Abstract
BACKGROUND:
Therapeutic approaches in pediatric populations are based on adult data because there is a lack of appropriate data for children. Consequently, there are many controversies regarding the proper treatment of pediatric patients.
OBJECTIVE:
The present study was designed to evaluate patients with differentiated thyroid carcinoma diagnosed before 20 years of age and to determine the factors associated with the response to the initial therapy.
METHODS:
Sixty‐five patients, treated in two tertiary‐care referral centers in Rio de Janeiro between 1980 and 2005 were evaluated. Information about clinical presentation and the response to initial treatment was analyzed and patients had their risk stratified in Tumor‐Node‐ Metastasis; Age‐Metastasis‐Extracapsular‐Size; distant Metastasis‐Age‐Completeness of primary tumor resection‐local Invasion‐Size and American‐Thyroid‐Association classification
RESULTS:
Patients ages ranged from 4 to 20 years (median 14). The mean follow‐up was 12,6 years. Lymph node metastasis was found in 61.5% and indicated a poor response to initial therapy, with a significant impact on time for achieving disease free status (p = 0.014 for response to initial therapy and p<0,0001 for disease‐free status in follow‐up). Distant metastasis was a predictor of a poor response to initial therapy in these patients (p = 0.014). The risk stratification systems we analyzed were useful for high-risk patients because they had a high sensitivity and negative predictive value in determining the response to initial therapy.
CONCLUSIONS:
Metastases, both lymph nodal and distant, are important predictors of the persistence of disease after initial therapy in children and adolescents with differentiated thyroid cancer.
Keywords: Thyroid cancer, Response to therapy, Radioactive‐iodine, Staging system, Childhood
INTRODUCTION
Differentiated thyroid carcinoma (DTC) is rare in pediatric patients. The incidence of this subtype of carcinoma has been reported to range from 2.6% to 12.9%.1-6 Papillary thyroid carcinoma (PTC) is the most common subtype of DTC in pediatric patients as well as in adult patients followed by its follicular variant.1,6 Radiation exposure in infancy has been reported to be associated with the possible occurrence of PTC.7,8 Advanced tumor manifestations, such as large or extensive tumor, multicentricity, lymphadenopathy, and synchronous lung metastasis at diagnosis, are more frequent and important risk factors in pediatric patients.1 In addition, young children have a worse prognosis compared to young adult or adolescent patients.6,9-16
Treatment of DTC consists of thyroidectomy and an ablative dosage of iodine‐131.17 Radioactive iodine therapy, after surgery, leads to a significantly improved prognosis, especially in patients who are at high risk of recurrence or death by thyroid cancer.18-22 These evidences have been well documented in adult patients and, due to the lack of data; have been used as the basis for in younger patients' treatment. Unfortunately, the first dosage of I‐131 is not always sufficient to achieve complete ablation of thyroid remnants.23-25 Zimmerman et al have proved that despite the more aggressive clinical presentation, the overall survival rate in patients under 20 years old is better than in older. Thus, because of the clinical trade‐off between aggressive manifestation and better outcomes, controversy still remains concerning the appropriate treatment for pediatric DTC patients.5
Regarding thyroid remnant ablation, little is known about the prognostic significance of achieving good response to initial therapy with the first dosage of I‐131 in patients with differentiated thyroid cancer. Verburg et al have shown that adult patients with good response to initial therapy had a better prognosis than those with poor response: disease‐free survival was 87% versus 49% after 10 years; additionally, thyroid‐cancer related survival was 93% versus 78%.26
The aim of this study is to evaluate patients with DTC who were diagnosed under 20 years old and analyze the factors that may influence the success of initial therapy.
PATIENT AND METHODS
This was a retrospective study of a cohort of patients diagnosed with DTC under 20 years old. The data was gathered in the files of the Universidade Federal do Rio de Janeiro and at the. Instituto Nacional do Cancer. Between January of 1980 and December of 2005, 73 pediatric patients (≤ 20 years) with DTC were initially treated in both institutions. Among the 73 consecutive patients, 8 were submitted to partial thyroidectomy and, so excluded from the analysis. The remaining 65 patients (17 males and 48 females) underwent total thyroidectomy and radioactive iodine initial therapy with I‐131 within 2‐6 months after surgery. Therapeutic neck dissection was performed only if the patients had enlarged and suspicious lymphadenopathy or biopsy proven lymph node metastases. No prophylactic neck dissection was performed. Pre‐operative neck ultrasounds were not routinely performed in all patients at the time they were included in the study. The age ranged from 4 to 20 years old (median 14) and the mean follow‐up was 12,6 years (ranging from 5‐32 years). Patients with positive thyroglobulin antibodies, medullary thyroid carcinoma or anaplastic thyroid carcinoma were excluded. Serum thyroglobulin was quantified by immunometric assay (Immulite®) with analytical sensitivity of 0.2 ng/ml, functional sensitivity of 0.9 ng/ml and interassay variation up to 8.8%.
