Abstract
Socioeconomic disadvantage experienced in early development predicts ill health in adulthood. However, the neurobiological pathways linking early disadvantage to adult health remain unclear. Lower parental education—a presumptive indicator of early socioeconomic disadvantage—predicts health-impairing adult behaviors, including tobacco and alcohol dependencies. These behaviors depend, in part, on the functionality of corticostriatal brain systems that 1) show developmental plasticity and early vulnerability, 2) process reward-related information, and 3) regulate impulsive decisions and actions. Hence, corticostriatal functionality in adulthood may covary directly with indicators of early socioeconomic disadvantage, particularly lower parental education. Here, we tested the covariation between parental education and corticostriatal activation and connectivity in 76 adults without confounding clinical syndromes. Corticostriatal activation and connectivity were assessed during the processing of stimuli signaling monetary gains (positive feedback [PF]) and losses (negative feedback). After accounting for participants’ own education and other explanatory factors, lower parental education predicted reduced activation in anterior cingulate and dorsomedial prefrontal cortices during PF, along with reduced connectivity between these cortices and orbitofrontal and striatal areas implicated in reward processing and impulse regulation. In speculation, adult alterations in corticostriatal functionality may represent facets of a neurobiological endophenotype linked to socioeconomic conditions of early development.
Keywords: anterior cingulate cortex, orbitofrontal cortex, reward processing, social health disparities, socioeconomic position, striatum
Introduction
Socioeconomic disadvantage experienced in childhood and adolescence confers risk for premature mortality and major medical conditions that develop in adulthood. As evidence, socioeconomic indicators of early disadvantage—which are commonly referenced to parental education, occupation, and income—are associated with higher all-cause mortality rates in adults (for reviews, see Power, Graham, et al. 2005; Turrell et al. 2007). Indicators of early socioeconomic disadvantage are also associated with morbidity and mortality rates that are specifically due to cardiovascular and cerebrovascular diseases (Lawlor et al. 2005; Galobardes et al. 2006) as well as chronic obstructive pulmonary disease and lung cancer (Power, Hypponen, et al. 2005). Further, indicators of early socioeconomic disadvantage are associated with the adult expression of biological risk factors and disadvantageous health behaviors that are implicated in the pathogenesis of the latter diseases. These risk factors include elevated levels of inflammatory proteins (Pollitt et al. 2007; Phillips et al. 2009), accelerated age-related declines in pulmonary function (Jackson et al. 2004), cigarette smoking, problematic alcohol use, and other behavioral habits that precipitate metabolic dysregulation, dyslipidemia, and obesity (Lynch et al. 1997; Poulton et al. 2002; Power, Graham, et al. 2005; Fergusson et al. 2007; Melchior et al. 2007; Batty et al. 2008). Moreover, cumulative epidemiological evidence indicates that the effects of early socioeconomic disadvantage on adult morbidity and mortality, as well as biological and behavioral risk factors for disease, persist even after accounting for adult socioeconomic position (please see Appendix for definition of socioeconomic position). In aggregate, this evidence has been taken to support lifecourse theories of social health disparities, which view disadvantaged socioeconomic circumstances of early development as playing a unique and formative role in shaping trajectories of risk for diseases that later emerge in adulthood—irrespective of the socioeconomic position that one attains in adulthood (Ben-Shlomo and Kuh 2002; Bradley and Corwyn 2002; Pollitt et al. 2005; Matthews and Gallo 2011). Nevertheless, the preclinical and neurobiological pathways by which early socioeconomic circumstances might relate to the later expression of adult health behaviors, and pathogenic processes remain unclear. There is broad speculation that these pathways involve early environmental, social, and familial influences on the developmental plasticity, assembly, and long-term functioning of brain circuitries that are important for 1) cognitive control functions and 2) self-regulatory behaviors that proximally affect peripheral physiology and health (Power and Hertzman 1997; Shonkoff and Phillips 2000; Evans 2004; Shonkoff et al. 2009; McEwen and Gianaros 2010; Taylor 2010).
