify the Hspa1a and Hspa1b polymorphs. Housekeeping gene glyceraldehid-3-phosphate dehydrogenase (GAPDH) expression was also detected for each sample and used for normalizing Hsp70 gene expression. The effects of i.p. injected recombinant dexamethasone (Sigma) at 40 μg/kg and glucocorticoid synthesis inhibitor metyrapone (Sigma) at 100 mg/kg on the expression of Hsp70 gene were also tested.
Hsp70 enzyme-linked immunosorbent assay
The levels of Hsp70 in mouse liver tissue homogenates and serum were determined using the enzyme-linked immunosorbent assay (ELISA) kit purchased from Assay Designs Inc. (Ann Arbor, MI, USA). The kit uses mouse anti-Hsp70 monoclonal antibody for capture while rabbit anti-Hsp70 polyclonal antibody combined with horseradish peroxidase-conjugated anti-rabbit IgG serves for detection. Blood was collected for serum Hsp70 samples only once with each mouse to minimize the effects of nonspecific stress.
Distribution of injected Hsp70 between PDT-treated tumor and liver
Recombinant mouse Hsp70 (Assay Designs) and mouse albumin (Sigma), biotinylated using AnaTag™ Biotin Protein Labeling Kit (AnaSpec Inc., Fremont, CA, USA), were injected intravenously (20 μg/mouse) at either 3.5 or 7.5 h after PDT light treatment. The mice were sacrificed for tumor and liver tissue collection at 30 min after biotinylated protein injection. The experimental groups (each containing four LLC tumor-bearing mice) were: control untreated mice (not injected biotinylated protein) [A], PDT-untreated mice injected with biotinylated Hsp70 [B], PDT-untreated mice injected with biotinylated albumin [C], PDT-treated mice injected with biotinylated Hsp70 and sacrificed at 4 h after PDT [D], PDT-treated mice injected with biotinylated albumin and sacrificed at 4 h after PDT [E], PDT-treated mice injected with biotinylated Hsp70 and sacrificed at 8 h after PDT [F], and PDT-treated mice injected with biotinylated albumin and sacrificed at 8 h after PDT [G]. The collected tumor and liver tissue homogenates were used for the determination of biotinylated Hsp70 and biotinylated albumin levels using the ELISA technique. For biotinylated Hsp70, the capture antibody was goat anti-mouse Hsp70 polyclonal antibody (K-20) obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and streptavidin–peroxidase polymer (Sigma, S2438) was used for detection. For biotinylated albumin, well plates were used from mouse albumin ELISA kit (Immunology Consultants Laboratory Inc., Newberg, OR, USA) that have rabbit anti-mouse albumin polyclonal antibody bound to solid phase while detection was again based on streptavidin–peroxidase polymer. Aliquots of biotinylated proteins prepared for injection into mice were used for the calibration curve standards. Nonspecific background value obtained from tumor and liver samples of group A was subtracted from the absorbance reading.
Flow cytometry
For testing the interaction of Hsp70 with PDT-treated cells, recombinant mouse Hsp70 and mouse albumin (Sigma) serving as control protein were labeled with 5-flourescein isothiocyanate (FITC) using a kit obtained from AnaSpec. After PDT treatment, consisting of the exposure to Photofrin™ (10, 20, or 40 μg/ml) followed 24 h later by 1 J/cm2 of 630 ± 10 nm light, LLC cells were incubated in full growth medium for 2.5 h and then FITC-Hsp70 or FITC-albumin was added at 10 μg per sample. These samples were left for another 30 min at 37°C and then the cells were collected for flow cytometry that was performed using a Coulter Epics Elite ESP (Coulter Electronics, Hialeah, FL, USA). Before flow cytometry, some samples were stained with PE-conjugated annexin V, or a combination of 7-aminoactinomycin D (7-AAD) and PE-conjugated rabbit anti-active caspase-3 monoclonal antibody, all from PharMingen (BD Biosciences, Mississauga, Ontario, Canada). Staining with the latter two dyes enabled the identification of cells as viable (negative with both stains), apoptotic (active caspase-3 positive, 7-AAD negative), and necrotic (positive with both stains).
Statistical analysis
The difference between means for various treatment groups was evaluated using nonparametric Mann–Whitney test. Statistical significance level threshold was set at 5%.
