Abstract
Objective
Both resistin and vitamin D have been associated with the renin-angiotensin-aldosterone system (RAAS). We investigated the association between resistin and the RAAS, and resistin and vitamin D under controlled dietary sodium conditions.
Design
Retrospective cross-sectional study of subjects from the HyperPATH Consortium, who were maintained in high dietary sodium (HS) and low dietary sodium (LS) balance for one week each.
Patients
Caucasian subjects with hypertension (n=177).
Measurements
25-hydroxyvitamin D (25[OH]D) levels were used to assess vitamin D status. Plasma resistin and RAAS measures were evaluated on each dietary intervention.
Results
Resistin levels were significantly higher in LS, where RAAS activity was high, when compared to HS balance, where RAAS activity was suppressed (6.36 vs. 5.86 µg/L, p<0.0001); however, resistin concentrations were not associated with plasma renin activity or serum aldosterone on either diet. 25(OH)D levels were positively and independently associated with resistin in both dietary conditions (HS: β=0.400, p-trend=0.027; LS: β=0.540, p-trend=0.014).
Conclusions
Dietary sodium loading reduced resistin levels, possibly by suppressing the RAAS; however, circulating RAAS components were not related to resistin concentrations within each specific dietary sodium condition. 25(OH)D was positively associated with resistin and may be involved in resistin regulation through an unknown mechanism. Further studies to better understand resistin regulation in human hypertension are warranted.
KEY TERMS: resistin, renin, angiotensin, sodium, vitamin D
INTRODUCTION
Adipose tissue is an endocrine organ that produces peptides with paracrine and endocrine functions termed adipokines 1. In animal models, resistin is an adipokine whose initial importance was underscored by its association with obesity and insulin resistance 2, 3. Subsequent human studies, however, have failed to consistently support these links 4–9. One potential explanation for these conflicting observations may relate to the fact that resistin, while generated by adipocytes in mice, is produced by inflammatory cells within adipose tissue in humans 10–13. While the role of resistin in human obesity and insulin pathobiology remains controversial, other studies have associated resistin concentrations with blood pressure 13–16, heart failure 17, and activity of the renin-angiotensin-aldosterone system (RAAS) 18, 19.
Experiments in transgenic mice have associated increased activity of the local adipose tissue RAAS with higher resistin levels 18. In humans, one study described higher resistin levels in patients with primary hyperaldosteronism when compared to essential hypertensives and normotensives 19. To our knowledge, further studies focused on elucidating the specific role of the RAAS in human resistin regulation are lacking.
Separate lines of evidence support vitamin D as a negative regulator of the RAAS 20; vitamin D has been shown to lower renin expression in mice 21, 22, and is inversely associated with plasma renin activity (PRA) in humans 23–26. 25-hydroxyvitamin D (25[OH]D) has been positively associated with the adipokine adiponectin 27–29, but to our knowledge has never been reported in association with resistin.
We sought to evaluate the relationship between resistin and the RAAS by studying hypertensive humans undergoing dietary sodium manipulation. We further explored the association between 25(OH)D and resistin given both their prior connections to RAAS activity.
METHODS
Study Population
This cross-sectional analysis was performed on data gathered from subjects studied in the International Hypertensive Pathotype (HyperPATH) Consortium. The HyperPATH study has been an on-going, multi-site study, aimed at investigating the pathophysiologic and genotypic mechanisms involved in hypertension and cardiovascular diseases. Participants were studied at four collaborating centers: Brigham and Women’s Hospital (Boston, MA), University of Utah Medical Center (Salt Lake City, UT), Vanderbilt University Hospital (Nashville, TN), and Hôpital Européen Georges Pompidou (Paris, France).
Subjects with chronic kidney disease, coronary heart disease, heart failure, suggested or known causes of secondary hypertension, and active malignancy were not enrolled in the HyperPATH study. All enrolled subjects had a glomerular filtration rate of > 60 mL/min. Enrolled subjects were classified as having hypertension if they had an untreated seated diastolic blood pressure (DBP) > 100 mmHg, a DBP > 90 mmHg with one or more antihypertensive medications, measured as the average of three readings with standard manual sphygmomanometer, or the use of two or more antihypertensive medications. Study procedures included dietary sodium modulation to maintain high-sodium (HS) and low-sodium balance (LS) in sequence.