Evaluation of outcomes
Clinicopathological features, treatments, and outcomes, such as age, gender, histology, specific variant, tumor size, extrathyroidal extension, tumor stage, presence of pathological node metastases, extension of thyroid resection and node dissection, surgical complications, and data from radioactive iodine therapy were obtained. These factors were compared among patients, independently from their responses to initial therapy. The patients were stratified in four systems of risk: TNM (Tumor, Node, Metastasis), employed by the American Joint Committee on Cancer (AJCC)/ International Union Against Cancer (UICC) (27), which is widely used for all types of cancers, AMES (Age, Metastasis, Extracapsular tumor and Size) 28 MACIS (distant metastasis, age, completeness of primary tumor resection, local invasion, tumor size),developed by the Mayo Clinic 29 and the recently published ATA risk classification for DTC30. These systems were designed for adults and consider only two different populations, older than 45 years old and younger than 45 years old, which leaves children, adolescents and young adults in the same group.
This study was approved by the Ethical boards of both institutions involved. Informed consent was obtained from patients and/or their parents.
Statistical Analysis
The results are expressed as the median and the range of variation. Frequencies were compared with the Fisher's exact probability test. We calculated relative risks [RR; 95% confidence interval (CI)] to evaluate the effects of each factor on the response to initial therapy. The performance of the different staging systems was measured by their sensitivity, specificity, positive and negative predictive value and their confidence interval at 95% (CI) considering as “positive test” the lower stage of each classification and for “negative test” the combination of stages two and three for ATA and MACIS classification. Differences were considered statistically significant when p‐values were less than 0.05. Statistical analyses were performed with Stata 9.0 (STATA CORPORATION, 2005).
Follow‐up and definition of Good response to initial therapy
Patient follow‐up was performed every 6‐12 months after initial treatment with surgery and radioactive iodine therapy. The success of initial therapy was defined as a negative whole body scan associated with undetectable thyroglobulin levels in hypothyroid patients and negative thyroglobulin antibodies after 6 to 12 months and no evidence of structural disease in cross‐sectional images when performed. When the patient had a partial response at the beginning such as shrinkage in structural disease and decrease in thyroglobulin levels and still had iodine avid tumor they were retreated with I131 with at least 6 months interval between the treatments.
RESULTS
There were 45 patients (69.2%) with classic papillary thyroid cancer, 16 with follicular variant types (24.7%), 2 with follicular thyroid carcinoma (2.7%) and 2 with Hurthle cell carcinoma (2.7%). Extrathyroidal extension was found in 39.5%, metastatic lymphadenopathy in 61.5%, multicentricity in 26.2% and distant metastasis in 29.2%, all of them in the lungs.
Overall, permanent surgical complications such as hypoparathyroidism were observed in 32.4% of the patients. There was no patient with damage to laryngeal nerves, neither recurrent laryngeal nerve nor superior laryngeal lesions. All the patients underwent total thyroidectomy, 50% lateral neck dissection as their first surgery and at least one initial therapeutic dose of radioactive iodine (I‐131), which. The (mean activity of I‐131 administered was 132 mCi (488.4 MBq) ranging from 50 to 200 mCi. The post therapy scan showed iodine uptake outside the thyroid bed in 29.3% of the patients while 70.7% had uptake only in the thyroid bed. However, the post therapy scan uptake pattern was not statistically significant in predicting the success of the initial therapy in this specific population. (Table 1)
Table 1.
