Abstract
Several studies suggest that the response to various stressors differs between the sexes. We aimed to study serum HSP70 and levels of oxidized-LDL (ox-LDL) as markers of oxidative stress in men and women with type 2 diabetes. We quantified serum HSP70 and levels of ox-LDL in three cohorts; patients with newly diagnosed diabetes, patients with long-standing diabetes and normal controls. The cohort of patients with newly diagnosed diabetes was followed up for 3 months under glucose-lowering therapy with metformin. Our findings showed that serum HSP70 level was increased in women with long-standing diabetes in comparison with men. HSP70 did not decrease after glucose lowering therapy in women with newly diagnosed diabetes, but it did decrease in men. There was no significant difference on ox-LDL between men and women in any of the studied cohorts. It decreased significantly in the cohort of patients with newly diagnosed diabetes after treatment, regardless of sex. There was no significant correlation between HSP70 and ox-LDL in any of the studied cohorts except among normal women. We suggest that diabetes induces an immune response and impairs cellular defense mechanisms against oxidative stress more commonly in women with type 2 diabetes than in men.
Keywords: HSP70, Ox-LDL, Oxidative stress, Type 2 diabetes, Female
Introduction
Diabetes has a greater impact on mortality from cardiovascular disorders and dyslipidemia in women than in men (Kragelund et al. 2007; Legato et al. 2006). However, the traditional risk factors for coronary heart disease such as hypertension, elevated serum cholesterol, and smoking, do not explain unusually high prevalence of coronary heart disease among women with type 2 diabetes (Wei et al. 2009).
One of the mechanisms underlying hyperglycemia-induced inflammation and vascular complications is oxidative stress and the action of reactive oxygen species within the cell nucleus (Wright et al. 2006). Heat shock proteins (HSP) are a family of stress-responsive proteins that modulate cell function and contribute to protein homeostasis (Asea 2008; Atalay et al. 2009; Mayer and Bukau 2005). HSPA1A/B (HSP70) is one of the members of the HSP family that have been most extensively studied (Kampinga et al. 2009; Hageman and Kampinga 2009). HSP70 expression increases under stressful conditions (Ireland et al. 2007; Pandey et al. 2009; Soti et al. 2005; Tamasi et al. 2009; Molvarec et al. 2009a) and is positively correlated with markers of inflammation, such as CRP, monocyte count, serum aspartate aminotrasferase, LDH activity, plasma malondialdehyde level and TNF-α (Njemini et al. 2004; Mayer and Bukau 2005; Molvarec et al. 2006, 2007a, 2009b,). We have previously shown that circulating levels of HSP70 is increased with duration of diabetes and is associated with the chronicity of the disease (Nakhjavani et al. 2010). Pockley et al. (2002) demonstrated that serum HSP70 level is significantly increased in subjects with hypertension and is a predictor of cardiovascular disease in humans (Pockley et al. 2002, 2003).
On the other hand, oxidized-LDL (ox-LDL) as a product of oxidative stress, has an important role in the early progression of atherosclerotic diseases and diabetes complications (Renie et al. 2007; Brownlee 2005). Several studies have reported a strong association between increased serum ox-LDL levels and the presence of auto-antibodies against ox-LDL with atherosclerosis, diabetes and its complications (Piarulli et al. 2005; Veiraiah 2005). Ox-LDL is an independent predictor of endothelial dysfunction (Woodman et al. 2005) in individuals suffering from oxidative stress, such as diabetic patients.
Svensson et al. (2006) reported that ox-LDL induces the release of HSP70 by human macrophages in vitro, and that extracellular HSP70 may be a major paracrine inducer of cytokine expression and secretion in human macrophage. There is one report that treatment of ketoacidosis resulted into lower level of HSP70 in patients with type 1 diabetes (Oglesbee et al. 2005). We are unaware of any study that discusses the response of HSP70 and ox-LDL to glucose-lowering therapy and also compares the results in men and women. We aimed to study:
The effect of metformin monotherapy on serum HSP70 and ox-LDL levels between men and women with type 2 diabetes
The correlation of HSP70 and ox-LDL in studied groups
A comparison of serum HSP70 and ox-LDL levels between men and women in 2 cohorts of patients defined as newly diagnosed diabetics and long-standing diabetics plus controls
Methods
Assembly of cohorts
We performed a cross-sectional analysis of serum samples from healthy normal controls and established cohorts of patients with type 2 diabetes, defined as: (1) patients with long-standing disease for more than 5 years, and (2) patients with newly diagnosed disease within the past 6 months who were not under any treatment. Patient recruitment was from the diabetes clinic of Vali-Asr Hospital, which is affiliated with Tehran University of Medical Sciences.