Following the original study, 25(OH)D measurements were performed on all the available frozen plasma of subjects with hypertension (n=345). Since race is known to influence 25(OH)D concentrations and RAAS activity 30, 31, the final study population for this analysis was restricted to Caucasian subjects with 25(OH)D measurements who were successfully maintained in sodium balance per study protocols (below) and with available frozen plasma in both HS and LS balance to measure resistin (n=177).
The HyperPATH Study Protocol
A particular strength of the HyperPATH study design was its rigorous study protocol, conducted in Clinical Research Centers, and designed to minimize notable confounders of the RAAS (dietary sodium intake, body posture, diurnal variation, and medications). To avoid interference with RAAS assessment, participants taking angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or mineralocorticoid receptor antagonists, were withdrawn from these medications three months before study initiation. Beta-blockers were withdrawn one month before study initiation. If needed for blood pressure control, subjects were treated with amlodipine and/or hydrochlorothiazide; however, these medications were stopped three weeks prior to laboratory evaluation.
Subjects were maintained in HS (≥200 mmol Na/24h) and then LS (≤10 mmol Na/24h), for 7 days each. Both study diets also included fixed quantities of potassium (80 mmol/day) and calcium (1000mg/day); vitamin D intake was not specifically controlled. After each diet phase, participants were admitted to the institutional Clinical Research Center and maintained in a supine position overnight. For each diet phase, baseline blood sampling was obtained in the morning, frozen without preservatives until assayed, and used to measure components of the RAAS and resistin. Baseline blood pressure was determined while supine between the hours of 8:00 AM and 10:00 AM, following 10 hours of overnight rest using the average of five readings from a Dinamap automated device (Critikon, Tampa, FL). External sodium balance and diet compliance were confirmed on admission to the Clinical Research Center with a 24-hr urine creatinine and sodium excretion of ≥ 150 mmol for HS, and ≤ 30 mmol for LS. Study protocols were approved by the Human Subjects Committees/Institutional Review Boards of each location, and informed written consent was obtained from each subject.
Biochemical Assessments
Plasma 25(OH)D levels were measured using the Diasorin assay (Stillwater, MN). Plasma renin activity (PRA) (Diasorin, Inc., Stillwater, MN), serum aldosterone (Siemens, Los Angeles, CA), plasma resistin (R&D Systems, Minneapolis, MN), and plasma glucose and insulin, were measured from the morning baseline blood sampling of both LS and HS phases. The intra-assay variation of the plasma resistin assay was 1.8–7.7% with an inter-assay variation of 3.4–9.3%, and a dynamic range of 0.21–50 µg/L. The homeostasis model assessment index (HOMA-IR) was calculated from plasma glucose and insulin values ([glucose] × [insulin] / 22.5), and used as a general representation of insulin resistance. All blood samples from the four participating study centers were processed and stored at a central laboratory (Brigham and Women’s Hospital, Boston, MA).
Statistical Methods
Analyses were performed to first evaluate the relationship between resistin, dietary sodium balance, and the RAAS, and subsequently the relationship between 25(OH)D and resistin.
Paired t-tests were used to compare data between HS and LS for normally distributed variables, which are presented as mean values with standard deviations (SD). Non-normally distributed variables (HOMA-IR, PRA, and aldosterone) are presented with median values and interquartile ranges and compared using the non-parametric Wilcoxon Ranks test. Vitamin D status was categorized based on clinically relevant definitions for 25(OH)D deficiency (<49.9 nmol/L, n=70), insufficiency (49.9–74.9 nmol/L, n=81), and sufficiency (≥74.9 nmol/L, n=26) 32.
Linear regression was employed to test the associations between resistin and demographic characteristics. Multivariable adjustment for age, gender, body-mass index (BMI), HOMA-IR, and systolic blood pressure (SBP) was applied when evaluating the relationship between resistin and 25(OH)D status. Linear regression results are reported with effect estimates (β), the 95% confidence intervals for β, and the corresponding p-value. HOMA-IR, PRA, and aldosterone were transformed to their natural logarithm when used in linear regression models. The level for significance for all tests conducted was set at α=0.05 and reported p-values are two-tailed. Data analyses were performed using SAS v9.1 (Cary, NC) and SPSS v15.0 (Chicago, Il) statistical software.