Results
PDT-induced Hsp70 gene expression in vitro
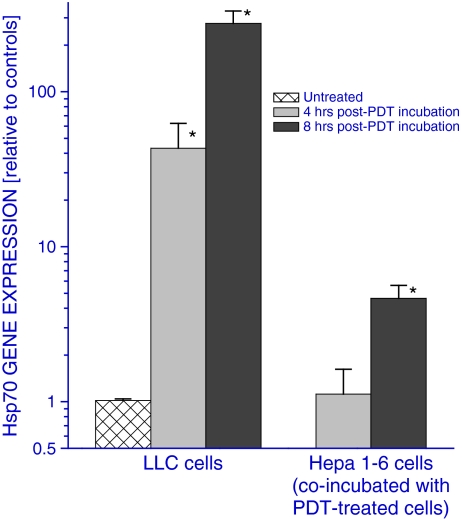
The insult inflicted in cells treated with PDT is known to provoke the elevated expression of heat shock proteins, including Hsp70 (Gomer et al. 1996; Zhou et al. 2008). This is also revealed when examining, based on quantitative RT-PCR analysis, the expression of gene encoding Hsp70 in LLC cells treated in vitro by a largely lethal dose of Photofrin™ PDT (Fig. 1). The upregulation of Hsp70 gene was prominent at 4 h after PDT and it raised even further reaching almost 300-fold increase (compared to the level in untreated cells) at 8 h after PDT. However, the expression of Hsp70 gene is not only affected in cells directly treated with PDT but also in untreated cells co-incubated with PDT-treated cells. This was documented by examining the Hsp70 gene expression in PDT-untreated Hepa 1-6 cells (malignantly transformed hepatocytes) that were in Petri dishes to which culture inserts were added containing PDT-treated LLC cells (Fig. 1). The Hsp70 gene expression in these cells increased when they were co-incubated for 8 h with PDT-treated LLC cells although the increase was not as dramatic as in PDT-treated LLC cells.
Fig. 1.
Direct and indirect effects of PDT treatment on Hsp70 gene expression in vitro. Cultured LLC cells were treated with PDT (Photofrin™ 20 μg/ml for 24 h followed by 1 J/cm2). They were then kept further in culture for 4 or 8 h either alone or co-incubated (contained in separate culture inserts) with untreated Hepa 1-6 cells. At the end of the incubation period, PDT-treated LLC cells and Hepa 1-6 cells were collected for quantitative RT-PCR-based analysis of Hsp70 gene expression. The results were normalized to corresponding controls using PDT-untreated LLC cells for the left columns and Hepa 1-6 cells co-incubated with PDT-untreated LLC cells for the right columns. N = 4, bars are SD; *p < 0.05, statistically significant difference compared to controls
Hsp70 gene expression in PDT-treated tumor and distant sites
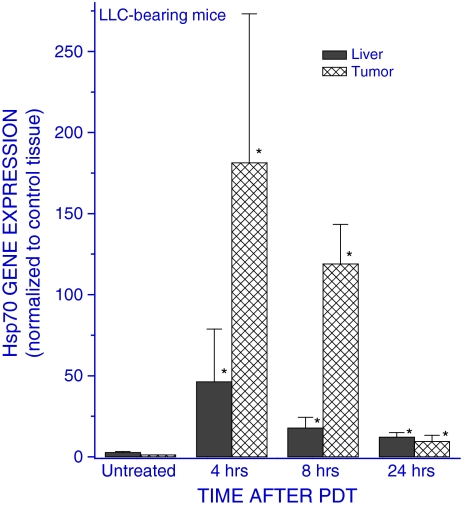
Since these results with Hepa 1-6 cells indicate that some factors released from PDT-treated cells may stimulate the upregulation of Hsp70 gene in untreated cells, it became warranted to examine whether such effect can be observed in vivo. Subcutaneous LLC tumors growing in syngeneic C57BL/6 mice were treated with PDT (dose producing a complete initial tumor ablation), and the mice were sacrificed at different time intervals after the therapy to collect their tumors, livers, and spleens for examining the Hsp70 gene expression in these tissues. The results reveal that the activity of this gene markedly increased not only in the PDT-treated tumors but also in the livers of host mice (Fig. 2). The fold increase was greater in the tumor than in the liver, but in both cases it was most prominent at 4 h after PDT and then subsided to remain only slightly elevated at 24 h post therapy. No significant changes in Hsp70 gene expression were found in tumor and liver tissues of control mice, including those that received photosensitizer only or tumor light treatment without prior photosensitizer administration. For instance, the values (means ± standard deviations) for ratios relative to those obtained with the untreated group were with the light-only controls 0.88 ± 0.28 and 1.71 ± 0.63 for tumor and liver tissues, respectively. Since mice had to be restrained for the exposure to light-only treatment, these results also demonstrate that the stress due to restraint itself cannot provoke significant Hsp70 gene upregulation in these tissues. It is of note that, judging from Ct values in quantitative RT-PCR analysis, the basic expression of Hsp70 gene was at similar levels in tumor and liver tissues. The upregulation of Hsp70 gene expression was also detected in the spleens of mice bearing PDT-treated tumors although the rise was only around 30% of that found in the livers (not shown).
Fig. 2.