Consistent with such speculation, there are electrophysiological and functional neuroimaging studies demonstrating that among children, indicators of parental socioeconomic disadvantage are associated with alterations in 1) baseline electroencephalographic activity suggestive of delayed prefrontal cortical development (Kishiyama et al. 2009); 2) event-related cortical potentials linked to impairments in cognitive control functions, such as selective attention (D'Angiulli et al. 2008); and 3) blood oxygen level–dependent (BOLD) activation patterns in cortical areas that may relate to alterations in a range of neurocognitive abilities that are themselves associated with early socioeconomic disadvantage, including delays in reading and language acquisition and higher order cognition (Noble et al. 2005, 2007; Noble, Farah, et al. 2006; Noble, Wolmetz, et al. 2006; Farah et al. 2008; Raizada et al. 2008; Hackman and Farah 2009; Raizada and Kishiyama 2010). In addition, indirect epidemiological evidence suggests that early socioeconomic disadvantage is associated with alterations in several cognitive functions in adulthood, particularly those that are presumably supported by prefrontal and networked cortical control systems (Kaplan et al. 2001; Singh-Manoux et al. 2005). For example, older British civil servants whose parents held a relatively lower socioeconomic position than others, as quantified by lower parental educational attainment and occupational grade, have been shown to perform more poorly on neuropsychological tests of visual and auditory memory, verbal fluency, and executive cognitive control (Singh-Manoux et al. 2005). Importantly, early socioeconomic disadvantage still accounted for adult neuropsychological performance in this cohort of civil servants after accounting for their adult socioeconomic position. Additional evidence from midlife community volunteers in the United States shows further that early socioeconomic disadvantage, as indexed by lower parental education, predicts impulsive decision making, again independent of adult socioeconomic position (Sweitzer et al. 2008). Yet, we are aware of no studies that have examined whether indicators of early socioeconomic disadvantage directly account for variation in the adult functionality of brain circuitries—specifically “corticostriatal brain circuitries”—that are thought to regulate the cognitive, decisional, and behavioral tendencies presumably conferring risk for ill health.
It is well established that corticostriatal circuitries are important not only for basic incentive motivation and instrumental learning functions but also for the higher order cognitive control over decisions, actions, and goal-directed behaviors that can promote or impair physical and mental health (Groenewegen et al. 1997; Masterman and Cummings 1997; Wilkinson 1997; Haber and Knutson 2010). More precisely, signaling patterns between networked corticostriatal brain systems are understood to reflect functions encompassing the encoding of salient and motivationally relevant environmental stimuli in the context of anticipated actions and learning histories to guide and behave according to decisions that are adaptive under the circumstances, while simultaneously suppressing actions that are maladaptive or disadvantageous (Haber 2003; Bechara 2005; Kable and Glimcher 2009). In this way, corticostriatal functionality is important for representing the relative value of rewards, along with punishing stimuli, to make decisions and flexibly regulate behavior (Haber et al. 1995; Rolls 2000; Schultz et al. 2000; Heimer and Van Hoesen 2006).
Anatomically, corticostriatal circuitries include networked areas of the dorsal, lateral, medial, parietal, and cingulate cortices, in addition to striatal regions of the basal ganglia (Ferry et al. 2000; Schultz et al. 2000; Middleton and Strick 2002; Haber 2003; Haber et al. 2006; Heimer and Van Hoesen 2006; Haber and Knutson 2010). Functionally, corticostriatal circuitries are modulated by ascending projections from midbrain dopaminergic cell groups (Jentsch et al. 2000; Haber and Knutson 2010). Within the basal ganglia, ventral and dorsal striatal targets of these ascending midbrain projections also receive input from cortical control regions and feed back onto cortical regions to form topographically organized circuits or “functional loops” that integrate aspects of cognitive, sensorimotor, and motivational information (Haber et al. 1995; Haber 2003). Importantly, a functional balance between activity in striatal regions and regulatory cortical inputs appear to be involved not only in registering the motivational salience of environmental stimuli but also abetting goal-directed decisions and actions as well as forming reward-dependent habits (Schultz et al. 2000; Breiter et al. 2001; Delgado et al. 2004; Voorn et al. 2004; Balleine et al. 2007; Delgado 2007). Thus, in the context of adult health and health-related behaviors, variation in corticostriatal functionality has long been implicated in vulnerability to conditions that have been linked to early socioeconomic disadvantage in the aforementioned epidemiological studies, namely, addictive behaviors and impulsive or disadvantageous decision making (Jentsch and Taylor 1999; London et al. 2000; Martin-Soelch et al. 2001; Volkow et al. 2004; Bechara 2005; Diekhof et al. 2008; Potenza 2008).