Initial presentation and good response to initial therapy with I‐131‐Therapy.
| N % | RR (95% CI )* | P‐value | ||
| Age | >10 | 56 | 1 | 0,328 |
| <10 | 9 | 0,38 (0,05‐2,5) | ||
| Gender | F | 48 | 1 | 0,188 |
| M | 16 | 0,4 (0,1‐1,5) | ||
| Size | >2cm | 57 | 1 | 0,372 |
| <2cm | 8 | 0,63(0,23‐1,73) | ||
| Lymph node metastasis | Y | 40 | 1 | 0,014 |
| N | 25 | 0,34 (0,14‐0,8) | ||
| Multicentricity | Y | 17 | 1 | 0,719 |
| N | 48 | 1,17 (0,48‐2,84) | ||
| Distant Metastasis | Y | 19 | 1 | 0,014 |
| N | 46 | 0,15 (0,02‐0,96) | ||
| Post therapy scan uptake | TB | 45 | 1 | 0,353 |
| OTB | 20 | 0,52 (0,17‐1,60) |
RR – risk relative; 95% CI – 95 confidence interval, Y‐ yes (present at diagnosis), N‐ No (absent at diagnosis; TB‐ Thyroid bed; OTB‐outside thyroid bed
Regarding the staging, 70.77% (46 patients) were TNM stage I and 29.23% stage II. The same proportion was found for the AMES staging system for low and high risk. For the MACIS staging system, 72.31% had less than 6 points, considered low risk, 10.77% had between 7 and 7.99 points, so called intermediate risk and 16.92% had above 8 points, high risk – there was no patient between 6 and 6.99 points. For the ATA classification, 21.54% were considered low risk, 52.31% intermediate, and 26.15% high risk.
The factors that negatively influenced the success of the initial therapy in this pediatric cohort were the presence of lymph node metastasis and distant metastasis. Neither age nor gender nor tumor size influenced in this outcome as shown in table 1.
All of the staging systems analyzed was able to predict a lower success of initial therapy among the high risk patients (high negative predictive value). The ATA classification was slightly superior to the others (table 2). However, all of the systems had a very low specificity and positive predictive value (table 3).
Table 2.
Staging systems and prediction of good response to initial therapy.
| I | II | III | IV | P‐value | ||
| TNM | NED | 16 | 1 | 0,014 | ||
| Persistent | 30 | 18 | ||||
| MACIS | NED | 16 | 0 | 1 | 3 | 0,019 |
| Persistent | 31 | 7 | 7 | |||
| AMES | NED | 16 | 1 | 0,014 | ||
| Persistent | 30 | 18 | ||||
| ATA | NED | 9 | 7 | 1 | 0,001 | |
| Persistent | 5 | 27 | 16 |
NED = no evidence of disease; Persistent = persistent disease; MACIS (I = <6 points; II = 6‐6.99; III‐ 7‐7.99 and IV‐ >8); AMES ( I‐ low risk; II‐high risk); ATA (I‐Low risk; II‐ Intermediate Risk and III‐ High risk)
Table 3.
Accuracy and the staging system on predicting good response to initial therapy.
| Measurements | TNM%(CI 95%) | MACIS%(CI 95%) | AMES% (CI 95%) | ATA% (CI 95%) |
| Sensitivity | 94.12 (71.31‐ 99.85) | 94.12 (71.31‐ 99.85) | 94.12 (71.31‐ 99.85) | 94.12 (71.31‐ 99.85) |
| Specificity | 37.5 (23.95‐ 52.65) | 35.42 (22.16‐ 50.54) | 37.5 (23.95‐ 52.65) | 33.33 (20.40‐48.41) |
| Positive predictive value | 34.78 (21.35‐ 50.25) | 34.04 (20.86‐ 49.31) | 34.78 (21.35‐50.25) | 33.33 (20.40‐ 48.41) |
| Negative predictive value | 94.74 (73.97‐ 99.87) | 94.44 (72.71‐ 99.86) | 94.74 (73.97‐ 99.87) | 94.12 (71.31‐ 99.85) |
95% CI – 95 confidence interval
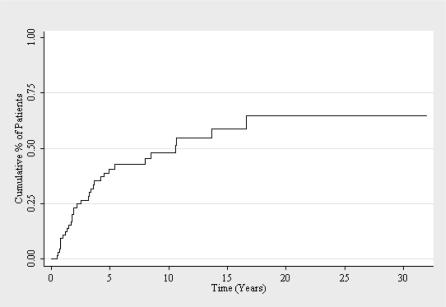
Patients classified as poor response to initial therapy underwent subsequent doses of radioactive iodine therapy.Total activity of radiopharmaceutical administered averaged 333 mCi (1232.1 MBq) ranging from 100 to 1150 mCi total activity. Transient leucopenia was observed in 2 patients, transient anemia in one with recovery 1 month after the last dose. After multiple doses of radioactive iodine, 50,8% achieved a disease free status at the end of the observation period (figure 1). Two patients had lymph node recurrence and two recurred in the thyroid bed and underwent further surgery. Only two patients developed new distant metastases, in both cases, lung metastases.
