Abstract
Although cognitive neuroscience has made remarkable progress in understanding the involvement of the prefrontal cortex in human memory, the necessity of the orbitofrontal cortex for key competencies of working memory remains largely unexplored. We therefore studied human brain lesion patients to determine whether the orbitofrontal cortex is necessary for working memory function, administering subtests of the Wechsler memory scale, the Wechsler adult intelligence scale, and the n-back task to 3 participant groups: orbitofrontal lesions (n = 24), prefrontal lesions not involving orbitofrontal cortex (n = 40), and no brain lesions (n = 54). Orbitofrontal damage was reliably associated with deficits on neuropsychological tests involving the coordination of working memory maintenance, manipulation, and monitoring processes (n-back task) but not on pure tests of working memory maintenance (digit/spatial span forward) or manipulation (digit/spatial span backward and letter–number sequencing). Our findings elucidate a central component of the neural architecture of working memory, providing key neuropsychological evidence for the necessity of the orbitofrontal cortex in executive control functions underlying the joint maintenance, manipulation, and monitoring of information in working memory.
Keywords: lesion data, orbitofrontal cortex, prefrontal cortex, working memory
Introduction
Accumulating functional neuroimaging evidence implicates the orbitofrontal cortex in working memory, demonstrating activity in this region for the coordination of multiple working memory operations (for meta-analytic reviews, see Wager and Smith 2003; Wager et al. 2004; Owen et al. 2005). The orbitofrontal cortex appears to mediate higher order processing requirements for working memory, enabling the joint maintenance, manipulation, and/or monitoring of representations for goal-directed behavior (for recent reviews, see Ramnani and Owen 2004; Kringelbach 2005; Badre 2008; Botvinick 2008). Despite the consistently observed correlation between activity in orbitofrontal cortex and working memory task performance, there is sparse neuropsychological evidence to corroborate the importance of orbitofrontal cortex in working memory. Of the neurological patient studies that have investigated the role of the prefrontal cortex in working memory (e.g., Ptito et al. 1995; D'Esposito and Postle 1999; Muller et al. 2002; Baldo and Dronkers 2006; D'Esposito et al. 2006; Volle et al. 2008; Tsuchida and Fellows 2009), all share one or more of the following features: 1) diffuse (rather than focal) orbitofrontal cortex lesions, 2) lack of comparison subjects carefully matched for pre- and post-injury performance measures, and 3) exclusive use of verbal/auditory or nonverbal/spatial working memory tests. Thus, there has been no comprehensive evaluation of working memory function in a relatively large group of patients with damage specifically involving orbitofrontal cortex and across a broad range of tasks and stimulus material. The absence of such data represents a substantial gap in the understanding of both orbitofrontal cortex function and the neural substrates of working memory. Here, we characterize working memory function in a group of patients with orbitofrontal cortex lesions.
Materials and Methods
Participant Data
We drew participants from the Phase 3 Vietnam Head Injury Study registry, which includes American veterans who suffered brain damage from penetrating head injuries in the Vietnam War (n = 199), as well as neurologically healthy Vietnam veterans (n = 54). To preclude the possibility that impaired performance on working memory and executive function tests could be secondary to deficits in the production and/or comprehension of language, we excluded any participant who had significant impairment (defined as performance at least 2 standard deviations below the mean of the neurologically healthy group) on a test of language production and language comprehension (i.e., the Boston Naming test). From the remaining brain-injured veterans, we selected those with lesions involving significant damage to the orbitofrontal cortex, which comprises the ventral surface of the frontal cortex (Kringelbach 2005) (Fig. 1; n = 24). We additionally assessed a comparison group of brain-injured veterans whose damage was confined to the prefrontal cortex but did not involve orbitofrontal cortex (non-orbitofrontal cortex lesion group; n = 40), as well as neurologically healthy veterans (no lesion group; n = 54). Demographic and background cognitive function data for the 3 groups are presented in Table 1. No significant group differences were observed with respect to basic demographic variables (age, sex, and years of education), pre- and post-combat measures of cognitive function, post-combat measures of verbal IQ and verbal comprehension, and total percent volume loss (Table 1). All patient groups were therefore well matched with respect to 1) basic demographic variables, 2) pre- and post-combat measures of cognitive function, and 3) lesion size.
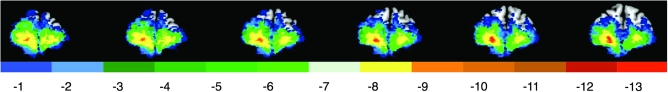
Figure 1.