The expression of Hsp70 gene in tumor and liver tissue of C57BL/6 mice bearing PDT-treated LLC tumors. Mice with LLC tumors were injected with Photofrin™ (10 mg/kg i.v.), and 24 h later, the tumors were exposed to 630 ± 10 nm light dose of 150 J/cm2. The mice were sacrificed at 4, 8, or 24 h after light treatment and their tumor and liver tissues collected for quantitative RT-PCR-based analysis of the expression of the Hsp70 gene. The results are presented as the GAPDH-normalized Hsp70 gene expression relative to that in the same tissue of untreated tumor-bearing mice. N = 4, bars are SD; *p < 0.05, values significantly different than the controls
Mediators of systemic Hsp70 gene upregulation
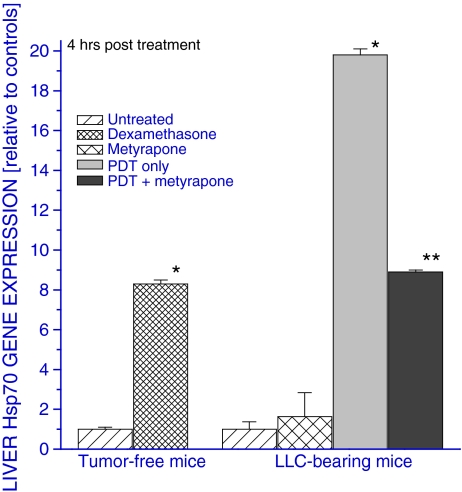
The upregulation of various genes in the livers of mice bearing PDT-treated tumors was recently described in our report demonstrating the induction of acute phase response by this therapy (Korbelik et al. 2008). The study identified glucocorticoid hormones as one of the major mediators responsible for this phenomenon. To test whether these hormones are involved in the observed modulation of Hsp70 gene activity, dexamethasone (synthetic glucocorticoid) was injected in naive tumor-free mice. The dexamethasone injection provoked a significant increase in the liver Hsp70 gene expression at 4 h after treatment (Fig. 3). In the second part of the experiment, Hsp70 gene expression was analyzed in the livers of mice with PDT-treated LLC tumors that were injected glucocorticoid synthesis inhibitor metyrapone or control solution and compared to that obtained from PDT-untreated mice. It can be seen that PDT-induced liver Hsp70 gene upregulation was strongly inhibited by the metyrapone treatment (Fig. 3). Without PDT, metyrapone treatment had no negative impact on Hsp70 gene expression (not shown).
Fig. 3.
The effects of dexamethasone and tumor PDT on liver Hsp70 gene expression. Tumor-free C57BL/6 mice were i.p. injected either with dexamethasone (40 μk/kg) or control solution. Alternatively, mice with LLC tumors were treated with PDT as described in Fig. 2 and some were given metyrapone (100 mg/kg, i.p.) 15 min before the onset of PDT light exposure. The mice were sacrificed at 4 h post treatment and their livers collected for quantitative RT-PCR-based analysis of Hsp70 gene expression. The results are presented as the GAPDH-normalized Hsp70 gene expression relative to that in the liver of tumor-free controls or untreated LLC tumor-bearing controls. N = 4, bars are SD; *p < 0.05, statistically significant difference compared to untreated controls; **p < 0.05, statistically significant difference compared to the PDT-only group
Hsp70 protein in the liver and serum
To investigate how PDT-induced changes in liver Hsp70 gene activity affect the levels of its protein product, the liver tissue was collected from mice bearing PDT-treated tumors at different time intervals and used for determining Hsp70 protein concentration based on ELISA assay. Despite the Hsp70 gene upregulation after PDT, the levels of liver Hsp70 protein were found to decrease from around 170 to about 115 ng/g of tissue (a decline by almost 40%) after the first 3 h; however, the levels increased over 20% between 3 and 5 h post PDT (consistent with the timing of Hsp70 gene upregulation seen in Fig. 2), but this was followed again by a 30% decrease after the next 4 h (Table 1).
Table 1.
The effect of tumor PDT on liver Hsp70 protein levels
| Experimental group | Hsp70 per gram of liver tissue (ng) |
|---|---|
| Tumor-free controls | 180.9 ± 21.0a |
| Untreated tumor control | 170.4 ± 22.7 |
| 3 h after PDT | 115.7 ± 4.3b,c |
| 5 h after PDT | 142.5 ± 7.1b |
| 9 h after PDT | 100.1 ± 5.5b,c |
Mice bearing LLC tumors were PDT-treated (Photofrin™ 10 mg/kg plus 150 J/cm2). They were sacrificed at indicated times after PDT, their livers excised and used for Hsp70 protein determination based on the ELISA assay (N = 4)
aStandard deviations
bSignificantly lower than the untreated tumor group (p < 0.05)
cSignificantly lower than the 5-h after PDT group
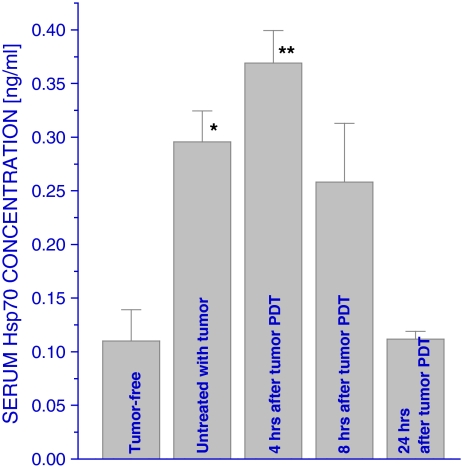
Since one of the most plausible explanations of the results in Table 1 is that the Hsp70 protein is released from the liver after PDT, changes in Hsp70 serum levels during this time period were examined next using ELISA. The results reveal that serum Hsp70 levels become elevated in host mice even when bearing untreated tumors while further increase was evident in mice with PDT-treated tumors at 4 h after therapy (Fig. 4); this was followed by a decrease to the pretreatment range at 8 h after PDT and further decline to the levels similar to tumor-free mice at 24 h after PDT.