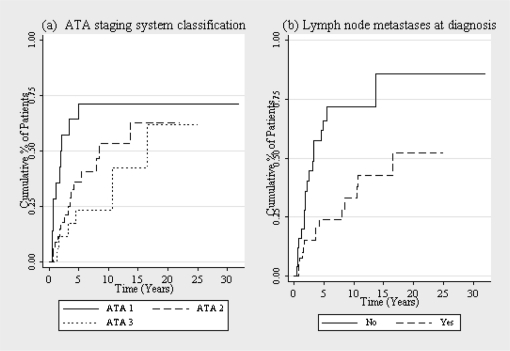
In this same analysis, the risk‐stratification systems failed to predict response to therapy, except for the ATA classification (p = 0.02) (figure 2). Between the patients that had no evidence of disease during some point in the follow up, patients that had lymph node metastases at the diagnosis took a longer time to be without evidence of disease (p<0.001) (figure 2). Sixty percent of the patients with distant metastases at diagnosis did not achieve the disease‐free status at the end of follow up, with the 40% that did, the analysis had no statistical significance. Only 6 patients had progressive disease and it was not possible to perform statistical analysis due to the small number. The overall survival was 100%.
DISCUSSION
Data regarding the management of DTC in pediatric patients is scarce in the literature. This present study had the aim to determine prognostic factors that may predict which children with DTC will become free of disease after initial therapy with radioactive iodine. The findings of the study demonstrated that a large number of children are not free of disease after initial therapy (even when considered “low risk” by conventional staging systems) and that clinical factor such as extent of initial disease presentation, especially lymph node metastasis, seemed to be important to predict outcomes after initial therapy in this population. In addition, most of the patients either are free of disease in some point in the follow up or they had persistent stable disease in this present study. The overall survival was very good as previously shown in other studies.
Pediatric series had already shown that thyroid cancer is the most common endocrine malignancy in children. The principal thyroid malignancies in children have the same histology as those afflicting adults, including papillary, follicular, and medullary thyroid cancer. However, children tend to present a more advanced disease, with a greater frequency of lymph‐node metastasis and distant metastasis at the time of diagnosis and high rates of recurrence during the first decade of life. In this study, there was a higher incidence in females (3 to 1) and it increases according to the age, with a high incidence of lymph node metastasis, (61.54%) and also distant metastasis (26.15%) all of them in the lung. The results from a large cohort of the SEER (Surveillance, Epidemiology and End Results) also confirm these data. In the study by Hogan et al., female patients outnumbered male patients by more than 4 to 1 and 95% of the patients were more than 10 years old. Distant metastases were found in 133 (7.6%) of the patients at the time of diagnosis and were most commonly found in the lung – rarely in the bone or liver. Regional lymph‐node metastases were found in 814 patients (46%), and disease confined to the thyroid gland was found in 741 (42%).31 The most common tumor histology, in the present study, was also papillary thyroid cancer followed by its follicular variant, in line with previous studies.31
The initial therapeutic approach for children and adolescents with DTC is still a controversial issue. Many authors32,33 advocate a routine of intensive approach because of the more advanced disease at diagnosis, propensity for recurrence, and greater radioiodine responsiveness in children. This intensive approach consists of total thyroidectomy followed by radioiodine administration. Although the prognostic significance of achieving good response to initial therapy with the first dosage of I‐131 in patients with differentiated thyroid cancer is still uncertain even in adults, Verburg et al, in a study with 180 adult patients with DTC with good response to initial therapy, leads to a better prognosis 26. The most important variables that influenced the good response to initial therapy in this present study were the absence of lymph node metastasis and absence of distant metastasis. Surprisingly, however, age at the time of initial therapy had no statistically significant influence on the outcome of the first dosage of I‐131 in our study population. Normally, age is considered to be one of the most important prognostic factors in thyroid cancer.30 The role of lymphadenopathy in low risk pediatric patients has been recently studied by Wada N et al by analyzing 57 consecutive TNM stage I patients (with 15 years or less) with DTC. The study showed that clinical lymphadenopathy (p = 0.006) was a risk factors for disease free survival in stage I pediatric patients.34