Lesion overlap of the orbitofrontal prefrontal lesion group. Color indicates the number of veterans in the orbitofrontal group (n = 24) with damage to a given voxel. Maximal overlap occurred in the orbitofrontal cortex. In each slice, the right hemisphere is on the reader's left.
Table 1.
Demographic and background data
| Group | OFC (n = 24) | Non-OFC (n = 40) | No lesion (n = 54) | ANOVA F value | ANOVA P value | Significant between-group differences |
| Age | 58.79 (2.62) | 58.35 (2.73) | 59.52 (3.42) | 1.86 | 0.16 | None |
| Sex (% male) | 100 | 100 | 100 | n/a | n/a | None |
| Years of education | 14.12 (1.80) | 14.33 (2.71) | 15.19 (2.47) | 2.28 | 0.10 | None |
| Pre-combat AFQT | 52.04 (24.44) | 57.71 (27.84) | 65.40 (22.91) | 2.03 | 0.13 | None |
| Post-combat AFQT | 68.00 (20.97) | 65.03 (24.90) | 72.34 (22.99) | 0.82 | 0.44 | None |
| Post-combat verbal IQ | 104.21 (11.28) | 103.87 (14.39) | 109.87 (12.38) | 3.00 | 0.05 | None |
| Post-combat verbal comprehension | 104.75 (12.00) | 105.60 (15.22) | 109.66 (12.04) | 1.62 | 0.20 | None |
| Total percent volume loss (cm3) | 2.15 (0.86) | 2.29 (1.39) | 0.00 (00.00) | 93.95 | 0.00 | OFC > no lesion |
| Non-OFC > no lesion |
Note: OFC, orbitofrontal cortex; n/a, not applicable. Data are presented as means with standard deviations in parentheses. One-way analyses of variance (ANOVAs) were conducted for each neuropsychological test. Significant between-group differences were determined with the Tukey's honestly significant difference test, P < 0.05. “Age” refers to age at the time of Phase 3 evaluation. “Sex” refers to the percentage of male veterans. “Years of education” refers to the total number of years of education the veterans completed. “Pre-combat AFQT” refers to index scores on the Armed Forces Qualification test, a battery of tests measuring basic cognitive function at the time of enlistment (pre-injury). “Post-combat AFQT” refers to index scores on the AFQT administered at Walter Reed Medical Center after injury. “Post-combat verbal IQ” refers to the Phase 3 verbal IQ index score from the WAIS. “Post-combat verbal comprehension” refers to the Phase 3 verbal comprehension index score from the WAIS.
Lesion Analysis
We acquired computed tomography (CT) data during the Phase 3 testing period. Axial CT scans without contrast were acquired at the Bethesda Naval Hospital on a General Electric Medical Systems Light Speed Plus CT scanner in helical mode. We reconstructed the images with an in-plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm, and a 1-mm slice interval. We determined lesion location and volume from CT images using the Analysis of Brain Lesion software (Makale et al. 2002; Solomon et al. 2007) contained in MEDx v3.44 (Medical Numerics) with enhancements to support the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al. 2002). We defined the orbitofrontal cortex region of interest by applying anatomical landmarks and selecting patients with damage to the ventral surface of the frontal lobe. As part of this process, we spatially normalized the CT image of each subject's brain to a CT template brain image in Montreal Neurological Institute space (Collins et al. 1994). We determined the percentage of AAL structures that the lesion entailed by analyzing the overlap of the spatially normalized lesion image with the AAL atlas image. We calculated lesion volume by manually tracing the lesion in all relevant slices of the CT image and then summing the traced areas and multiplying by slice thickness. The tracing technique isolated areas of missing brain and regions affected by metallic artifacts and penetrating objects. A trained neuropsychiatrist carried out the manual tracing, which was then reviewed by an observer that was blind to the results of the neuropsychological testing.