On the basis of the results of an exploratory analysis, we designed a follow-up of patients with newly diagnosed diabetes before and after 3 months of treatment. Treatment was started with metformin monotherapy and the patients had a 3-month period of therapy with three phases of initiation, titration and maintenance. The average dose of metformin was 1,000–1,500 mg/day. To avoid the confounding effect of measurement techniques, all serums of patients with newly diagnosed diabetes, before and after treatment, underwent measurements of HSP70 and ox-LDL twice with two different kits.
Diabetes was diagnosed according to the criteria of the American Diabetes Association (Diagnosis and classification of diabetes mellitus 2009). Cohorts were matched for age, sex, and body mass index (BMI). Exclusion criteria were smoking, pregnancy, proteinuria (creatinine >1.5 mg/dl or GFR <70 cm3/min), glomerulonephritis, congestive heart failure and hospital admission in the past 6 months. None of the participants had overt diabetic complications. Demographic and anthropometric data including age, sex duration of diabetes, height, weight in light clothing, and blood pressure in sitting position were recorded. Blood pressure was remeasured twice after 5 min and averaged. The BMI (kg/m2) was calculated according to the Quetelet formula.
The research was carried out according to the principles of the Declaration of Helsinki. Because the study involved data that was routinely collected, consent was orally obtained. The local ethics review committee of Tehran University of Medical Science approved the study protocol.
Blood samples
Blood samples were collected after 12 h of fasting, centrifuged and were kept at −70°C until analysis. Serum creatinine, fasting blood sugar (FBS), total cholesterol, triglycerides, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and HbA1c were measured for all participants. Glucose measurements (intra-assay coefficient of variants [CV] 2.1%, inter-assay CV 2.6%) were carried out using the glucose oxidase method. Cholesterol, HDL-C, LDL-C and triglycerides were determined using direct enzymatic methods (Parsazmun, Karaj, Iran).
Serum HSP70 and ox-LDL analysis
Measurements of serum HSP70 and ox-LDL were performed for all studied populations. Serum samples of patients with newly diagnosed diabetes underwent analysis of HSP70 and ox-LDL for the second time, before and after treatment with different kits from the primary analysis. Primary measurement of soluble HSP70 level was performed using a quantitative sandwich ELISA immunoassay (EKS-700B, Stressgen, USA). The intra- and inter-assay CV ranged between 4.5% and 7%. A heat shocked Hela cell lysate (Stressgen, USA) was included as positive control. Blood serums were extracted using centrifugation. The procedure was followed according to the instructions provided in the kit’s brochure. Primary measurement of ox-LDL was performed using a commercially available sandwich enzyme-linked immunosorbent assays (ELISA, Mercodia, Uppsala, Sweden). The intra- and inter-assay CV for the assay ranged between 4.5% and 7%. The detection limit was 0.03 U/l.
We should clarify that the manufacturer has not recommended EKS-700B for serum or plasma analysis. In order to check the validity of EKS-700B kit for our serum samples, we performed serial dilutions of our positive controls and demonstrated that HSP70 detection is within in the linear range for the amounts presented in our control group (range: 0.1–3 ng/ml). Also, the detection limit was ≤0.03 ng/ml, which was lower than the levels present in our samples.
Secondary measurements of HSP70 were performed using a quantitative sandwich ELISA immunoassay (EKS-715, Stressgen). The intra- and inter-assay CV ranged between 4.5% and 7%. Measurement of ox-LDL-β2GPI was performed using a commercially available sandwich enzyme-linked immunometric assays (ELISA, Cayman, USA). The intra- and inter-assay CV for the assay ranged between 6.4% and 3.4%.