Fig. 4.
Serum Hsp70 levels in mice after tumor PDT. LLC tumors growing in C57BL/6 mice were PDT-treated as described in Fig. 2, and blood was collected from the mice at either 4, 8, or 24 h post therapy for serum Hsp70 measurement based on the ELISA assay. N = 4, bars are SD; *p < 0.05, statistically significant difference compared to tumor-free controls; **p < 0.05, statistically significant difference compared to the control group with untreated tumors
Since the average liver weights in mice used in the experiments described in Table 1 were around 600 mg, from the results in this table, it can be estimated that at least around 30 ng of Hsp70 disappeared from the liver of tumor PDT-treated mice during the first 3 h after therapy. As the total blood volume of a mouse is less than 2 ml (Wu et al. 1981), if all of the Hsp70 depleted from the liver was released into the blood and remained in circulation, it would increase the serum level of this protein to over 15 ng/ml. This is over five times more than the actual increase in serum Hsp70 detected by our measurement (Fig. 4) that translates to about 2.7 ng/ml for the area under curve covering 0 to 8 h time interval (after which the levels drop below the pretreatment range). To address the possibility that some of serum Hsp70 was contained within secretory vesicles (exosomes) and remained undetected (Bausero et al. 2005), some samples were pretreated with 1% Lubrol WX (a detergent which solubilizes exosomes) but this produced no significant difference in the ELISA measurement (not shown). The implication is that either most of this Hsp70 was catabolized in the liver or that it was rapidly sequestered from the circulation. Since the latter consideration is consistent with the possibility that Hsp70 protein produced and released from the liver of mice with PDT-treated tumors is attracted to the tumor, we tested the capacity of Hsp70 to bind to PDT-treated tumor cells using an in vitro model. For that purpose, recombinant mouse Hsp70 and a control protein (albumin) were conjugated with the fluorescent marker FITC.
Hsp70 protein binding to PDT-treated cells
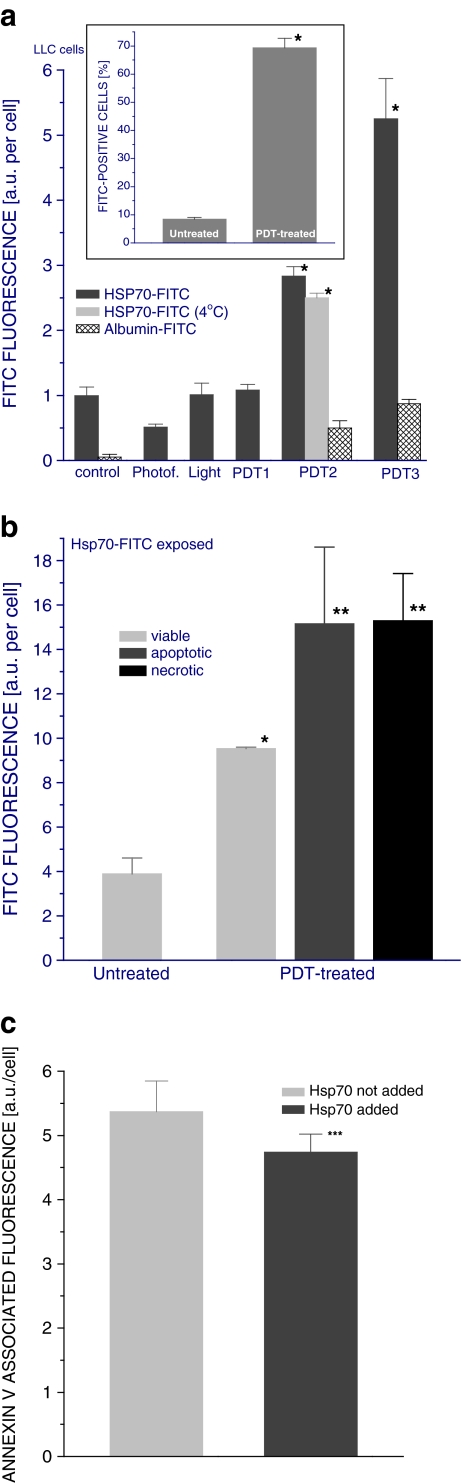
The results presented in Fig. 5a show that increasing amounts of Hsp70 become associated with tumor cells treated with increasing doses of PDT while this was not evident with albumin. This effect was evident from the increased Hsp70-associated fluorescence on tumor cells (main graph) as well as from the increased percentage of fluorescence-positive tumor cells (insert). The association of Hsp70-FITC with tumor cells was not promoted by treatment of cells with Photofrin™ only or light alone. It can be also seen that incubating PDT-treated cells with Hsp70 at 4°C (instead of 37°C as done with the other samples in this experiment) had no major impact on the levels of cell-associated protein (Fig. 5a). Since incubation at such low temperature prevents metabolic activity that could lead to active internalization of bound Hsp70, the lack of difference between 4°C and 37°C suggests no significant loss of Hsp70 from the cell surface by this process.