Neuropsychological Tests
We administered subtests of the Wechsler memory scale, third edition (WMS III; Wechsler 1997a), the Wechsler adult intelligence scale, third edition (WAIS III; Wechsler 1997b), and an experimental test of working memory, the n-back task (Cohen et al. 1993, 1997), to investigate the necessity of the orbitofrontal cortex for specific 1) cognitive operations (maintenance, manipulation, and monitoring) and 2) modalities of information (verbal and spatial) in working memory. Working memory “maintenance” refers to the temporary online retention of information and is measured by simple retention tasks (e.g., the digit span forward task). “Manipulating” items in working memory refers to the rearrangement and transformation of representations for goal-directed behavior and is measured by active retention tasks (e.g., the letter–number sequencing task). The joint maintenance, manipulation, and monitoring of items in working memory is investigated by executive control tasks that draw upon multiple components of working memory (e.g., the n-back task). The reported neuropsychological data from the WMS III and WAIS III represent standardized scores based on the published norms in Wechsler (1997a, 1997b). Data for the n-back task represent the mean number of errors in each patient group.
Maintenance Tasks
We investigated the patient's ability to maintain information in working memory, administering a verbal/auditory maintenance measure, WAIS III: digit span forward task, and a nonverbal/spatial maintenance measure, WMS III: spatial span forward task. In digit span forward, the patient hears a sequence of digits and attempts to repeat the sequence in order (Wechsler 1997b). In spatial span forward, the patient watches the examiner tap a sequence of locations on a board and attempts to repeat the tapping sequence in order (Wechsler 1997a). Together, these tasks provide an assessment of the simple retention of verbal/auditory and nonverbal/spatial representations in working memory.
Manipulation Tasks
We investigated the patient's ability to manipulate items in working memory, employing 2 measures of the rearrangement of verbal/auditory information (WMS III: letter–number sequencing and WMS III: digit span backward) and a measure of the manipulation of nonverbal/spatial representations (WMS III: spatial span backward). In letter–number sequencing, the patient hears a sequence of alternating digits and letters and attempts to rearrange the order of each item by repeating the digits in numerical order followed by the letters in alphabetical order (Wechsler 1997a). Digit span backward (Wechsler 1997a) and spatial span backward (Wechsler 1997a) are the same as their forward counterparts, except that the subject attempts to repeat each sequence in reverse order. Together, these measures provide an assessment of the manipulation and rearrangement of verbal and spatial representations in working memory.
Joint Maintenance, Manipulation, and Monitoring Tasks
Accumulating neuroscience evidence implicates the orbitofrontal cortex in executive control processes underlying the coordination of multiple working memory operations (for meta-analytic reviews, see Wager and Smith 2003; Wager et al. 2004; Owen et al. 2005). We therefore investigated the patient's ability to jointly maintain, manipulate, and monitor information in working memory, administering the n-back task. In this task, the patient receives a sequence of visually presented letters and indicates whether each letter matches the stimulus that appeared n trials previously (1-, 2-, or 3-back; Cohen et al. 1993, 1997). The n-back task provides a continuous recognition measure of the patient's ability to 1) process and simultaneously monitor a series of stimuli, 2) maintain activation of recently processed and potentially relevant items, 3) discard recently processed but irrelevant information, and 4) make comparisons between various items in the series to identify a correct match. Thus, the n-back task examines the joint maintenance, manipulation, and monitoring of information in working memory (for experimental validation of the n-back task in working memory, see Kane et al. 2007).
Reasoning Tasks
To investigate whether the orbitofrontal cortex is necessary for performance on reasoning tasks that do not exclusively depend on working memory, we administered neuropsychological tests of mental arithmetic (WAIS III: arithmetic) and visuospatial reasoning (WAIS III: matrix reasoning). In arithmetic (Wechsler 1997a), the subject hears numerical problems in story format, performs mental arithmetic (i.e., without paper and pencil), and provides a verbal response. In matrix reasoning, the patient receives pictures of geometric shapes and draws an analogical inference about the missing shape that completes the pattern (Wechsler 1997b). The inclusion of verbal and spatial reasoning tasks complements our analysis of these operations in working memory, supporting an assessment of the contribution of the orbitofrontal cortex to cognitive operations for manipulating information in a broader range of contexts.
Statistical Analyses
For each neuropsychological test, we conducted a one-way analysis of variance examining the performance of orbitofrontal lesion patients (n = 24) relative to patients with prefrontal lesions not involving orbitofrontal cortex (n = 40) and neurologically healthy participants (n = 54). We then applied Tukey's honestly significant difference test to determine significant between-group differences.