RESULTS
Characteristics of the Study Population
The study population had a mean age of 49.4 years (SD=7.8, range 25–71), a mean BMI of 27.9 kg/m2 (SD=3.5, range 19.8–37.3), and a mean 25(OH)D of 56.0 nmol/L (SD=21.5, range 15.7–148.9). As expected, HS balance was associated with a suppression of RAAS activity, an elevation of blood pressure, and a lower HOMA-IR when compared to LS balance 33 (Table 1). Furthermore, in LS balance where RAAS activity was high, plasma resistin concentrations were significantly higher when compared to HS balance (Table 1).
Table 1.
Study population characteristics in HS and LS balance.
| HS | LS | p | |
|---|---|---|---|
| n | 177 | - | |
| Age (y) | 49.4 (7.8) | - | |
| Gender (% female) | 43.5 | ||
| BMI (kg/m2) | 27.9 (3.7) | - | |
| 25(OH)D (nmol/L) | 56.0 (21.5) | - | |
| 24h Urine Sodium (mmol) | 231.1 (62.4) | 12.4 (7.6) | <0.0001 |
| Systolic Blood Pressure (mmHg) | 146.5 (20.8) | 131.4 (19.3) | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 86.7 (11.1) | 78.9 (10.5) | <0.0001 |
| PRA (µg/L/h) | 0.40 (0.20, 0.80) | 1.80 (0.90, 3.35) | <0.0001 |
| Aldosterone (nmol/L) | 0.108 (0.069, 0.188) | 0.387 (0.268, 0.591) | <0.0001 |
| Glucose (mmol/L) | 5.20 (1.26) | 5.50 (1.46) | 0.02 |
| Insulin (pmol/L) | 67.4 (49.2) | 76.0 (46.9) | 0.001 |
| HOMA-IR | 11.7 (8.2, 19.2) | 15.2 (9.6, 23.5) | <0.0001 |
| Plasma resistin (µg/L) | 5.86 (1.60) | 6.36 (1.85) | <0.0001 |
Results reported as mean (SD) for normally distributed variables and median (interquartile range) for non-normally distributed variables. (LS=low dietary sodium; HS=high dietary sodium; HOMA-IR=homeostatic model assessment; PRA=plasma renin activity; BMI=body-mass index; 25(OH)D=25-hydroxyvitamin D).
The Association between Components of the RAAS and Resistin
To further investigate the changes in resistin levels seen with manipulation of dietary sodium, we evaluated the relationship between resistin and individual components of the RAAS. Plasma resistin did not significantly associate with PRA or serum aldosterone concentrations under either dietary condition; although a non-significant positive trend between aldosterone and resistin was seen (Table 2). Additionally, consistent with on-going controversies in prior human studies 4–9, we observed no association between plasma resistin with BMI, insulin resistance, or blood pressure (Table 2).
Table 2.
The univariate association between plasma resistin and other study variables in HS and LS balance.
| Variable | HS | LS | ||
|---|---|---|---|---|
| β | p | β | p | |
| BMI (kg/m2) | 0.011 | 0.76 | 0.022 | 0.59 |
| 24h Urine Sodium (mmol) | 0.002 | 0.36 | −0.001 | 0.96 |
| SBP (mmHg) | −0.004 | 0.53 | −0.011 | 0.14 |
| DBP (mmHg) | −0.013 | 0.25 | −0.008 | 0.57 |
| PRA (µg/L/h) | −0.132 | 0.31 | 0.138 | 0.33 |
| Aldosterone (nmol/L) | 0.312 | 0.16 | 0.252 | 0.27 |
| Glucose (mmol/L) | −0.23 | 0.08 | −0.08 | 0.53 |
| Insulin (pmol/L) | 0.0001 | 0.74 | 0.001 | 0.72 |
| HOMA-IR | −0.226 | 0.21 | 0.003 | 0.99 |
(HS=high dietary sodium balance; LS=low dietary sodium balance; β=univariate effect estimate; BMI=body-mass index; SBP=systolic blood pressure; DBP=diastolic blood pressure; PRA=plasma renin activity; HOMA-IR=homeostatic model assessment).