Regarding the staging systems analyzed, all of them were able to identify high risk with a good correlation with poor response to initial therapy, expressed as high negative predictive value. Nonetheless, none of the examined staging classifications was accurate to identify the low risk patients. Likewise other authors showed, the current used staging systems, especially TNM, was not accurate to determinate disease free survival in low risk patients.34 In fact, most of these systems were designed to predict mortality, which might explain slight superiority of the ATA classification (p = 0,014 for TNM vs p = 0,001for ATA) that was designed to predict recurrence and takes into account information about histology and response to initial treatment such as uptake outside the thyroid bed on the first post‐treatment whole‐body radioactive iodine scan and thyroglobulin levels.30
The role of radioactive iodine therapy in children and adolescents is still unclear. Chow et al,32 in a univariate analysis showed that adjuvant therapy with I‐131 decreased the recurrence rate from 42% to 6,3%, however they did not risk‐stratified the patients. On the other hand, the Mayo Clinic studies did not show a major impact in the overall survival and in the recurrence rates.5,35 In this study, after multiple treatments with radioactive iodine, the disease‐free survival status increased from 26.5% to 50.8% but as most of the patients showed uptake in the thyroid bed, this might be due to the ablation of normal thyroid tissue and there is not enough data to analyze its impact on the recurrence rates and overall survival.
In conclusion, the present study, which analyzed a Brazilian cohort of 65 patients diagnosed with DTC under 20 years of age, suggests that young patients have a more aggressive clinical presentation with more frequent lymph node and distant metastasis comparing to what is usually seen in adults. Those, in fact, seem to be the most important prognostic factors for the good response to initial therapy in these patients. Unfortunately, most of the risk stratification systems do not give enough emphasis to the presence of lymph node metastasis in this population which might decrease the ability to identify “real low‐risk patients'. Since DTC has a very long overall survival, this fact might leads to undertreatment with a less aggressive initial therapy. Certainly, further studies are needed to determinate the prognostic significance of a good response to initial therapy in pediatric population and to propose other ways of stratifying young patients based on disease free survival and recurrence rate instead of on cause specific death, which will more accurate identify low risk patients.
Kaplan‐Meier curve of disease‐free state in young papillary thyroid carcinoma patients after the first treatment.
Kaplan‐Meier curve of disease‐free state in young papillary thyroid carcinoma patients after the first treatment according to: (a) ATA staging system classification (1 = low risk; 2 = intermediate risk; 3 = high risk); (b) Lymph node metastases at diagnosis.
REFERENCES
- 1.Danese D, Gardini A, Farsetti A, Sciacchitano S, Andreoli M, Pontecorvi A. Thyroid carcinoma in children and adolescents. Eur J Pediatr. 1997;156:190–4. doi: 10.1007/s004310050580. 10.1007/s004310050580 [DOI] [PubMed] [Google Scholar]
- 2.Dottorini ME, Vignati A, Mazzucchelli L, Lomuscio G, Colombo L. Differentiated thyroid carcinoma in children and adolescents: a 37‐year experience in 85 patients. J Nucl Med. 1997;38:669–75. [PubMed] [Google Scholar]