Results
To summarize the results reported in Table 2, no significant group differences in the orbitofrontal patient sample were observed for neuropsychological tests of working memory maintenance (digit span forward and spatial span forward) or manipulation (letter–number sequencing, digit span backward, and spatial span backward) or for measures of mathematical (arithmetic) or spatial reasoning (matrix reasoning). However, deficits were found in the orbitofrontal patient group for tests of working memory requiring the coordination of maintenance, manipulation, and monitoring processes (total errors for 1-back, 2-back, and 3-back). This pattern of findings suggests that the orbitofrontal cortex may be critical for executive control functions underlying the joint maintenance, manipulation, and monitoring of information in working memory. To substantiate this conclusion, however, it is necessary to further examine the anatomical specificity of the observed results.
Table 2.
Neuropsychological tests of working memory and reasoning
| Group | OFC (n = 24) | Non-OFC (n = 40) | No lesion (n = 54) | ANOVA F value | ANOVA P value | Significant between-group differences |
| Digit span forward | 6.54 (1.06) | 6.21 (1.01) | 6.68 (1.22) | 2.04 | 0.13 | None |
| Spatial span forward | 9.86 (2.45) | 9.76 (2.97) | 10.22 (2.69) | 2.61 | 0.34 | None |
| 1-Back errors | 3.35 (2.66) | 3.06 (2.66) | 2.57 (2.48) | 0.76 | 0.47 | None |
| 2-Back errors | 5.06 (2.79) | 4.87 (2.31) | 3.85 (2.39) | 2.54 | 0.08 | None |
| 3-Back errors | 6.06 (2.19) | 5.48 (2.72) | 4.68 (2.40) | 2.39 | 0.09 | None |
| Total errors for 1-, 2-, and 3-Back | 4.82 (2.75) | 4.47 (2.74) | 3.70 (2.56) | 4.64 | 0.01 | OFC > no lesion |
| Letter–number sequencing | 9.83 (2.49) | 9.38 (2.61) | 11.04 (2.66) | 4.96 | 0.01 | Non-OFC < no lesion |
| Digit span backward | 4.79 (1.21) | 4.38 (1.18) | 4.96 (1.41) | 2.24 | 0.11 | None |
| Spatial span backward | 11.43 (2.54) | 11.13 (3.13) | 12.02 (3.12) | 1.00 | 0.36 | None |
| Arithmetic | 10.29 (2.62) | 9.65 (3.08) | 11.00 (2.25) | 2.76 | 0.06 | None |
| Matrix reasoning | 11.81 (2.80) | 10.92 (3.32) | 12.28 (2.94) | 2.20 | 0.11 | None |
Note: OFC, orbitofrontal cortex. Means are presented with standard deviations in parentheses. One-way analyses of variance (ANOVAs) were conducted for each neuropsychological test. Significant between-group differences were determined with Tukey's honestly significant difference test, P < 0.05.
Medial versus Lateral Orbitofrontal Cortex Lesions
As can be seen in Figure 1, the orbitofrontal patient group includes lesions involving medial and lateral aspects of the orbitofrontal cortex and, in approximately 5 or 6 cases, entails damage that extends beyond the orbitofrontal cortex. Thus, impaired performance in the orbitofrontal patient sample may reflect the contribution of medial and/or lateral prefrontal subregions or areas outside orbitofrontal cortex. To further isolate the specific orbitofrontal subregions underlying working memory function, we performed a supplementary analysis that specifically examined the relative importance of medial and lateral orbitofrontal subregions in working memory. We constructed 2 patient groups to examine this issue. First, we selected the subset of patients in the orbitofrontal sample whose lesions were highly focal, entailing selective damage to medial subregions that did not also encompass lateral orbitofrontal subregions (n = 6; Fig. 2). Second, we selected the subset of patients in the orbitofrontal sample with focal lesions primarily involving lateral orbitofrontal cortex (i.e., with minimal involvement of medial subregions; n = 12; Fig. 3). Demographic and background cognitive function data for each patient group are presented in Table 3. No significant group differences were observed with respect to basic demographic variables (age, sex, and years of education), pre- and post-combat measures of cognitive function, post-combat measures of verbal IQ and verbal comprehension, and lesion size (Table 3). In summary, the focal patient groups were well matched with respect to 1) basic demographic variables, 2) pre- and post-combat measures of cognitive function, and 3) lesion size.
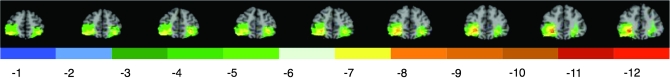
Figure 2.