Statistical analysis
The statistical package SPSS 17 for windows (Chicago, IL, USA), was used for analysis. Variables distributed normally are presented as mean and standard deviation (SD). Kolmogorov–Smirnov test was employed to test the normality of HSP70 distribution in each group. Circulating levels of stress proteins are typically log normally (not normally) distributed, so HSP 70 is presented as mean and interquintile range. Parametric and non parametric tests including independent sample t-test and Mann–Whitney U-test was employed for comparison of variables between males and females as appropriate. The Wilcoxon matched pairs test was used for paired comparisons of serum HSP70 level before and after treatment. Pearson's and Spearman's correlation tests were employed to test the association of HSP70 with ox-LDL and ox-LDL/LDL ratio within each group. For the last analysis, serum HSP70 was transformed into a logarithmic scale to change its distribution to normal. General linear model was employed to study the effect of gender on serum HSP70 level in the groups of the primary cohort as the impact of treatment in the patients with newly diagnosed diabetes.
Results
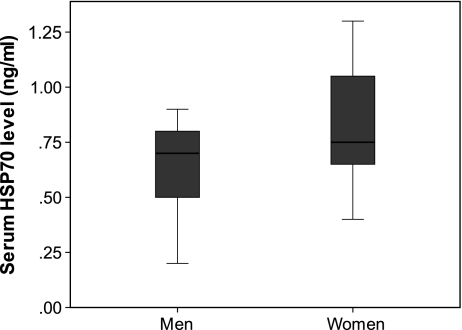
Primary analysis of the cohorts of patients and controls are presented in Table 1. None of the patients with newly diagnosed diabetes received satins or any type of antihypertensive therapy. Men and women with long-standing diabetes were similar regarding statin and antihypertensive therapy: ten men (55%) and nine women (47%) with long-standing diabetes were on antihypertensive therapy; and 12 men (66%) and 14 women (73%) were on statins. Women in the long-standing diabetes group had higher serum HSP70 levels than men (p < 0.05) (Fig. 1). This was significant (p = 0.005), after controlling for age, BMI, FBS and HbA1C, using a general linear model. Controls and patients with newly diagnosed diabetes did not show any gender difference in serum HSP70, ox-LDL levels and ox-LDL/LDL ratio, even after controlling for age, BMI, FBS and HbA1C (Table 1). There was not any significant correlation between HSP70 and ox-LDL in any of the studied cohorts except normal women (r = −0.42, p < 0.05).
Table 1.
Baseline characteristic of three cohorts of participants
| Normal controls | Newly diagnosed patients with diabetes | Patients with long-standing diabetes (diabetes duration ≥5 years) | ||||
|---|---|---|---|---|---|---|
| Male (n = 18) | Female (n = 18) | Male (n = 18) | Female (n = 18) | Male (n = 18) | Female (n = 19) | |
| Age (years) | 48.11 ± 8.44 | 47.72 ± 7.75 | 49.22 ± 10.11 | 49.11 ± 9.08 | 49.50 ± 9.85 | 49.52 ± 9.71 |
| BMI (%) | 26.58 ± 2.30 | 26.21 ± 3.92 | 26.03 ± 3.22 | 30.64 ± 4.30** | 28.04 ± 5.71 | 29.53 ± 4.80 |
| FBS (mg/dl) | 91.05 ± 6.27 | 90.16 ± 8.24 | 188.05 ± 46.89 | 186.38 ± 45.23 | 191.72 ± 92.03 | 224.15 ± 63.88 |