In extension, behavioral correlates of corticostriatal functionality have been increasingly understood to stem not only from heritable factors (e.g., genetic variation in dopaminergic neurotransmission, Yacubian et al. 2007; Dreher et al. 2009; Forbes et al. 2009) but also from environmental influences over neurodevelopment. Indeed, there is growing in vivo neuroimaging evidence that corticostriatal circuitries show marked developmental plasticity, likely reflecting maturational changes in cell proliferation, synaptogenesis, dendritic pruning and arborization, and myelination (Barnea-Goraly et al. 2005; Kelly et al. 2009; Ostby et al. 2009). Moreover, given the protracted course of prefrontal cortical development relative to reward-related striatal regions, there has been a recent empirical focus (e.g., May et al. 2004; Galvan et al. 2006; Van Leijenhorst, Moor, et al. 2010; Van Leijenhorst, Zanolie, et al. 2010) on the notion that childhood and adolescence correspond to periods of vulnerability in which experience-dependent imbalances or disturbances in the interplay between prefrontal and cortical or “top-down” regulatory inputs to downstream striatal regions may increase risk for impulsive decision making, maladaptive risk taking, and adopting health-impairing habits that will have long-term mental and physical health consequences over the life span (Chambers et al. 2003; Fareri et al. 2008; Ernst and Fudge 2009; Geier and Luna 2009; Marsh et al. 2009; Cohen et al. 2010).
Hence, in view of separate, but converging, lines of epidemiological and neurodevelopmental evidence, it is reasonable to question whether early socioeconomic factors are associated with alterations in adult corticostriatal functionality. To address this question, we tested whether lower parental education, a widely used indicator of early socioeconomic disadvantage, would be associated with alterations in adult corticostriatal activation to positive feedback (PF) and negative feedback (NF) stimuli signaling monetary gains and losses. We also tested whether parental education would be associated with alterations in the correlated activity (both functional and effective connectivity) between those corticostriatal regions that varied in activation as a function of parental education, focusing specifically on prefrontal and networked striatal regions. Next, we tested whether associations between parental education and corticostriatal activation or connectivity could be alternatively explained by multilevel indicators of adult socioeconomic position, adult demographic factors, or adult dispositional factors that may affect reward processing. And finally, we explored whether other indicators of parental socioeconomic position (occupation and perceived family economic status) could explain additional variation in measures of adult corticostriatal functionality after first accounting for parental education. If more advantaged socioeconomic circumstances of childhood and adolescence are associated broadly with early environmental, social, and familial influences on the maturation and long-term functioning of cortical systems important for 1) top-down control functions and 2) self-regulatory behaviors that proximally affect peripheral physiology and health in adulthood, then adults from more advantaged socioeconomic backgrounds could be expected to show greater cortical activation in prefrontal regions when processing PF stimuli signaling monetary rewards—particularly in association with markers of stronger functional and effective connectivity between networked corticostriatal brain systems.
Materials and Methods
Participants
Participants were 76 adults (aged 31–54 years, 41 women) recruited from a parent epidemiological study, the Adult Health and Behavior (AHAB) Project. AHAB is a community-based registry of 1379 adults from Southwestern Pennsylvania, United States, who completed assessment protocols for diverse behavioral and biological factors predictive of chronic mental and physical illnesses. A sample of AHAB participants also completed the neuroimaging protocol described below. To 1) maintain consistent between-family comparisons regarding parental education and 2) avoid the confounding effect of parental absence, the present sample of 76 participants were limited to those raised in intact families by 2 biological parents. All participants in this sample were further screened to exclude those with any current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis-I psychiatric disorder (including substance, alcohol, mood, or anxiety disorders) determined by structured clinical interview (First et al. 1996), any cardiovascular or cerebrovascular disease affecting cerebral blood flow or metabolism, any current chronic health condition, and any medication regimen that could confound study findings. Participants’ average IQ was 115.7 (standard deviation [SD]: 10.9; range: 83–139), as estimated by the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1997). Please refer to Supporting Information for further recruitment and screening methods and to Supplementary Table S1 for participants’ demographic and related health characteristics. All participants consented to study protocols, approved by The University of Pittsburgh Institutional Review Board.
Measurement of Parental and Participants’ Own (Adult) Education
Participants reported their biological mothers’ and fathers’ educational attainment, defined as the highest level of education attained by the time the participants were aged 18 years. Educational attainment for each parent was coded as: no high school diploma; high school diploma or some technical training; some college without a degree; associate’s degree; bachelor’s degree; master’s degree; or doctoral degree. Consistent with prior epidemiological studies, analyses of neuroimaging data were performed using the higher of the 2 parents’ education to capture the probable socioeconomic advantages afforded to a particular family (e.g., Karlamangla et al. 2005; Taylor et al. 2006). To further achieve an approximate distributional balance for the purpose of creating matched comparison groups, participants were divided into 1) those from households in which neither parent attained a postsecondary or higher college degree (i.e., no high school diploma, high school diploma, or some technical training) and 2) those from households in which at least one parent attained a postsecondary or higher degree (i.e., at least an associate’s or higher degree). Hereafter, these groups are nominally referred to as the lower (n = 43) and higher (n = 33) parental education groups (for group characteristics, see Supplementary Tables S1 and S2).