Lesion overlap of the medial orbitofrontal lesion group. Color indicates the number of veterans in the focal medial orbitofrontal prefrontal group (n = 6) with damage to a given voxel. Maximal overlap occurred in the medial orbitofrontal cortex. In each slice, the right hemisphere is on the reader's left.
Figure 3.
Lesion overlap of the lateral orbitofrontal lesion group. Color indicates the number of veterans in the lateral orbitofrontal group (n = 12) with damage to a given voxel. Maximal overlap occurred in the lateral orbitofrontal cortex. In each slice, the right hemisphere is on the reader's left.
Table 3.
Demographic and background data
| Group | Medial OFC (n = 6) | Lateral OFC (n = 12) | No lesion (n = 54) | Kruskal–Wallis χ2 | Kruskal–Wallis P value | Significant between-group differences |
| Age | 57.83 (1.47) | 59.25 (3.31) | 59.52 (3.42) | 2.62 | 0.27 | None |
| Sex (% male) | 100 | 100 | 100 | n/a | n/a | None |
| Years of education | 14.00 (3.16) | 13.08 (2.99) | 15.19 (2.47) | 4.50 | 0.11 | None |
| Pre-combat AFQT | 56.50 (23.46) | 53.91 (27.86) | 65.40 (22.91) | 2.06 | 0.36 | None |
| Post-combat AFQT | 57.50 (24.96) | 74.78 (17.22) | 72.34 (22.99) | 2.45 | 0.29 | None |
| Post-combat verbal IQ | 104.17 (9.83) | 104.42 (12.48) | 109.87 (12.38) | 2.64 | 0.27 | None |
| Post-combat verbal comprehension | 103.83 (10.87) | 105.33 (13.92) | 109.66 (12.04) | 2.03 | 0.36 | None |
| Total percent volume loss (cm3) | 2.26 (0.73) | 2.26 (1.10) | 0.00 (00.00) | 71.15 | 0.00 | Medial OFC > no lesion |
| Lateral OFC > no lesion |
Note: OFC, orbitofrontal cortex; n/a, not applicable. Data are presented as means with standard deviations in parentheses. “Age” refers to age at the time of Phase 3 evaluation. “Sex” refers to the percentage of male veterans. “Years of education” refers to the total number of years of education the veterans completed. “Pre-combat AFQT” refers to index scores on the Armed Forces Qualification test, a battery of tests measuring basic cognitive function at the time of enlistment (pre-injury). “Post-combat AFQT” refers to index scores on the AFQT administered at Walter Reed Medical Center after injury. “Post-combat verbal IQ” refers to the Phase 3 verbal IQ index score from the WAIS. “Post-combat verbal comprehension” refers to the Phase 3 verbal comprehension index score from the WAIS. Nonparametric statistics were used to test for group effects and for the pairwise comparisons given the small number of participants in each sample. Significant between-group differences were determined with the Mann–Whitney U test, P < 0.05.
To determine the specific effect of medial versus lateral orbitofrontal damage on working memory, we compared the performance of the medial orbitofrontal patient group (n = 6) with the lateral orbitofrontal patient sample (n = 12) and neurologically healthy participants (n = 54). Because the assumptions underlying parametric statistics were not satisfied (Supplementary Table 1, Supplementary Figs 1–22), nonparametric statistics were applied to test for group effects and for pairwise comparisons. As Table 4 illustrates, no significant between-group differences were observed for tests of working memory maintenance (digit span forward and spatial span forward) or manipulation (letter–number sequencing, digit span backward, and spatial span backward) or for measures of mathematical (arithmetic) or spatial reasoning (matrix reasoning). However, neuropsychological tests involving the coordination of working memory maintenance, manipulation, and monitoring processes revealed significant deficits in the medial orbitofrontal patient group (total errors for 1-, 2-, and 3-back), relative to both the lateral orbitofrontal lesion sample and the neurologically healthy participant group, particularly at high levels of cognitive load (2-back and 3-back). Lateral orbitofrontal lesions were not associated with pervasive deficits on the administered working memory measures. This pattern of findings indicates that the medial orbitofrontal cortex is critical for executive control functions underlying the joint maintenance, manipulation, and monitoring of information in working memory.
Table 4.