| HbA1c (%) | 8.07 ± 2.26 | 8.28 ± 1.73 | 7.81 ± 1.946 | 8.95 ± 2.00 | ||
| Triglyceride (mg/dl) | 161.22 ± 64.54 | 130.05 ± 49.84 | 243.83 ± 194.81 | 183.66 ± 56.82 | 197.56 ± 85.30 | 261.42 ± 158.60 |
| Cholesterol (mg/dl) | 195.94 ± 37.52 | 204.77 ± 51.50 | 202.50 ± 47.00 | 200.94 ± 29.83 | 190.77 ± 43.71 | 213.47 ± 48.73 |
| HDL (mg/dl) | 40.77 ± 12.03 | 50.77 ± 10.14* | 41.77 ± 6.44 | 43.33 ± 12.25 | 39.00 ± 10.58 | 45.73 ± 12.30 |
| LDL (mg/dl) | 108.16 ± 22.99 | 104.11 ± 27.83 | 118.44 ± 32.13 | 114.81 ± 18.58 | 98.00 ± 30.17 | 101.78 ± 24.66 |
| Systolic blood pressure (mmHg) | 119.44 ± 14.33 | 120.83 ± 8.44 | 129.11 ± 17.69 | 121.38 ± 14.63 | 125.27 ± 25.11 | 128.68 ± 19.0 |
| Diastolic blood pressure (mmHg) | 77.50 ± 6.91 | 80.00 ± 7.66 | 83.05 ± 11.38 | 76.94 ± 9.41 | 74.72 ± 11.17 | 81.84 ± 10.16 |
| HSP70 (ng/ml) | 0.26 [0.22–0.30] | 0.20 [0.18–0.33] | 0.46 [0.36–0.61] | 0.41 [0.36–0.62] | 0.65 [0.49–0.73] | 0.8* [0.74–1.03] |
| OX-LDL (U/L) | 40.0 [37.6–46.17] | 41.5 [38.2–47.6] | 45.00 [40.5–49.1] | 48 [40.7–49.6] | 84.00 [78.5–89.0] | 81.00 [74.9–85.6] |
| Ox-LDL/LDL | 0.39 [0.34–0.47] | 0.40 [0.37–0.49] | 0.40 [0.34–0.45] | 0.41 [0.35–0.48] | 0.86 [0.78–1.11] | 0.74 [0.70–0.85] |
Variables distributed normally are expressed as mean ± standard deviation, otherwise median [interquartile range]
*p < 0.05, **p < 0.01, when comparing men and women
Fig. 1.
Box plot demonstrating serum level of HSP-70 (ng/ml) in women vs. men in long-standing diabetes group
Serum HSP70 levels in patients with diabetes was higher than in controls (0.70 [0.59–0.81] vs. 0.23 [0.22–0.30] ng/ml; p < 0.001). Likewise, HSP70 levels were higher in patients with long-standing diabetes than newly diagnosed patients (0.80 [0.70–1.05] vs. 0.42 [0.41–0.64]; p < 0.001). Ox-LDL was significantly higher in patients with prolonged diabetes versus newly diagnosed patients (82.0 [78.4–85.8] vs. 48.0 [42.0–47.9]; p < 0.001). The ox-LDL/LDL ratio was significantly (p < 0.001) higher in patients with long-standing diabetes (0.77 [0.76–0.97] in comparison to newly diagnosed (0.40 [0.36–0.44]) patients and healthy (0.39 [0.37–0.46]) participants.
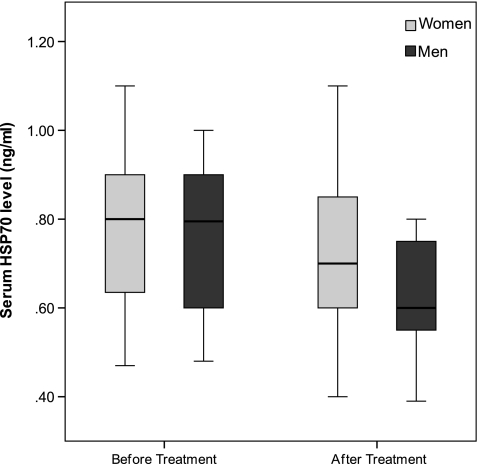
The characteristics of the cohort of patients with newly diagnosed diabetes before and after treatment are presented in Table 2. There was a significant decrease in serum HSP70 levels (Fig. 2), total cholesterol, and LDL in men before and after treatment, but treatment did not change any of the studied variables in women (Table 2). The observed change in serum HSP70 level in men remained significant (p < 0.001) after multiple adjustments for age, BMI, FBS and HbA1c, using general linear models. Serum ox-LDL and ox-LDL/LDL ratio decreased regardless of gender (Table 2) after metformin therapy in patients with newly diagnosed diabetes.
Table 2.