Participants’ own adult educational attainments were assessed and coded according to the same educational categories applied to their parents. By comparison, the 2 parental education groups did not differ in their own educational attainments according to the above coding scheme at a conventional statistical significance level, χ2 = 2.46, P = 0.12 by Fisher’s Exact Test. However, when adult education was recoded into binary form, as was done for parental education (e.g., holding less than a postsecondary degree vs. a postsecondary or higher degree), there was a trend percentage difference between the groups, such that those in the higher parental education group were more likely than the lower parental education group to have attained at least a postsecondary degree (28/33 vs. 29/43), χ2 = 2.98, P = 0.08 by Fisher’s Exact Test. Accordingly, an adult education variable coded according to having 1) less than a postsecondary degree and 2) having a postsecondary or higher degree was included a covariate (control variable) in all analyses.
Measurement of Other Socioeconomic, Demographic, Behavioral, and Dispositional Factors
We evaluated the influence of alternative explanatory factors (potential confounders) that might, in part, account for observed associations between parental education and corticostriatal activation and connectivity. These included participants’ annual household income, a composite indicator of community-level socioeconomic position, age, sex, self-reported frequency of alcohol use, depressive symptoms, and dispositional reward responsiveness (for summaries of these variables and parental education group comparisons, see below and Supplementary Table S1).
Participants’ current (adult) pretax household income in US dollars was coded as: <$10 K; $10–14 999 K; $15–24 999 K; $25–34 999 K; $35–49 999 K; 50–64 999 K; 65–79 999 K; and ≥$80 K. To compute an indicator of adjusted income, reported earnings were weighted by the number of occupants currently residing in the participants’ households. An indicator of community-level socioeconomic position was derived from US Census Bureau variables measured at the level of census tracts, which in this sample geographically subsumed blocks and groups of blocks comprising 71–2784 households. For all tracts, the following variables were extracted from the year 2000 U.S. census report (available at http://factfinder.census.gov/): 1) median household income (in 1999 U.S. dollars); 2) percentage of adults over 25 years of age holding a bachelor’s degree or higher; 3) proportion of households with incomes falling beneath the federally designated poverty line; and 4) percentage of households with a female head of household, with no spouse present, and one or more children under 18 years of age (i.e., single mother households, as a proportion of all households with 2 or more residents). Following our prior reports (Manuck et al. 2005; Petersen et al. 2006), census variables were standardized (by z-score transformation) and averaged (after inverse coding the latter 2 variables) to compute a community-level socioeconomic indicator variable. As a result, higher community-level socioeconomic indicator scores were taken to reflect socioeconomic advantage at the tract level. One participant did not provide a zip code and was assigned the mean z-score value of 0.
Frequency of alcohol use, a potentially confounding reward-related health behavior that could affect corticostriatal functionality, was computed as the average number of drinks consumed per week, as estimated over the 4 weeks prior to testing. Depressive symptoms, also possibly associated with alterations in positive affect or reward processing, were assessed using the Beck Depression Inventory (BDI; Beck et al. 1961). Self-reported dispositional reward responsiveness, which arguably could be associated with activation to PF stimuli signaling monetary gains in our paradigm, was assessed from the reward responsiveness subscale of the Behavioral Activation System–Behavioral Inhibition System inventory (Carver and White 1994). Prior to analysis, BDI and alcohol use values were natural log transformed.
For completeness of reporting and exploratory purposes, 2 other indicators of parental socioeconomic position were evaluated. First, the maximum occupational status attained by either parent was computed from the job-coding catalog of the Hollingshead Index of Social Position (Hollingshead and Redlich 1958). Second, an indicator of perceived family economic status was derived from a scale that asked participants whether they considered their families to have been poor (0), of average income (1), or well-to-do (2) when they were aged 13–17 years (for summaries of these indicators, see Supplementary Tables S2 and S3).