Fig. 5.
Binding of Hsp70 protein to PDT-treated tumor cells. LLC cells were exposed to Photofrin™ at 10 μg/ml (for PDT1), 20 μg/ml (for PDT2, same dose as in Fig. 1), or 40 μg/ml (for PDT3) for 24 h and then treated with 1 J/cm2 of 630 ± 10 nm light. The cells were thereafter incubated in full growth medium at 37°C. At 2.5 h post-PDT light treatment, Hsp70-FITC or albumin-FITC was added at 10 μg/sample and the cells were further incubated for 30 min at 37°C (except for one group incubated at 4°C) before they were collected for flow cytometry analysis. a Hsp70- or albumin-associated fluorescence of cells treated with PDT based on three different Photofrin™ doses and controls including untreated, Photofrin™-only (20 μg/ml), and light-only (1 J/cm2)-treated cells (the fluorescence values were corrected by subtracting the background measured with cells receiving the same treatment but no FITC-labeled protein)—the insert depicts FITC fluorescence-positive fraction size for untreated and PDT2-treated cells exposed to Hsp70-FITC; b Hsp70-associated fluorescence on cells treated with PDT2 identified as viable, apoptotic, or necrotic; c PE fluorescence obtained with annexin V–PE staining of PDT2-treated cells exposed or not exposed to Hsp70-FITC. N = 4, bars are SD; *p < 0.05, statistically significant difference compared to PDT-untreated controls; **p < 0.05, statistically significant difference compared to viable cells in the PDT-treated group; ***p < 0.05, statistically significant difference compared to Hsp70 not added group
In order to determine whether cell death induced by PDT treatment influences Hsp70 binding, the level of Hsp70-associated fluorescence was examined separately on viable, apoptotic, and necrotic cells. The results reveal that Hsp70 was associated with cells that appeared viable after PDT treatment, but this was much less pronounced than with dead cells (Fig. 5b). There was no detectable difference in the extent of Hsp70 interaction between apoptotic and necrotic cells.
Phosphatidylserine, which becomes expressed on dead cells and Hsp70 is known to bind to it (Arispe et al. 2004), is one of the obvious candidates for the molecule on PDT-treated cells that could be responsible for the observed fixation of Hsp70. To test this, PDT-treated tumor cells exposed to Hsp70 were stained with PE-conjugated annexin V (classical phosphatidylserine-binding protein). The results show that annexin V-associated fluorescence was significantly reduced on cells incubated with Hsp70 compared to cells not incubated with Hsp70 (Fig. 5c), suggesting that phosphatidylserine is indeed one of the molecules localized on PDT-treated cells that interacts with Hsp70. The fact that the annexin V-associated fluorescence was only partially diminished implies that other molecules are also involved in Hsp70 binding and/or Hsp70 interaction with phosphatidylserine does not completely prevent subsequent binding of annexin V.
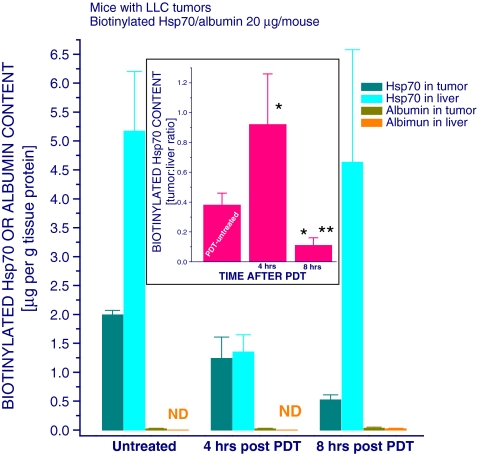
Accumulation of circulating Hsp70 in PDT-treated tumor
Since our flow cytometry data suggest that extracellular Hsp70 can get captured bound to PDT-treated cells, it was of interest to determine whether Hsp70 from peripheral blood circulation converges to and becomes entrapped in the tumor after PDT. For that purpose, biotinylated recombinant mouse Hsp70 or biotinylated mouse albumin (serving as a control reference protein) was injected intravenously into mice bearing PDT-treated LLC tumors, and the animals were sacrificed 30 min later to collect their liver and tumor tissue for analysis. The results show that, expressed in micrograms per gram of tissue protein, about 2.6 times more of injected Hsp70 accumulated in the liver than in the tumor in mice with untreated tumors (Fig. 6). However, as seen by the tumor/liver ratio depicted in the figure insert, this changed dramatically at 4 h after PDT when the preference of Hsp70 accumulation in tumors increased substantially (with tumor to liver ratio becoming close to 1). Circulating Hsp70 attracted to PFT-treated tumor may not be retained long at that site; after being captured complexed with client damaged proteins by phagocytes, it can be degraded or transported to draining lymph nodes. Such occurrence would explain the fact that, quantitatively, the amount of injected Hsp70 detected in the PDT-treated tumors at the 4-h time point has not increased compared to untreated tumors.