Characteristics of patients with newly diagnosed diabetes before and after treatment
| Males (n = 19) | Females (n = 25) | |||
|---|---|---|---|---|
| Age (years) | 56.26 ± 11.76 | 48.5 ± 9.84 | ||
| Waist (cm) | 95.66 ± 7.316 | 98.75 ± 11.52 | ||
| Hip (cm) | 103.40 ± 6.71 | 107.42 ± 10.33 | ||
| Systolic blood pressure (mmHg) | 133.29 ± 23.01 | 123.40 ± 19.68 | ||
| Diastolic blood pressure (mmHg) | 85.1 ± 9.31 | 76.55 ± 13.34 | ||
| Before treatment | After treatment | Before treatment | After treatment | |
| FBS (mg/dl) | 186.94 ± 63.26 | 140.27 ± 42.03 | 190.52 ± 48.51 | 148.80 ± 28.34 |
| Blood Sugar (mg/dl) | 274.07 ± 95.14 | 218.92 ± 63.15 | 261.84 ± 91.60 | 201.38 ± 64.73 |
| HbA1c (%) | 8.62 ± 2.68* | 7.38 ± 1.23 | 8.17 ± 1.91* | 7.31 ± 1.12 |
| Creatinine (mg/dl) | 1.02 ± 0.22 | 1.02 ± 0.24 | 0.78 ± 0.13 | 0.86 ± 0.18 |
| HDL-C (mg/dl) | 43.94 ± 11.38 | 44.75 ± 9.49 | 45.45 ± 12.01 | 46.38 ± 15.44 |
| Cholesterol(mg/dl) | 206.15 ± 52.21* | 166.41 ± 33.13 | 209.68 ± 32.71 | 197.78 ± 26.93 |
| LDL-C (mg/dl) | 126.23 ± 44.81* | 91.18 ± 27.70 | 122.03 ± 29.26 | 107.44 ± 27.35 |
| Triglyceride (mg/dl) | 195.63 ± 116.69 | 173.12 ± 131.64 | 207.71 ± 70.02 | 200.05 ± 104.40 |
| HSP70 (ng/ml) | 0.8* [0.68–0.87] | 0.60 [0.57–0.7] | 0.8 [0.66–0.89] | 0.72 [0.61–0.82] |
| Ox-LDL β2GPI (nm/l) | 9.5** [7.8–13.9] | 6.00 [4.8–7.96] | 10.5** [9.4–15.8] | 6.5 [5.8–8.38] |
| Ox-LDL/LDL | 0.088** [0.072–0.120] | 0.059 [0.048–0.072] | 0.072 [0.077–0.141]** | 0.071 [0.052–0.081] |
Variables distributed normally are expressed as mean ± standard deviation, otherwise median [interquartile range]
*p < 0.05, **p < 0.01, when comparing patients before and after treatment
Fig. 2.
Box plot demonstrating serum level of HSP-70 (ng/ml) in women vs. men before and after treatment in newly diagnosed patients
To study the effect of menopause, we stratified women into the pre- and postmenopausal state. There was not any significant difference in serum levels of HSP70, ox-LDL and ox-LDL/LDL ratio between pre- (n = 9) and postmenopausal (n = 10) women in the long-standing diabetes group. Also, there was no significant difference in serum levels of HSP70, ox-LDL and ox-LDL/LDL ratio between premenopausal (n = 15) and postmenopausal (n = 8) women in the group of patients with newly diagnosed diabetes, both before and after treatment. Similarly in the control group, there was no significant difference in serum levels of HSP70, ox-LDL and ox-LDL/LDL ratio between premenopausal (n = 10) and postmenopausal (n = 8) women.
Discussion
Gender has been repeatedly shown to affect physiological function and disease presentation in a variety of conditions. Studies have also noted substantially greater risk factors for cardiovascular disorders in women with type 2 diabetes (Gu et al. 1999; Donahoe et al. 2007; White et al. 2010). However, why women with diabetes tend to suffer more cardiovascular events compared to men is not yet known. Our findings clearly showed that serum HSP70 was decreased by glucose lowering therapy in men with newly diagnosed diabetes while it caused no significant decrease in women. We also demonstrated that serum HSP70 level was significantly higher in women with long duration of diabetes in comparison with men. Furthermore we showed that ox-LDL and ox-LDL levels corrected for LDL-cholesterol concentration, were significantly decreased by glucose lowering treatment in both sexes and did not differ according to gender. Although these findings do not allow us to determine whether HSP70 is a link in the chain of pathogenesis of higher mortality in women with type 2 diabetes, they do support the hypothesis that increased serum HSP70 level reflects more pronounced inflammation in women with type 2 diabetes.