Measurement of Early Family Environments
Also for completeness of reporting and to characterize the potential parental socioeconomic correlates of participants’ early family environments (see Discussion), we conducted exploratory correlation analyses using scores from the subscales of the Family Environment Scale (FES) (Moos RH and Moos BS 1981), as modified by Plomin et al. (1988). On the FES, participants rated their early family environments for their degree of relationship quality (conflict and cohesion), organization, and cultural–intellectual orientation (see Supplementary Table S3). In addition, a composite indicator of parental and adult supervision between the ages of 10 and 17 was derived from a customized early environmental inventory (see Supplementary Table S3). Composite supervision scores showed significant associations in expected directions with FES subscale scores, but supervision scores did not differ between the 2 parental education groups. Nor did they correlate significantly with any other measure of parental socioeconomic position in this sample (Supplementary Table S3). Hence, it appears unlikely that findings reported below can be attributed to early neglect or lack of early adult supervision in this sample.
Corticostriatal Activation Protocol
To elicit corticostriatal activation during a single functional magnetic resonance imaging (fMRI) run of 342 s, participants completed a standardized protocol comprised of receiving PF and NF in the context of gaining or losing a monetary reward. In this blocked-design fMRI protocol, participants performed a variant of a card-guessing game developed by Delgado et al. (2000), which we have detailed previously (Hariri et al. 2006). For this guessing game, participants were pseudorandomly presented with trials that signaled winning (PF) or losing (NF) a monetary reward to be received at the end of the game. On each trial, participants had 3 s to guess (via button press) whether the value of a forthcoming visually presented playing card would be higher or lower than the value 5, with guesses made by index and middle finger button presses, respectively. After a guess, the value of the card was presented for 500 ms. The card value was then followed by PF (a green upward arrow for a “correct” guess, signaling reward) or NF (a red downward arrow for an “incorrect” guess, signaling loss) for an additional 500 ms. After receiving feedback, participants viewed a crosshair fixation for 3 s, yielding a total trial length of 7 s. In all, participants completed 9 blocks of 5 trials as follows: 3 blocks of predominantly PF (75% correct guesses), 3 blocks of predominantly NF (25% correct guesses), and 3 interleaved blocks of control (C) trials. For C trials, participants made alternating button presses during the presentation of an “X” (3 s), followed by an asterisks (500 ms) and a yellow circle (500 ms). As explained previously (Hariri et al. 2006), an incongruent trial was randomly presented within each feedback block (e.g., 1 of 4 trials during PF blocks was incorrect, resulting in NF) to prevent participants from anticipating specific feedback for each trial and to maintain engagement, motivation, stimulus saliency, and unpredictability—thus decreasing the likelihood of habitation. All feedback and C blocks were preceded by a 2 s instruction (“guess number” or “press button,” respectively), resulting in total block lengths of 38 s each. All participants were unaware of the fixed outcome probabilities associated with feedback blocks, and they were falsely led to believe that their performance would determine net monetary gains. Instead, all participants received US $10.
Neuroimaging Data Acquisition
Neuroimaging data were acquired on a 3-T Siemens MAGNETOM Allegra head-dedicated scanner. For spatial normalization of BOLD images, T2-weighted structural images were acquired prior to the corticostriatal activation protocol (time repetition [TR]/time echo [TE]: 6530/95 ms; flip angle 150°; 34 axial oblique slices; field of view [FOV] 200 mm; in-plane resolution 0.8 × 0.8 mm; slice thickness 3 mm [no gap]). BOLD images were then acquired during the protocol with a gradient-echo echo planar imaging (EPI) sequence that encompassed the entire cerebrum and majority of the cerebellum (TR/TE: 2000/25 ms; flip angle 70°; 34 axial oblique slices; FOV 200 mm; in-plane resolution 3.125 × 3.125 mm; slice thickness 3 mm [no gap]; 4 initial discarded volumes). BOLD image quality was enhanced through fast gradients (slew rate 400 T/m/s), which minimized echo spacing and reduced EPI geometric distortion. Before data acquisition, a reference BOLD image was obtained and inspected for artifacts (e.g., ghosting), ensuring signal quality across the acquisition volume, particularly in slices encompassing the orbitofrontal cortex (OFC) and striatum.
Neuroimaging Data Preprocessing
Neuroimaging data were preprocessed and analyzed with statistical parametric mapping software (SPM2; http://www.fil.ion.ucl.ac.uk/spm). For preprocessing, BOLD images were realigned to the first image of the series, coregistered to each participant’s T2-weighted structural image, normalized to the International Consortium for Brain Mapping 152 template (Montreal Neurological Institute [MNI]), and smoothed with an 8-mm Gaussian kernel.