The Association between 25(OH)D and Resistin
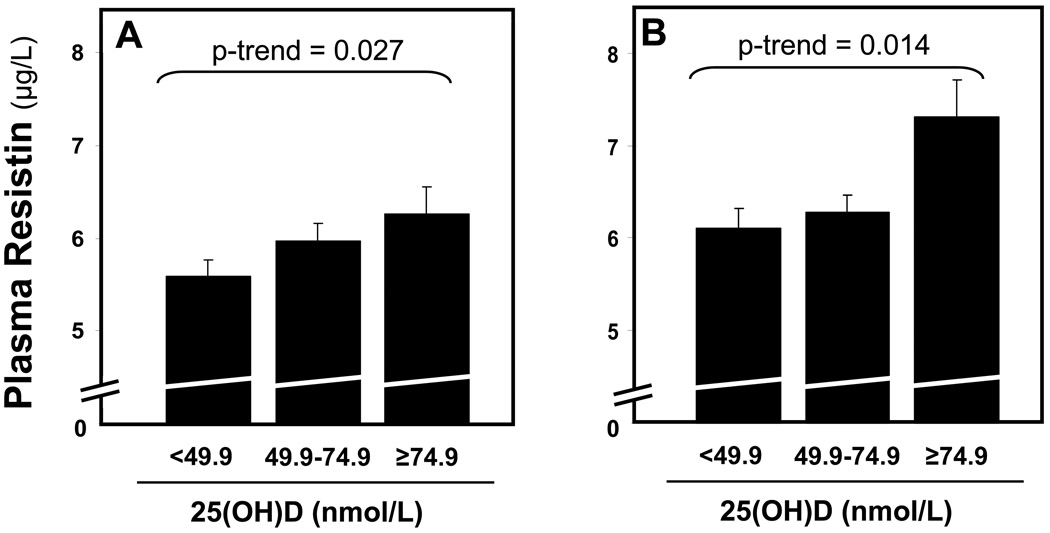
We explored the relationship between 25(OH)D and plasma resistin levels in both HS and LS balance. Increasing 25(OH)D status was associated with higher resistin concentrations in both HS (β=0.345, 95% C.I.=[0.005, 0.685], p-trend=0.047) and LS (β=0.502, 95% C.I.=[0.112, 0.892], p-trend=0.012) balance. Following multivariable adjustment for age, gender, BMI, HOMA-IR, and systolic blood pressure (SBP), 25(OH)D status remained independently associated with plasma resistin levels in both HS (β=0.400, 95% C.I.=[0.046, 0.754], p-trend=0.027) and LS (β=0.540, 95% C.I.=[0.112, 0.967], p-trend=0.014) balance (Figure 1).
Figure 1. The relationship between 25(OH)D and plasma resistin concentrations in high-dietary sodium balance (A) and low-dietary sodium balance (B).
Bars represent the mean resistin concentration in each 25(OH)D category while whiskers represent the standard error of means. P-values represent the test statistic for multivarvariate linear regression trends.
DISCUSSION
Resistin is an adipokine whose role in human physiology continues to be explored; in addition to its implications in obesity and metabolism, it has been associated with hypertension 13–16 and the RAAS 18, 19. We investigated the relationship between resistin, the RAAS, and vitamin D in Caucasians strictly phenotyped as hypertensive, studied under meticulous dietary conditions, and observed three notable findings. First, one week of dietary sodium loading resulted in suppression of RAAS activity and decreased resistin concentrations. However, within each specific dietary intervention, resistin levels were not associated with PRA or aldosterone; this suggests that either dietary sodium manipulation may affect resistin independently of circulating RAAS activity, or possibly that the variability in RAAS activity within each dietary intervention was too small to observe an association with resistin. Second, higher 25(OH)D levels were associated with higher resistin concentrations; this novel positive relationship parallels that seen previously between 25(OH)D and adiponectin 27–29 and raises new questions regarding the role of vitamin D in adipokine regulation in humans. Third, we observed no relationship between resistin and BMI or HOMA-IR, supporting several human studies that also failed to see these relationships 4–9.