Recent studies have shown the role of sexual dimorphism in the mechanisms regulating oxidant/antioxidant pathways (Kamper et al. 2009). Women have been shown to secrete higher levels of interlukin-6 and to have a greater response to stress (Jankord et al. 2007). It is shown that the consumption of a high-fat food is associated with an elevation of plasma IL-6 concentrations that is greater in women than in men (Payette et al. 2009). Data from healthy middle-aged postmenopausal women show that CRP and interleukin-6 predict cardiovascular disease risk in women and are of added value in the Framingham risk score for general cardiovascular disease (Albert and Ridker 2006). To date, we are unaware of any study demonstrating the impact of gender and diabetes on markers of oxidative stress such as serum HSP70 and ox-LDL level. Glucose-lowering therapy resulted in decreased ox-LDL levels in men and women with type 2 diabetes. It could be questioned why treatment was not effective in lowering HSP70 in women. HSP70 was higher in women with long duration of diabetes despite conventional treatments, when there was no difference between men and women in serum HSP70 levels in normal controls and newly diagnose diabetes before treatment.
There are at least two possible reasons that could explain our results. The first one is that serum HSP70 levels are independent predictors of oxidative stress from ox-LDL. We also found no significant correlation between serum ox-LDL and HSP70 concentration in any of our studied cohorts except normal women. This may be in line with the results of Svensson et al. (2006), who report that ox-LDL induces the release of HSP70 by human macrophage in vitro. The major functions of HSPs are protection against apoptotic stimulus and de novo folding of nascent polypeptides within the cell (Atalay et al. 2009; Mayer and Bukau 2005). On the other hand, it has been suggested that ox-LDL could be formed enzymatically within the circulation (Steinberg 1997). One would think that intracellular inflammation, which is reflected by serum HSP70 levels, is more severely pronounced in women with type 2 diabetes. Ox-LDL, as a marker of extracellular stress was similar in both men and women with type 2 diabetes.
Furthermore, it has been recently recognized that HSPs play a dual role in regulating immune responses (Atalay et al. 2009). While intracellular HSP70 induction in response to pro-inflammatory stimuli can exert anti-inflammatory effects, extracellular HSPs may signal danger, activating immune cells (Atalay et al. 2009). It is well known that the immune system is much more active in women (Grimaldi 2006; De Leon-Nava and Morales-Montor 2006), so oxidative stress may result in an exacerbation of a chronic inflammatory state. On the other hand there was no sexual dimorphism on serum ox-LDL levels, and thus may be concluded that the process is not immune modulated.
A second explanation of the results is that serum HSP70 levels are directly or indirectly affected by sexual hormones, where as ox-LDL production is not. Perez-Torres et al. (2009) reported that ovariectomy decreases the activity of superoxide-dismutase, catalase and endothelial nitric oxide synthase, which will be modulated after estrogen therapy. Whereas in normal women estrogen results in increased NO production and has protective effects; it has been reported that the diabetic state is associated with estrogen-stimulated production of superoxide and a reduced level of NO within the vasculature (White et al. 2010). Increase in superoxide production and a reduced level of NO are also reported in the postmenopausal state (White et al. 2010). Both thee mechanisms are associated with HSP70 induction (Gebhardt et al. 1999; Wang et al. 1996). Consistent with our presumptions, Molvarec et al. (2007b, 2010) demonstrated that serum HSP70 concentrations are decreased in normal human pregnancy, which is associated with down regulation of the immune system. We did not find any study addressing the impact of sex hormones on serum ox-LDL levels.
Whether our findings are due to differences in hormonal status or due to differences in the course of disease between women and men with type 2 diabetes has to be studied in the future. We suggest that diabetes induces an immune response and impair cellular defense mechanisms against oxidative stress in women with type 2 diabetes more than in men.
The principal limitation of the present study is that we used the EKS700 B kit to study the cohort of patients with long-standing diabetes and normal controls our. As previously stated, EKS700B Stressgen, which was used for measurements of primary analyses of cohorts, is not recommended for serum HSP70 analysis by the manufacturer. In order to use this kit, we checked the linearity of HSP70 detection within our sample range (0.1–3 ng/ml). Since this kit could linearly detect the differences between higher and lower values of HSP70 in our sample range, we assumed it to be appropriate for signifying the differences between our participants. There might be some disturbances in HSP70 detection for ranges other than our evaluated range and we confirm that this kit is not generally suitable for detection of serum HSP70 levels. However, the two kits gave results that were in agreement with each other in patients with newly diagnosed diabetes. In this study, we did not measure serum CRP so we could not report any possible correlation; however this is an interesting topic which can be covered in future studies. On the other hand, we took advantage of a relatively large sample size, precisely selected cohorts and the close similarity between the groups in most of the potentially confounding clinical and laboratory variables.