Neuropsychological tests of working memory and reasoning
| Group | Medial OFC (n = 6) | Lateral OFC (n = 12) | No lesion (n = 54) | Kruskal–Wallis χ2 | Kruskal–Wallis P value | Mann–Whitney U | Mann–Whitney UP value | Significant between-group differences |
| Digit span forward | 6.83 (1.17) | 6.50 (0.91) | 6.68 (1.22) | 0.69 | 0.71 | n/a | n/a | None |
| Spatial span forward | 7.83 (2.99) | 10.90 (1.97) | 10.22 (2.70) | 4.06 | 0.13 | n/a | n/a | None |
| 1-Back errors | 3.67 (1.97) | 3.25 (2.38) | 2.57 (2.48) | 2.72 | 0.26 | n/a | n/a | None |
| 2-Back errors | 6.83 (1.94) | 4.75 (2.92) | 3.85 (2.39) | 9.46 | 0.00 | 40.50 | 0.00 | Medial OFC > no lesion |
| 3-Back errors | 8.00 (2.28) | 5.38 (1.30) | 4.68 (2.40) | 8.39 | 0.01 | 48.50 | 0.00 | Medial OFC > no lesion |
| 7.50 | 0.03 | Medial OFC > lateral OFC | ||||||
| Total errors for 1-, 2-, and 3-Back | 6.17 (2.71) | 4.46 (2.38) | 3.70 (2.56) | 13.94 | 0.00 | 710.00 | 0.00 | Medial OFC > no lesion |
| 128.00 | 0.02 | Medial OFC > lateral OFC | ||||||
| Letter–number sequencing | 9.17 (1.84) | 9.67 (2.31) | 11.04 (2.66) | 5.76 | 0.06 | n/a | n/a | None |
| Digit span backward | 5.00 (1.09) | 4.58 (0.79) | 4.96 (1.41) | 0.73 | 0.69 | n/a | n/a | None |
| Spatial span backward | 10.33 (1.37) | 11.60 (2.79) | 12.02 (3.12) | 1.34 | 0.51 | n/a | n/a | None |
| Arithmetic | 10.33 (2.73) | 10.50 (2.71) | 11.00 (2.25) | 0.62 | 0.73 | n/a | n/a | None |
| Matrix reasoning | 11.00 (2.97) | 11.90 (3.14) | 12.28 (2.94) | 1.07 | 0.59 | n/a | n/a | None |
Note: OFC, orbitofrontal cortex; n/a, not applicable. Means are presented with standard deviations in parentheses. Because the assumptions of parametric statistics were not satisfied (Supplementary Table 1, Supplementary Figs 1–22), nonparametric statistics were used to test for group effects and for the pairwise comparisons. Significant between-group differences were determined with the Mann–Whitney U test, P < 0.05.
Discussion
The aim of the current study was to investigate the necessity of the orbitofrontal cortex for key competencies of working memory. Using a relatively large sample of patients with orbitofrontal damage (n = 24) and a wide-ranging assessment of cognitive function, we report several main findings: 1) medial orbitofrontal lesions are associated with reliable deficits in executive control functions underlying the joint maintenance, manipulation, and monitoring of information in working memory, particularly at high levels of cognitive load; 2) medial orbitofrontal lesions are not associated with pervasive deficits for tests of working memory maintenance or manipulation; 3) medial orbitofrontal lesions are not associated with reliable deficits in tests of verbal or spatial reasoning; 4) lateral orbitofrontal lesions do not yield significant impairment in working memory function. Together, these results indicate that the neural architecture of the medial orbitofrontal cortex is computationally necessary for the coordination of working memory maintenance, manipulation, and monitoring processes.
Accumulating evidence indicates that the orbitofrontal cortex mediates executive control functions underlying the coordination of multiple working memory processes (for meta-analytic reviews, see Wager and Smith 2003; Wager et al. 2004; Owen et al. 2005). According to this framework, the orbitofrontal cortex is engaged when problems involve more than one discrete cognitive process, namely, when the application of one cognitive operation is not sufficient to solve the problem and the integration of 2 or more separate cognitive operations is required to fulfill the higher behavioral goal. The n-back task is an ideal example of such conditions, requiring the participant to simultaneously monitor a series of stimuli, maintain activation of recently processed and potentially relevant items, discard recently processed but irrelevant information, and make comparisons between various items in the series to identify a correct match. Multiple cognitive operations can be carried out successfully only if they are coordinated, and it is suggested that the coordination of information processing and information transfer between multiple operations is an important aspect of orbitofrontal cortex function.