Understanding a potential relationship between the RAAS and resistin may provide a mechanistic basis for associations between resistin and blood pressure. Like adiponectin, components of the RAAS, except for aldosterone, are produced by adipocytes 34. Activity of the RAAS is known to be inversely related to circulating adiponectin 18, 35; however, the effect of the RAAS on resistin is not well established. Kim et al. showed that increased activity of the adipose tissue RAAS resulted in higher resistin concentrations in transgenic mice 18. The only human study to analyze the association between the RAAS and resistin demonstrated that individuals with primary hyperaldosteronism had more circulating resistin than those with essential hypertension or normotension 19. Since patients with primary hyperaldosteronism have suppression of other RAAS components, the observation in transgenic mice that adipose-tissue specific RAAS activity (which is not known to include aldosterone) raised resistin levels is not necessarily consistent with human findings demonstrating a positive association between aldosterone and resistin. On the other hand, correlating murine findings to human physiology may not be straightforward since human resistin is produced by inflammatory cells within adipose tissue rather than adipocytes 10–13.
Our findings extend the work of these prior investigations; we observed significantly higher resistin levels during LS conditions, when RAAS activity was several fold higher than in HS balance. However, when each diet was analyzed separately, neither PRA nor aldosterone were associated with resistin. Within each dietary condition, RAAS neared the extremes of its spectrum; the inter-individual variability in PRA and aldosterone were much smaller than the variability when comparing LS to HS balance. Thus, we may have lacked sufficient power to observe significant associations between individual RAAS components and resistin in our effort to meticulously control dietary sodium. On the other hand, our results may implicate sodium balance, independent of the circulating RAAS, as a determinant of resistin concentrations. It is also possible that local adipose tissue RAAS activity (not directly evaluated in our study) is related to resistin regulation in humans, as previously seen in mice 18.
Vitamin D has been implicated as an inhibitor of the RAAS via antagonism of renin gene expression 20–26, and positively associated with adiponectin levels27–29; however, to our knowledge, the relationship between vitamin D and resistin has never been reported. Based on the inverse relationship between vitamin D and the RAAS 20, and the proposed positive relationship between the RAAS and resistin 18, 19, an inverse association between 25(OH)D and human resistin concentrations could be hypothesized. In contrast, we observed that higher 25(OH)D status independently predicted higher resistin levels. Though the lack of any other corroborative or ancillary data preclude us from making further conclusions, we speculate that the relationship between vitamin D and resistin in humans may not involve the RAAS, or may be more complicated than our current state of understanding. This may again be underscored by the fact that the majority of human resistin is not produced by adipocytes, as is the case with adiponectin 10–13.
Our results must be interpreted within the context of our study design. This analysis was cross-sectional, and thus cannot prove causality or directionality of associations. We studied a population of Caucasians with hypertension, thus the generalizability of our results to other races and blood pressure status remains uncertain. On the other hand, a major strength of our study was that subjects underwent a paired intervention design with meticulous control for race, sodium/RAAS status, and hypertension phenotype; all of these could otherwise confound measures of the RAAS and result in unreliable observations 30, 31. Our study design controlled dietary sodium and calcium intake, but because calcium and parathyroid hormone were not directly measured, we cannot comment on whether associations between 25(OH)D and resistin were independent of these factors. Vitamin D intake was not controlled in the HyperPATH study design, but we speculate that the vast majority of the study population was not taking vitamin D supplements given that the mean 25(OH)D level was only 56.0 nmol/L (22.4 ng/mL); thus, it is unlikely that many subjects had significant vitamin D intake during the one week of HS and LS diet phases. Though our study population had normal renal function on enrollment, the majority of them did not have assessments of their glomerular filtration rate under each dietary condition. Since renal function has been inversely associated with resistin36, 37, it is possible that the change in resistin we observed with dietary sodium manipulation could have been influenced by changes in glomerular filtration rate. Both resistin and the RAAS have been implicated in the inflammatory response 38–41; because we did not assess inflammatory markers in our study population, our study does not address the potential role inflammation may play in our observations. Our findings do not exclude a relationship between the adipose tissue RAAS and resistin; since we did not directly investigate adipose RAAS activity, we are unable to comment on the role it may play. Future studies focused on further characterizing these associations are required.
Resistin remains an interesting molecule; although its putative role in human obesity and insulin resistance is yet to be determined, it continues to be implicated in both hypertension and cardiovascular disease 13, 17. We observed increased resistin concentrations with sodium restriction and higher resistin levels with higher 25(OH)D status; whether these phenomena were related directly to dietary sodium manipulation, modulation of local or circulating RAAS activity, or other mechanisms, remains unresolved. Our findings raise new interest into the role dietary sodium, the adipose tissue RAAS, and/or vitamin D may play in human resistin physiology; future studies to investigate these relationships are warranted.