Fig. 6.
Distribution of biotinylated Hsp70 in mice with PDT-treated tumors. LLC tumors growing in C57BL/6 mice were PDT-treated as described in Fig. 2. The mice were injected with biotinylated recombinant mouse Hsp70 or biotinylated mouse albumin (20 μg/mouse, i.v.) at either 3.5 or 7.5 h after PDT light treatment. Thirty minutes after biotinylated protein injection, the mice were sacrificed and their tumor and liver tissue collected for the determination of biotinylated Hsp70 and biotinylated albumin levels using ELISA technique. The graph insert shows PDT-induced changes in the tumor/liver ratio of biotinylated Hsp70 content. N = 4, bars are SD; *p < 0.05, statistically significant difference compared to PDT-untreated controls; **p < 0.05, statistically significant difference compared to the 4-h post-PDT group
The results of the same experiments further show that at a later phase after PDT (8 h), the levels of injected Hsp70 found in the tumors compared to the liver declined dramatically and become lower than those in the untreated tumors (Fig. 6). In contrast to Hsp70, the injected albumin manifested no particular tendency to accumulate either in the liver or tumor irrespective of PDT treatment.
Discussion
Injury causing breakdown of local homeostasis, such as acute physical trauma, prompts a rapid host reaction calling into action two major innate defense effector processes, inflammation and acute phase response. Characterized as a stress response at the level of the organism, the acute phase response is a dynamic homeostatic process integrating complex regulated (predetermined) physiological and biochemical events involving distant systemic changes and including many organ systems (Gabay and Kushner 1999). The aim of acute phase response is mobilizing resources from the entire organism in order to create an overall protective environment to deal with tissue injury (isolating and removing damaged tissue, and then ensuring healing and regeneration of normal function). A major hallmark of the acute phase response is the radically altered biosynthetic profile of the liver that is regulated by inflammatory cytokines, adrenal hormones (products of activated hypothalamic–pituitary–adrenal axis), and other mediators (Gabay and Kushner 1999; Chrousos 1995). This results in dramatic changes in the levels of various plasma proteins known as acute phase reactants. Among acute phase reactants, which have a wide range of activity contributing to the eventual restoration of homeostasis at the affected site, are proteins (such as complement components and pentraxins) engaged in clearance of host dead cells and tissue debris (Gabay and Kushner 1999; Steel and Whitehead 1994). Thus, these acute phase reactants promote local accumulation of phagocytes and other inflammatory effector cells, stimulate their activation, and/or opsonize damaged cells to facilitate their efferocytosis (phagocytic removal) (Nauta et al. 2003; Manfredi et al. 2002).
Rapid destruction of tumor tissue by PDT is perceived by the host not much differently than a physical trauma inflicted in normal tissue, as evidenced by a strong inflammatory reaction and acute phase response observed following this therapy (Korbelik 2006; Korbelik et al. 2008). Acute phase reactants found to be produced in the host mice bearing PDT-treated tumors include complement and pentraxin proteins with important roles in the disposal of dead cells (Merchant et al. 2007; Korbelik et al. 2008). The present investigation demonstrated that the gene encoding Hsp70 becomes upregulated following tumor-targeted PDT at distant sites including the liver and spleen. Moreover, the results reveal that this increase in the expression of liver Hsp70 gene is mediated at least in part by adrenal glucocorticoid hormones. The levels of these hormones are known to become elevated in acute phase response (Gabay and Kushner 1999). We have recently demonstrated that mice bearing PDT-treated tumors have increased serum levels of corticosterone (primary glucocorticoid in these animals) as early as 1 h after therapy (Merchant et al. 2010), and it is becoming increasingly evident that glucocorticoid hormones play an important role in tumor PDT response (Korbelik and Merchant 2008). Such effect of glucocorticoids is in accordance with the reported induction of Hsp70 production in rat cardiac myocytes by dexamethasone treatment (Sun et al. 2000). The full extent of upregulation of liver Hsp70 gene expression is most likely attained by the action of glucocorticoids in conjunction with other mediators, primarily interleukin-6 (IL-6) that is known as a major inducer of various acute phase reactants (Heinrich et al. 1990). We detected an increase in the expression of Hsp70 gene in the liver of naive mice injected with 1 μg of recombinant mouse IL-6 (data not shown). The results in vitro with Hepa 1-6 cells (Fig. 1) also indicate that there are mediators released from PDT-treated cells capable of triggering signal transduction pathways leading to the increased expression of Hsp70 gene.