Extensive functional neuroimaging data support this conclusion, demonstrating that the orbitofrontal cortex mediates higher order processing requirements for goal-directed behavior across several cognitive domains, including working memory (Wager and Smith 2003; Wager et al. 2004; Owen et al. 2005), planning and subgoal processing (e.g., Koechlin et al. 1999), relational integration (Christoff et al. 2001; Kroger et al. 2002; Patterson & Barbey, forthcoming), and social and emotional processing (e.g., Amodio and Frith 2006; Barbey et al. 2009a, 2009b, forthcoming; Krueger et al. 2009; Passingham et al. 2009; Barbey and Grafman forthcoming a, forthcoming b), and for the evaluation of reward-based contingencies in prediction and decision making (e.g., Bechara et al. 1999; Manes et al. 2002; Sanfey et al. 2003; Camille et al. 2004; Fellows and Farah 2005; Hsu et al. 2005). The observed functional profile of orbitofrontal cortex suggests that this region is critical for the coordination of multiple cognitive operations, particularly the monitoring and flexible updating of dynamic task-relevant information, thus providing a nexus for the integration of social, affective, and cognitive representations underlying executive control and goal-directed decision making. The shared role of orbitofrontal cortex in working memory and reward-related behavior further indicates that these processes are fundamentally linked, suggesting that working memory operations may have evolved from or co-opted neural mechanisms for the representation and processing of reward—increasing the likelihood that cognitive operations could be sustained over longer periods of time to support goal achievement and attainment of rewards.
Our neuropsychological patient data further complement the nonhuman primate (e.g., Jacobsen 1936; Malmo 1942; Butters and Pandya 1969; Butters et al. 1971, 1972; Goldman-Rakic 1987, 1995; Fuster 1997; Levy and Goldman-Rakic 1999, 2000) and functional neuroimaging literatures (e.g., Ptito et al. 1995; D'Esposito and Postle 1999; Muller et al. 2002; Baldo and Dronkers 2006; D'Esposito et al. 2006; Volle et al. 2008; Tsuchida and Fellows 2009), which have traditionally focused on dorsal and ventrolateral prefrontal contributions to working memory. The reported findings are consistent with the observed involvement of the dorsolateral prefrontal cortex in the manipulation of information in working memory (i.e., impairments in the letter–number sequencing task for the non-orbitofrontal lesion group are primarily attributable to dorsolateral prefrontal lesions involving Brodmann area 9; Barbey AK, Colom R, Krueger F, Grafman J, unpublished data; Barbey AK, Koenigs M, Grafman J, unpublished data). Our results, however, failed to corroborate neuroscience evidence implicating the ventrolateral prefrontal cortex in the maintenance of information in working memory, suggesting that this region may not be a necessary component of the neural systems mediating human working memory. Although this finding is consistent with functional neuroimaging evidence (i.e., the ventrolateral prefrontal cortex may be associated with but not necessary for working memory), divergence from the nonhuman primate literature may reflect significant macroscopic anatomical differences known to prevent mapping the precise localization of working memory function from monkeys to humans (e.g., Fuster 1997; Petrides 2000, 2005).
In conclusion, our findings elucidate a central component of the neural architecture of working memory, demonstrating that the medial orbitofrontal cortex is necessary for the coordination of working memory maintenance, manipulation, and monitoring processes. Although the computational bases of working memory operations remain to be fully characterized (i.e., uncovering algorithms for the active maintenance, manipulation, and monitoring of items in working memory), our findings provide key evidence for the neural architecture of these cognitive processes. The results of our neuropsychological patient study, together with emerging functional neuroimaging evidence, indicate that the medial orbitofrontal cortex is necessary for key competencies of working memory and demonstrate that this prefrontal subregion is a central component of the neural systems underlying the joint maintenance, manipulation, and monitoring of representations for goal-directed behavior.
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/. See also http://www.DecisionNeuroscienceLab.org/.
Funding
US National Institute of Neurological Disorders and Stroke Intramural Research Program; Project grant from the US Army Medical Research and Material Command administered by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase 3: a 30-year post-injury follow-up study, grant DAMD17-01-1-0675).