ACKNOWLEDGMENTS
We would like to thank the staff of the Clinical Research Center’s at our collaborating institutions, including the Brigham and Women’s Hospital, the Centre Investigation Clinique, Hôpital Européen Georges Pompidou, the University of Utah Medical Center, and Vanderbilt University Hospital. Funding support courtesy of National Institutes of Health grants F32 HL104776-01 (AV), F31 NR011108-01 (PCU), K08 HL079929 (JPF), KL2 RR025757 (LHP), SDG 0735609T (LHP), K23 HL08236-03 (JSW), U54LM008748 from the National Library of Medicine and UL1 RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources and M01-RR02635, Brigham & Women's Hospital, General Clinical Research Center, from the National Center for Research Resources, and the Specialized Center of Research (SCOR) in Molecular Genetics of Hypertension P50HL055000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Library of Medicine, the National Institutes of Health or the National Center for Research Resources.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES: The authors have nothing to disclose.
REFERENCES
- 1.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. Journal of Clinical Endocrinology and Metabolism. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 2.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 3.McTernan CL, McTernan PG, Harte AL, et al. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–47. doi: 10.1016/s0140-6736(02)07281-1. [DOI] [PubMed] [Google Scholar]
- 4.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochemical and Biophysical Research Communications. 2001;285:561–564. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Chan JL, Yiannakouris N, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. Journal of Clinical Endocrinology and Metabolism. 2003;88:4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 6.Heilbronn LK, Rood J, Janderova L, et al. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. Journal of Clinical Endocrinology and Metabolism. 2004;89:1844–1848. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- 7.Stumvoll M, Haring H. Resistin and adiponectin--of mice and men. Obesity Research. 2002;10:1197–1199. doi: 10.1038/oby.2002.162. [DOI] [PubMed] [Google Scholar]
- 8.Savage DB, Sewter CP, Klenk ES, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 9.Owecki M, Miczke A, Nikisch E, et al. Serum Resistin Concentrations are Higher in Human Obesity but Independent from Insulin Resistance. Experimental and Clinical Endocrinology & Diabetes. 2010 doi: 10.1055/s-0030-1263111. [DOI] [PubMed] [Google Scholar]
- 10.Fain JN, Cheema PS, Bahouth SW, et al. Resistin release by human adipose tissue explants in primary culture. Biochemical and Biophysical Research Communications. 2003;300:674–678. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang RZ, Huang Q, Xu A, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochemical and Biophysical Research Communications. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 12.Curat CA, Wegner V, Sengenes C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 13.Yiannikouris F, Gupte M, Putnam K, et al. Adipokines and blood pressure control. Current Opinion in Nephrology and Hypertension. 2010;19:195–200. doi: 10.1097/MNH.0b013e3283366cd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takata Y, Osawa H, Kurata M, et al. Hyperresistinemia is associated with coexistence of hypertension and type 2 diabetes. Hypertension. 2008;51:534–539. doi: 10.1161/HYPERTENSIONAHA.107.103077. [DOI] [PubMed] [Google Scholar]
- 15.Thomopoulos C, Daskalaki M, Papazachou O, et al. Association of resistin and adiponectin with different clinical blood pressure phenotypes. Journal of Human Hypertension. 2010 doi: 10.1038/jhh.2010.22. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Curhan GC, Forman JP. Plasma resistin levels associate with risk for hypertension among nondiabetic women. Journal of the American Society of Nephrology. 2010;21:1185–1191. doi: 10.1681/ASN.2009101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel DS, Vasan RS, D'Agostino RB, Sr, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. Journal of the American College of Cardiology. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Soltani-Bejnood M, Quignard-Boulange A, et al. The Adipose Renin-Angiotensin System Modulates Systemic Markers of Insulin Sensitivity and Activates Intrarenal Renin-Angiotensin System. Journal of Biomedicine and Biotechnology. 2006;5 doi: 10.1155/JBB/2006/27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iacobellis G, Petramala L, Cotesta D, et al. Adipokines and cardiometabolic profile in primary hyperaldosteronism. Journal of Clinical Endocrinology and Metabolism. 2010;95:2391–2398. doi: 10.1210/jc.2009-2204. [DOI] [PubMed] [Google Scholar]