The analysis of liver Hsp70 protein levels (Table 1) shows that tumor PDT treatment may stimulate the liberation of this protein from the liver and this includes, as suggested by the temporary rise between 3 and 5 h after PDT, newly synthesized Hsp70 produced as a result of the PDT-induced liver Hsp70 gene upregulation. The Hsp70 released from the liver (also from the spleen and possibly other sites following PDT) appears to remain in the circulation for a very limited time since the blood Hsp70 levels, although temporarily elevated, never reach correspondingly high concentrations. Notably, even the presence of untreated growing tumors provoked a two- to threefold increase in liver Hsp70 gene expression in the host mice (not shown) that was reflected in a threefold increase in serum Hsp70 content (Fig. 4). At 4 h after tumor PDT, there was a further rise in serum Hsp70 levels by about 25%, but no elevation was detected at later post-therapy time intervals. Moreover, at 24 h after PDT, when the treated tumors became impalpable, the serum Hsp70 levels declined to those found in tumor-free mice. These results suggest that elevated concentration of Hsp70 in the circulation is a sensitive signal of the presence of malignant growth and potential indicator of the effectiveness of therapy-mediated tumor destruction. This consideration merits further verification and is being pursued in our ongoing investigation.
Prompted by the discovery from our in vitro studies that Hsp70 avidly associates with PDT-treated cells, especially those undergoing apoptotic or necrotic death (Fig. 5), we investigated the relevance of this phenomenon in vivo. Our findings reveal that while the liver, as a normal site of Hsp70 catabolism, captures much greater amount of this protein injected into the circulating blood than the untreated tumor, PDT-treated tumors at 4 h after therapy sequester similar amounts of Hsp70 as the liver (Fig. 6). Thus, changes precipitated by PDT treatment render the tumor tissue strongly attracting for Hsp70 from circulation. The fact that absolute amounts of injected Hsp70 found retained in both liver and tumor are actually lower at 4 h after PDT can have several possible causes. Tumor-associated phagocytes activated after PDT may scavenge Hsp70 together with its captured target and either rapidly degrade it or transport it out of the tumor to lymph nodes. Another possibility is that PDT-activated immune cells in the spleen or other sites become more engaged in entrapping circulating Hsp70.
Based on these findings, we suggest that the presence of damaged tumor tissue such as induced by PDT prompts a rapid sequestration of circulating Hsp70 to the lesion site. This Hsp70 originates from two sources, either from the fraction of this protein produced by genes upregulated in the treated tumor as a consequence of PDT-induced stress that is known to translate to the cell surface (Korbelik et al. 2005), or from the Hsp70 stimulated after PDT to be produced in the liver (also in the spleen) as acute phase reactant. It appears that both these Hsp70 sources are important: the first due to its local character (that can secure immediate supply) and the second for the mobilization of high producing capacity of organs such as the liver.
Binding to surface-exposed phosphatidylserine on dying cells (Arispe et al. 2004) seems to be one of the underlying molecular interactions, but it appears that other molecular structures (remaining to be identified) are also involved. This phenomenon would be in accordance with the function of Hsp70 as a major facilitator of physiological removal of dying cells and their debris. For performing such role, Hsp70 can act as one of the phagocytosis-promoting bridging molecules (Májai et al. 2006; Krysko et al. 2006) due to its ability to bind phagocytic receptors on macrophages and other phagocytes (Calderwood et al. 2007). It can also interact with other bridging molecules, notably complement proteins (Prohászka et al. 2002). Additional amounts of Hsp70 could originate from a fraction of this protein produced in the same cells that is translated to the surface as a consequence of PDT insult (Korbelik et al. 2005).
Impairment of disposal of dead cells leads to autoimmune disease (Manfredi et al. 2002) because this process is critical for maintaining immune tolerance (Mahoney and Rosen 2005). Immune rejection of antigens from dead cells or their tolerance depends largely on which phagocytic receptors are engaged and is greatly influenced by the involved bridging molecules. For instance, binding of pentraxins C-reactive protein or serum amyloid P component to dying cells could direct the interaction towards immunodownregulating type of Fcγ receptors on the phagocytes (Mold et al. 2001; Nimmerjahn and Ravetch 2008) while the engagement of Hsp70 as bridging molecule would steer the phagocytosis through scavenger receptors such as LOX-1 that may trigger immunoactivating signaling and recognition of antigens associated with Hsp70 (Calderwood et al. 2007). Another possible consequence of the fixation of Hsp70 on dying cells is providing the opportunity for this HSP to bind Toll-like receptors 2 and 4 as well as other receptors (CD91, CD14, CD40, and CCR5) on various immune effector cells (Calderwood et al. 2007; Gehrmann et al. 2008), which would modulate their activity. Thus, the mobilization of Hsp70 in the process of disposal of tumor tissue destroyed by PDT may represent an important element in the development of immune response against the treated tumor that is known to develop following treatment by this modality (Korbelik 2006; Castano et al. 2006).
In conclusion, the presented study used PDT as a tool to uncover an important property of Hsp70 to be produced as an acute phase reactant. Released from the liver and other distant sites (spleen), it appears to be rapidly sequestered from the circulation to the tumor tissue damaged by treatments such as PDT in order to facilitate the disposal of dying cells and debris. Cancer therapy that elicits such engagement of Hsp70 has increased potential for promoting the development of antitumor immune response. Serum Hsp70 emerges as a highly sensitive indicator of tumor burden and its monitoring may be useful for valorizing the effectiveness of cancer therapy.