References
- Albert MA, Ridker PM. C-reactive protein as a risk predictor: do race/ethnicity and gender make a difference? Circulation. 2006;114(5):e67–e74. doi: 10.1161/CIRCULATIONAHA.106.613570. [DOI] [PubMed] [Google Scholar]
- Asea A. Hsp70: a chaperokine. Novartis Found Symp. 2008;291:173–179. doi: 10.1002/9780470754030.ch13. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci. 2009;10(1):85–95. doi: 10.2174/138920309787315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Leon-Nava MA, Morales-Montor J. Immune sexual dimorphism: can sex steroids affect the th1/th2 cytokine profile? Rev Invest Clin. 2006;58(2):161–169. [PubMed] [Google Scholar]
- Diagnosis and classification of diabetes mellitus Diabetes Care. 2009;32 Suppl 1(1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- Gebhardt BR, Ries J, Caspary WF, Boehles H, Stein J. Superoxide: a major factor for stress protein induction in reoxygenation injury in the intestinal cell line caco-2. Digestion. 1999;60(3):238–245. doi: 10.1159/000007664. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive b cells. Curr Opin Rheumatol. 2006;18(5):456–461. doi: 10.1097/01.bor.0000240354.37927.dd. [DOI] [PubMed] [Google Scholar]
- Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in us adults. JAMA. 1999;281(14):1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- Hageman J, Kampinga HH. Computational analysis of the human hsph/hspa/dnaj family and cloning of a human hsph/hspa/dnaj expression library. Cell Stress Chaperones. 2009;14(1):1–21. doi: 10.1007/s12192-008-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland HE, Leoni F, Altaie O, Birch CS, Coleman RC, Hunter-Lavin C, Williams JH. Measuring the secretion of heat shock proteins from cells. Methods. 2007;43(3):176–183. doi: 10.1016/j.ymeth.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, Keisler DH, Laughlin MH. Sex difference in link between interleukin-6 and stress. Endocrinology. 2007;148(8):3758–3764. doi: 10.1210/en.2006-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, Kamper M, Dalla C, Pitychoutis PM, Papadopoulou-Daifoti Z. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 2009;98(1–2):215–222. doi: 10.1016/j.physbeh.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragelund C, Kober L, Faber J, Steffensen R, Hildebrandt P. Metabolic syndrome and mortality in stable coronary heart disease: relation to gender. Int J Cardiol. 2007;121(1):62–67. doi: 10.1016/j.ijcard.2007.04.068. [DOI] [PubMed] [Google Scholar]
- Legato MJ, Gelzer A, Goland R, Ebner SA, Rajan S, Villagra V, Kosowski M. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3(2):131–158. doi: 10.1016/S1550-8579(06)80202-0. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. J Hum Hypertens. 2006;20(10):780–786. doi: 10.1038/sj.jhh.1002060. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Prohaszka Z, Nagy B, Kalabay L, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of increased serum heat shock protein 70 and c-reactive protein concentrations and decreased serum alpha(2)-hs glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol. 2007;73(2):172–179. doi: 10.1016/j.jri.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Rigo J, Jr, Nagy B, Walentin S, Szalay J, Fust G, Karadi I, Prohaszka Z. Serum heat shock protein 70 levels are decreased in normal human pregnancy. J Reprod Immunol. 2007;74(1–2):163–169. doi: 10.1016/j.jri.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Derzsy Z, Kocsis J, Boze T, Nagy B, Balogh K, Mako V, Cervenak L, Mezes M, Karadi I, Prohaszka Z, Rigo J., Jr Circulating anti-heat-shock-protein antibodies in normal pregnancy and preeclampsia. Cell Stress Chaperones. 2009;14(5):491–498. doi: 10.1007/s12192-009-0102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A, Rigo J, Jr, Lazar L, Balogh K, Mako V, Cervenak L, Mezes M, Prohaszka Z. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones. 2009;14(2):151–159. doi: 10.1007/s12192-008-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A, Tamasi L, Losonczy G, Madach K, Prohaszka Z, Rigo J., Jr Circulating heat shock protein 70 (hspa1a) in normal and pathological pregnancies. Cell Stress Chaperones. 2010;15(3):237–247. doi: 10.1007/s12192-009-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, Outeiro TF. Increased serum hsp70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010 doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2004;5(1):31–38. doi: 10.1023/B:BGEN.0000017684.15626.29. [DOI] [PubMed] [Google Scholar]