- 20.Vaidya A, Forman JP. Vitamin D and Hypertension: Current Controversies and Future Directions. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.109.140160. epub October. [DOI] [PubMed] [Google Scholar]
- 21.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. Journal of Clinical Investigation. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YC. Vitamin D regulation of the renin-angiotensin system. Journal of Cellular Biochemistry. 2003;88:327–331. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 23.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Annals of Internal Medicine. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 24.Resnick LM, Nicholson JP, Laragh JH. Calcium metabolism in essential hypertension: relationship to altered renin system activity. Federation Proceedings. 1986;45:2739–2745. [PubMed] [Google Scholar]
- 25.Tomaschitz A, Pilz S, Ritz E, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Clinica Chemica Acta. 2010 doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Vaidya A, Forman JP, Williams JS. 25-hydroxyvitamin D is Associated with Plasma Renin Activity and the Pressor Response to Dietary Sodium Intake in Caucasians. Journal of the Renin-Angiotensin-Aldosterone System. 2010 doi: 10.1177/1470320310391922. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gannage-Yared MH, Chedid R, Khalife S, et al. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. European Journal of Endocrinology. 2009;160:965–971. doi: 10.1530/EJE-08-0952. [DOI] [PubMed] [Google Scholar]
- 28.Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, et al. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine. 2009;36:205–210. doi: 10.1007/s12020-009-9216-9. [DOI] [PubMed] [Google Scholar]
- 29.Vaidya A, Forman JP, McGowan L, et al. Vitamin D is Associated with Favorable Adiponectin and Renin-Angiotensin System Profiles in Obesity. Hypertension. 2010;56:e50–e166. (P376). [Google Scholar]
- 30.Price DA, Fisher ND. The renin-angiotensin system in blacks: active, passive, or what? Current Hypertension Reports. 2003;5:225–230. doi: 10.1007/s11906-003-0025-x. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. Journal of Clinical Endocrinology and Metabolism. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 33.Townsend RR, Kapoor S, McFadden CB. Salt intake and insulin sensitivity in healthy human volunteers. Clinical Science (London) 2007;113:141–148. doi: 10.1042/CS20060361. [DOI] [PubMed] [Google Scholar]
- 34.Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- 35.Lely AT, Krikken JA, Bakker SJ, et al. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. Journal of Clinical Endocrinology and Metabolism. 2007;92:1821–1826. doi: 10.1210/jc.2006-2092. [DOI] [PubMed] [Google Scholar]
- 36.Dimitriadis K, Tsioufis C, Selima M, et al. Independent association of circulating resistin with glomerular filtration rate in the early stages of essential hypertension. Journal of Human Hypertension. 2009;23:668–673. doi: 10.1038/jhh.2009.12. [DOI] [PubMed] [Google Scholar]
- 37.Kawamura R, Doi Y, Osawa H, et al. Circulating resistin is increased with decreasing renal function in a general Japanese population: the Hisayama Study. Nephrology Dialysis and Transplantation. 2010;25:3236–3240. doi: 10.1093/ndt/gfq155. [DOI] [PubMed] [Google Scholar]
- 38.Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin, an adipokine with potent proinflammatory properties. Journal of Immunology. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 39.Straburzynska-Lupa A, Nowak A, Pilaczynska-Szczesniak L, et al. Visfatin, resistin, hsCRP and insulin resistance in relation to abdominal obesity in women with rheumatoid arthritis. Clinical Experimental Rheumatology. 2010;28:19–24. [PubMed] [Google Scholar]
- 40.Fargnoli JL, Sun Q, Olenczuk D, et al. Resistin is associated with biomarkers of inflammation while total and high-molecular weight adiponectin are associated with biomarkers of inflammation, insulin resistance, and endothelial function. European Journal of Endocrinology. 2010;162:281–288. doi: 10.1530/EJE-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Androulakis ES, Tousoulis D, Papageorgiou N, et al. Essential hypertension: is there a role for inflammatory mechanisms? Cardiology Reviews. 2009;17:216–221. doi: 10.1097/CRD.0b013e3181b18e03. [DOI] [PubMed] [Google Scholar]