Acknowledgments
The authors wish to thank Dr. Polly Matzinger for helpful advice in conducting this study. Expert assistance with flow cytometry was provided by Denise McDougal. Axcan Pharma has provided their product (Photofrin™) free of charge for this study.
Disclosure of funding received This study was supported by the National Cancer Institute of Canada, with funds from the Canadian Cancer Society.
References
- Arispe N, Doh M, Simakova O, Kurganov B, Maio A. Hsc70 and Hsp70 interact with phospahatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18:1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- Asea A. Chaperone-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- Bausero MA, Gastpar R, Multhoff AA. Alternative mechanism by which IFN-γ enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Theriault J, Gray PJ, Gong J. Cell surface receptors for molecular chaperons. Methods. 2007;43:199–206. doi: 10.1016/j.ymeth.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumor immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Radons J, Molls M, Multhoff G. The therapeutic implications of clinically applied modifiers of heat shock protein 70 (Hsp70) expression by tumor cells. Cell Stress Chaperones. 2008;13:1–10. doi: 10.1007/s12192-007-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AMR. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56:2355–2360. [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperons in cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin 6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283–294. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38:500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- Korbelik M. Complement upregulation in photodynamic therapy-treated tumors: role of Toll-like receptor pathway and NFκB. Cancer Lett. 2009;281:232–238. doi: 10.1016/j.canlet.2009.02.049. [DOI] [PubMed] [Google Scholar]
- Korbelik M, Merchant S. Hormonal component of tumor photodynamic therapy response. Proc SPIE. 2008;6857:051–058. [Google Scholar]
- Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- Korbelik M, Cecic I, Merchant S, Sun J. Acute phase response induction by cancer treatment with photodynamic therapy. Int J Cancer. 2008;122:1411–1417. doi: 10.1002/ijc.23248. [DOI] [PubMed] [Google Scholar]
- Krysko DV, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17:583–588. doi: 10.1016/j.coi.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Májai G, Petrovski G, Fésüs L. Inflammation and the apopto-phagocytic system. Immunol Lett. 2006;104:94–101. doi: 10.1016/j.imlet.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Manfredi AA, Iannacone M, D’Auria F, Rovere-Querini P. The disposal of dying cells in living tissue. Apoptosis. 2002;7:153–161. doi: 10.1023/A:1014366531885. [DOI] [PubMed] [Google Scholar]
- Merchant S, Sun J, Korbelik M. Dying cells program their expedient disposal: serum amyloid P component upregulation in vivo and in vitro induced by photodynamic therapy of cancer. Photochem Photobiol Sci. 2007;6:1284–1289. doi: 10.1039/b709439f. [DOI] [PubMed] [Google Scholar]
- Merchant S, Huang N, Korbelik M (2010) Expression of complement and pentraxin proteins in acute phase response elicited by tumor photodynamic therapy: the engagement of adrenal hormones. Int Immunopharmacol (in press) [DOI] [PubMed]
- Mold C, Gresham HD, Clos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Prohaska Z, Nagy B, Kalabay L, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of increased serum heat shock protein 70 and C-reactive protein concentration and decreased serum alpha(2)-HS glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol. 2006;73:172–179. doi: 10.1016/j.jri.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Daha MR, Kooten C, Roos A. Recognition and clearance of apoptotic cells: a role of complement and pentraxins. Trends Immunol. 2003;24:148–154. doi: 10.1016/S1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune response. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Prohászka Z, Singh M, Nagy K, Lakos G, Duba J, Füst G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7:17–22. doi: 10.1379/1466-1268(2002)007<0017:HSPIAP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Sun L, Chang J, Kirchhoff SR, Knowlton AA. Activation of HSF and selective increase in heat-shock proteins by acute dexamethasone treatment. Am J Physiol Heart Circ Physiol. 2000;278:H1091–H1097. doi: 10.1152/ajpheart.2000.278.4.H1091. [DOI] [PubMed] [Google Scholar]
- Terry DF, Wyszynski DF, Nolan VG, Atzmon G, Schoenhofen EA, Pennington JY, Andersen SL, Wilcox MA, Farrer LA, Barzilai N, Baldwin CT, Asea A. Serum heat shock protein 70 level as a biomarker of exceptional longevity. Mech Ageing Dev. 2006;127:862–868. doi: 10.1016/j.mad.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Wu MS, Robbins JC, Bugianesi RL, Ponpipom MM, Shen Y. Modified in vivo behavior of liposomes containing synthetic glycolipids. Biochim Biophys Acta. 1981;674:19–29. doi: 10.1016/0304-4165(81)90342-1. [DOI] [PubMed] [Google Scholar]
- Zhou F, Xing D, Chen WR. Dynamics and mechanism of HSP70 translocation induced by photodynamic therapy treatment. Cancer Lett. 2008;264:135–144. doi: 10.1016/j.canlet.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Zhou F, Xing D, Chen WR. Regulation of HSP70 on activating macrophages using PDT-induced apoptotic cells. Int J Cancer. 2009;125:1380–1389. doi: 10.1002/ijc.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]