- 3.Lee YM, Lo CY, Lam KY, Wan KY, Tam PK. Well‐differentiated thyroid carcinoma in Hong Kong Chinese patients under 21 years of age: a 35‐year experience. J Am Coll Surg. 2002;194:711–6. doi: 10.1016/s1072-7515(02)01139-0. 10.1016/S1072‐7515(02)01139‐0 [DOI] [PubMed] [Google Scholar]
- 4.Popovtzer A, Shpitzer T, Bahar G, Feinmesser R, Segal K. Thyroid cancer in children: management and outcome experience of a referral center. Otolaryngol Head Neck Surg. 2006;135:581–4. doi: 10.1016/j.otohns.2006.04.004. 10.1016/j.otohns.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman D, Hay ID, Gough IR, Goellner JR, Ryan JJ, Grant CS, McConahey WM. Papillary thyroid carcinoma in children and adults: long‐term follow‐up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988;104:1157–66. [PubMed] [Google Scholar]
- 6.Bal CS, Padhy AK, Kumar A. Clinical features of differentiated thyroid carcinoma in children and adolescents from a sub‐Himalayan iodine‐deficient endemic zone. Nucl Med Commun. 2001;22:881–7. doi: 10.1097/00006231-200108000-00006. 10.1097/00006231‐200108000‐00006 [DOI] [PubMed] [Google Scholar]
- 7.Spinelli C, Bertocchini A, Antonelli A, Miccoli P. Surgical therapy of the thyroid papillary carcinoma in children: experience with 56 patients < or = 16 years old. J Pediatr Surg. 2004;39:1500–5. doi: 10.1016/j.jpedsurg.2004.06.016. 10.1016/j.jpedsurg.2004.06.016 [DOI] [PubMed] [Google Scholar]
- 8.Farahati J, Bucsky P, Parlowsky T, Mäder U, Reiners C. Characteristics of differentiated thyroid carcinoma in children and adolescents with respect to age, gender, and histology. Cancer. 1997;80:2156–2. doi: 10.1002/(sici)1097-0142(19971201)80:11<2156::aid-cncr16>3.0.co;2-y. 10.1002/(SICI)1097‐0142(19971201)80:11<2156::AID‐CNCR16>3.0.CO;2‐Y [DOI] [PubMed] [Google Scholar]
- 9.Borson‐Chazot F, Causeret S, Lifante JC, Augros M, Berger N, Peix JL. Predictive factors for recurrence from a series of 74 children and adolescents with differentiated thyroid cancer. World J Surg. 2004;28:1088–92. doi: 10.1007/s00268-004-7630-y. 10.1007/s00268‐004‐7630‐y [DOI] [PubMed] [Google Scholar]
- 10.Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Svec RL, Adair C, et al. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf) 1998;49:619–28. doi: 10.1046/j.1365-2265.1998.00584.x. 10.1046/j.1365‐2265.1998.00584.x [DOI] [PubMed] [Google Scholar]
- 11.Palmer BA, Zarroug AE, Poley RN, Kollars JP, Moir CR. Papillary thyroid carcinoma in children: risk factors and complications of disease recurrence. J Pediatr Surg. 2005;40:1284–8. doi: 10.1016/j.jpedsurg.2005.05.012. 10.1016/j.jpedsurg.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 12.Chaukar DA, Rangarajan V, Nair N, Dcruz AK, Nadkarni MS, Pai PS, et al. Pediatric thyroid cancer. J Surg Oncol. 2005;92:130–3. doi: 10.1002/jso.20339. 10.1002/jso.20339 [DOI] [PubMed] [Google Scholar]
- 13.Jarzab B, Handkiewicz Junak D, Włoch J, Kalemba B, Roskosz J, et al. Multivariate analysis of prognostic factors for differentiated thyroid carcinoma in children. Eur J Nucl Med. 2000;27:833–41. doi: 10.1007/s002590000271. 10.1007/s002590000271 [DOI] [PubMed] [Google Scholar]
- 14.Vassilopoulou‐Sellin R, Klein MJ, Smith TH, Samaan NA, Frankenthaler RA, Goepfert H, et al. Pulmonary metastases in children and young adults with differentiated thyroid cancer. Cancer. 1993;71:1348–52. doi: 10.1002/1097-0142(19930215)71:4<1348::aid-cncr2820710429>3.0.co;2-3. 10.1002/1097‐0142(19930215)71:4<1348::AID‐CNCR2820710429>3.0.CO;2‐3 [DOI] [PubMed] [Google Scholar]
- 15.Newman KD, Black T, Heller G, Azizkhan RG, Holcomb GW, 3rd, Sklar C, et al. Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children's Cancer Group. Ann Surg. 1998;227:533–41. doi: 10.1097/00000658-199804000-00014. 10.1097/00000658‐199804000‐00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massimino M, Gasparini M, Ballerini E, Del Bo R. Primary thyroid carcinoma in children: a retrospective study of 20 patients. Med Pediatr Oncol. 1995;24:13–7. doi: 10.1002/mpo.2950240104. 10.1002/mpo.2950240104 [DOI] [PubMed] [Google Scholar]