- Oglesbee MJ, Herdman AV, Passmore GG, Hoffman WH. Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kda heat shock protein. Clin Biochem. 2005;38(10):900–904. doi: 10.1016/j.clinbiochem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem. 2009;23:23. doi: 10.1016/j.clinbiochem.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Payette C, Blackburn P, Lamarche B, Tremblay A, Bergeron J, Lemieux I, Despres JP, Couillard C. Sex differences in postprandial plasma tumor necrosis factor-alpha, interleukin-6, and C-reactive protein concentrations. Metabolism. 2009;58(11):1593–1601. doi: 10.1016/j.metabol.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Perez-Torres I, Roque P, El Hafidi M, Diaz-Diaz E, Banos G. Association of renal damage and oxidative stress in a rat model of metabolic syndrome. Influence of gender. Free Radic Res. 2009;43(8):761–771. doi: 10.1080/10715760903045296. [DOI] [PubMed] [Google Scholar]
- Piarulli F, Lapolla A, Sartore G, Rossetti C, Bax G, Noale M, Minicuci N, Fiore C, Marchioro L, Manzato E, Fedele D. Autoantibodies against oxidized ldls and atherosclerosis in type 2 diabetes. Diab Care. 2005;28(3):653–657. doi: 10.2337/diacare.28.3.653. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20(9):1815–1820. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42(3):235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Renie G, Maingrette F, Li L. Diabetic vasculopathy and the lectin-like oxidized low-density lipoprotein receptor-1 (lox-1) Curr Diabetes Rev. 2007;3(2):103–110. doi: 10.2174/157339907780598225. [DOI] [PubMed] [Google Scholar]
- Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146(6):769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272(34):20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- Svensson PA, Asea A, Englund MC, Bausero MA, Jernas M, Wiklund O, Ohlsson BG, Carlsson LM, Carlsson B. Major role of hsp70 as a paracrine inducer of cytokine production in human oxidized LDL treated macrophages. Atherosclerosis. 2006;185(1):32–38. doi: 10.1016/j.atherosclerosis.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamasi L, Bohacs A, Tamasi V, Stenczer B, Prohaszka Z, Rigo J, Jr, Losonczy G, Molvarec A. Increased circulating heat shock protein 70 levels in pregnant asthmatics. Cell Stress Chaperones. 2009;15(3):295–300. doi: 10.1007/s12192-009-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiraiah A. Hyperglycemia, lipoprotein glycation, and vascular disease. Angiology. 2005;56(4):431–438. doi: 10.1177/000331970505600411. [DOI] [PubMed] [Google Scholar]
- Wang YR, Xiao XZ, Huang SN, Luo FJ, You JL, Luo H, Luo ZY. Heat shock pretreatment prevents hydrogen peroxide injury of pulmonary endothelial cells and macrophages in culture. Shock. 1996;6(2):134–141. doi: 10.1097/00024382-199608000-00009. [DOI] [PubMed] [Google Scholar]
- Wei W, Liu Q, Tan Y, Liu L, Li X, Cai L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin. 2009;33(5):370–377. doi: 10.3109/03630260903212175. [DOI] [PubMed] [Google Scholar]
- White RE, Gerrity R, Barman SA, Han G (2010) Estrogen and oxidative stress: a novel mechanism that may increase the risk for cardiovascular disease in women. Steroids Epub ahead of print [DOI] [PMC free article] [PubMed]
- Woodman RJ, Watts GF, Playford DA, Best JD, Chan DC. Oxidized LDL and small LDL particle size are independently predictive of a selective defect in microcirculatory endothelial function in type 2 diabetes. Diabetes Obes Metab. 2005;7(5):612–617. doi: 10.1111/j.1463-1326.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60(3):308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]