Acknowledgments
We are grateful to S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, V. Raymont, K. Reding, and G. Tasick for their invaluable help with the testing of participants and organization of this study. Conflict of Interest: None declared.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20:529–538. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Grafman J. The prefrontal cortex and goal-directed social behavior. In: Decety J, Cacioppo J, editors. The handbook of social neuroscience. New York, NY: Oxford University Press; Forthcoming a. [Google Scholar]
- Barbey AK, Grafman J. Hoboken, NJ: Wiley Interdiscip Rev Cogn Sci. Forthcoming b. An integrative cognitive neuroscience theory for social reasoning and moral judgment. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. An evolutionarily adaptive neural architecture for social reasoning. Trends Neurosci. 2009a;32:603–610. doi: 10.1016/j.tins.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. Structured event complexes in the prefrontal cortex support counterfactual representations for future planning. Philos Trans R Soc Lond B Biol Sci. 2009b;364:1291–1300. doi: 10.1098/rstb.2008.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. Structured event complexes and mental models for counterfactual inference. In: Bar M, editor. Predictions in the brain: using our past to prepare for the future. Oxford University Press; Forthcoming. [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM. Hierarchical models of behavior and prefrontal function. Trends in Cogn Sci. 2008;12:201–208. doi: 10.1016/j.tics.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters N, Pandya DN. Retention of delayed alternation effect of selective lesions of sulcus principalis. Science. 1969;165:1271–1273. doi: 10.1126/science.165.3899.1271. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya DN, Sanders K, Dye P. Behavior deficits in middle third of the sulcus principalis. J Comp Physiol Psychol. 1971;94:8–14. doi: 10.1037/h0031037. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya DN, Stein D, Rosen J. A search for the spatial engram within the frontal lobes of the monkeys. Acta Neurobiol Exp. 1972;32:305–329. [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapp. 1993;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- D'Esposito M, Cooney JW, Gazzaley A, Gibbs SEB, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and fMRI data. J Int Neuropsychol Soc. 2006;12:248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:89–101. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex. New York: Raven Press; 1997. [Google Scholar]
- Goldman-Rakic P. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology. Vol. 5. Bethesda, MD: American Psychological Society; 1987. pp. 373–417. [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Jacobsen CF. Studies of cerebral function in primates. Comp Psychol Monogr. 1936;13:1–68. [Google Scholar]
- Kane MJ, Conway ARA, Miura TK, Colflesh GJH. Working memory, attention control, and the n-back task: a question of construct validity. J Exp Psychol Learn Mem Cogn. 2007;33:615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci. 2009;13:103–109. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. J Neurosci. 1999;19:5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput. 2002;34:6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Malmo RB. Interference factors in delayed response in monkeys after removal of frontal lobes. J Neurophysiol. 1942;5:295–308. [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Muller NG, Machado L, Knight RT. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J Cogn Neurosci. 2002;14:673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Bengtsson SL, Lau HC. Medial frontal cortex: from self-generated action to reflection on one's own performance. Trends Cogn Sci. 2009;14:16–21. doi: 10.1016/j.tics.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R, Barbey AK. Causal simulation theory: An integrative cognitive neuroscience framework for causal reasoning. In: Grafman J, Krueger F, editors. The neural basis of belief systems. London, UK: Psychological Press; Forthcoming. [Google Scholar]
- Petrides M. Frontal lobes and memory. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. 2nd ed. Vol. 2. Amsterdam: Elsevier; 2000. pp. 67–84. [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptito A, Crane J, Leonard G, Amsel R, Caramanos Z. Visual-spatial localization by patients with frontal lobe lesions invading or sparing area 46. Neuroreport. 1995;6:45–48. doi: 10.1097/00001756-199509000-00018. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Hastie R, Colvin MK, Grafman J. Phineas gauged: decision-making and the human prefrontal cortex. Neuropsychologia. 2003;41:1218–1229. doi: 10.1016/s0028-3932(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions ABLe. Comput Methods Programs Biomed. 2007;86:245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Lesion evidence that two distinct regions within prefrontal cortex are critical for n-back performance in humans. J Cogn Neurosci. 2009;12:2263–2275. doi: 10.1162/jocn.2008.21172. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volle E, Kinkingnehun S, Pochon J, Mondon K, de Schotten MT, Seassau M, Duffau H, Samson Y, Dubois B, Levy R. The functional architecture of the left posterior and lateral prefrontal cortex in humans. Cereb Cortex. 2008;10:1093–1103. doi: 10.1093/cercor/bhn010. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence test administration and scoring manual. San Antonio (TX): The Psychological Corporation; 1997b. [Google Scholar]
- Wechsler D. Wechsler memory test administration and scoring manual. San Antonio (TX): The Psychological Corporation; 1997a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.