- 17.Schlumberger MJ. Papillary and follicular thyroid carcinoma. New Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. 10.1056/NEJM199801293380506 [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferri EL, Jhiang SM. Long‐term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. 10.1016/0002‐9343(94)90321‐2 [DOI] [PubMed] [Google Scholar]
- 19.Simpson WJ, McKinney SE, Carruthers JS, Gospodarowicz MK, Sutcliffe SB, Panzarella T. Papillary and follicular thyroid cancer. Prognostic factors in 1,578 patients. Am J Med. 1987;83:479–88. doi: 10.1016/0002-9343(87)90758-3. 10.1016/0002‐9343(87)90758‐3 [DOI] [PubMed] [Google Scholar]
- 20.DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 71:414–24. doi: 10.1210/jcem-71-2-414. 10.1210/jcem‐71‐2‐414 [DOI] [PubMed] [Google Scholar]
- 21.Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–20. doi: 10.1210/jcem.75.3.1517360. 10.1210/jc.75.3.714 [DOI] [PubMed] [Google Scholar]
- 22.Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. Clinical review 170: a systematic review and metaanalysis of the effectiveness of radioactive iodine remnant initial therapy for well‐differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89:3668–76. doi: 10.1210/jc.2003-031167. 10.1210/jc.2003‐031167 [DOI] [PubMed] [Google Scholar]
- 23.Doi SA, Woodhouse NJ. Initial therapy of the thyroid remnant and 131I dose in differentiated thyroid cancer. Clin Endocrinol (Oxf) 2000;52:765–73. 10.1046/j.1365‐2265.2000.01014.x [PubMed] [Google Scholar]
- 24.de Klerk JM, de Keizer B, Zelissen PM, Lips CM, Koppeschaar HP. Fixed dosage of 131I for remnant initial therapy in patients with differentiated thyroid carcinoma without pre‐ablative diagnostic 131I scintigraphy. Nucl Med Commun. 2000;21:529–32. doi: 10.1097/00006231-200006000-00005. 10.1097/00006231‐200006000‐00005 [DOI] [PubMed] [Google Scholar]
- 25.Cailleux AF, Baudin E, Travagli JP, Ricard M, Schlumberger M. Is diagnostic iodine‐131 scanning useful after total thyroid initial therapy for differentiated thyroid cancer? J Clin Endocrinol Metab. 2000;85:175–8. doi: 10.1210/jcem.85.1.6310. 10.1210/jc.85.1.175 [DOI] [PubMed] [Google Scholar]
- 26.Verburg F A, Keizer B, Lips CJM, Zelissen PMJ, de Klerk JMH. Prognostic significance of good response to initial therapy with radioiodine of differentiated thyroid cancer patients. Eur J Endocrinol. 2005;152:33–7. doi: 10.1530/eje.1.01819. 10.1530/eje.1.01819 [DOI] [PubMed] [Google Scholar]
- 27.International Union Against Cancer (UICC) New York: Wiley; 2009. “TNM classification of malignant tumors.”7th ed. [Google Scholar]
- 28.Cady B. Presidential address: beyond risk groups–a new look at differentiated thyroid cancer. Surgery. 1998;124:947–57. 10.1016/S0039‐6060(98)70034‐0 [PubMed] [Google Scholar]
- 29.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–7. [PubMed] [Google Scholar]
- 30.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 31.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156:167–72. doi: 10.1016/j.jss.2009.03.098. 10.1016/j.jss.2009.03.098 [DOI] [PubMed] [Google Scholar]
- 32.Chow SM, Law SC, Mendenhall WM, Au SK, Yau S, Mang O, et al. Differentiated thyroid carcinoma in childhood and adolescence‐clinical course and role of radioiodine. Ped Blood Cancer. 2004a;42:176–83. doi: 10.1002/pbc.10410. 10.1002/pbc.10410 [DOI] [PubMed] [Google Scholar]
- 33.Jarzab B, Handkiewicz‐Junak D, J Włoch J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: a qualitative review Endocrine‐Related Cancer. 2005;12:773–803. doi: 10.1677/erc.1.00880. [DOI] [PubMed] [Google Scholar]
- 34.Wada N, Sugino K, Mimura T, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, et al. Pediatric differentiated thyroid carcinoma in stage I: risk factor analysis for disease free survival BMC Cancer. 2009;9:306–8. doi: 10.1186/1471-2407-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay ID, Gonzalez‐Lousada T, Reunalda MS, Honetschalger JA, Richards ML, Thompson GB. Long‐Term Outcome in 215 Children and Adolescents with Papillary Thyroid cancer Treated During 1940 though 2008. World J Surg. 2010;34:1192–202. doi: 10.1007/s00268-009-0364-0. 10.1007/s00268‐009‐0364‐0 [DOI] [PubMed] [Google Scholar]