Analysis of Parental Education and Corticostriatal Activation
After preprocessing, contrast images reflecting 4 types of feedback-related BOLD activation were estimated for each individual. These included: 1) PF > NF, 2) PF > (C), 3) NF > PF, and 4) NF > C. These contrasts permitted a determination of whether activation differences between the parental education groups generalized across types of feedback or were specific to activation patterns differentially elicited by monetary gains PF versus losses NF. For this purpose, PF, NF, and C blocks were modeled with rectangular waveforms that were convolved with the default SPM hemodynamic response function. Contrast images were then generated by general linear model (GLM) estimation. Prior to estimation, voxel-wise low-frequency BOLD signal drift was removed by high-pass filtering (128 s cutoff); serial BOLD signal autocorrelations were also corrected with a first-order autoregressive model.
To determine the covariation between parental education and corticostriatal BOLD activation at the group level, individual contrast images were submitted to second-level, mixed effects GLMs in SPM2. In the models, parental education was entered as an explanatory factor of interest, with participants’ own (adult) education entered as a covariate. The covariation between parental education and corticostriatal activation across individuals was then tested with control for multiple statistical testing in whole-brain analyses. To this end, we maintained a corrected false-positive detection rate of P < 0.05 for parental education analyses by employing a voxel-wise threshold of P < 0.001, which was combined with a cluster (k) extent threshold that was determined individually by the spatial correlations between BOLD signal changes in neighboring voxels for each of the following mixed-effects parametric maps: 1) k = 77 contiguous voxels for PF > NF, 2) k = 85 for PF > C, 3) k = 77 for NF > PF, and 4) k = 89 for NF > C. The number of contiguous voxels needed to maintain these corrected false-positive detection rates was empirically determined by the spatial smoothness (in full-width at half-maximum [FWHM]) of each contrast map and 2000 iterative Monte Carlo simulations implemented in the software program AlphaSim (Forman et al. 1995; Ward 2000).
Analysis of Parental Education and Functional Connectivity
In an orthogonal and separate set of functional connectivity analyses, we tested whether parental education covaried with temporally correlated BOLD signal changes between the only 4 brain areas that showed significant reward-related (PF > NF and PF > C) activation differences between the parental education groups, as revealed by the BOLD activation analyses detailed above (see Results and Fig. 1). For these connectivity analyses, we first used MarsBaR to extract a BOLD signal time series from the following areas for each participant: 1) Brodmann area (BA) 32 of the perigenual anterior cingulate cortex (pACC, x, y, z coordinates: −2, 40, −2), 2) BA45 of the lateral prefrontal cortex (LPFC, −42, 18, 20), 3) BA10 of the dorsomedial prefrontal cortex (dMPFC, 0, 60, 26), and 4) BA39 of the inferior parietal cortex (IPC, −44, −60, 32). Specifically, a BOLD signal time series was extracted and computed as the first principal component of the aggregate BOLD signal from all voxels within in the cluster corresponding to the above areas that met corrected statistical thresholds in mixed-effects analyses. Extracted time series were then mean centered, drift corrected, and computationally inspected for outliers. To this end, any values >3 SD of the mean of the time series were replaced by averaging 2 surrounding values. These corrected time series were then entered as regressors in individual GLM design matrices in SPM2. In the design matrices, BOLD signal variance attributable to task conditions were partitioned by entering a regressor corresponding to the feedback and C blocks, which was convolved with the SPM default hemodynamic response function. Hence, connectivity values generated from this approach are taken to reflect so-called intrinsic or task-independent functional coupling between brain regions (see below). Furthermore, given that functional connectivity analyses are prone to confounding by cardiac, respiratory, and other nonspecific sources of noise, we extracted a cerebrospinal fluid time series from the fourth ventricle (x, y, z coordinates: 0, −43, −26), and we included this series as a nuisance regressor in all individual GLM matrices (Van Dijk et al. 2010). Also included as nuisance regressors were the 6-directional movement parameters defined by spatial realignment in image preprocessing. Finally, for all individual-level connectivity analyses, voxel-wise low-frequency BOLD signal drift was high-pass filtered (128 s cutoff), and BOLD signal autocorrelations were corrected with a first-order autoregressive model. For each individual, this procedure yielded a functional connectivity map identifying areas where BOLD signal changes cross-correlated with signal changes in the 4 cortical seeds described above, net the contributions of experimental condition (feedback) variation.
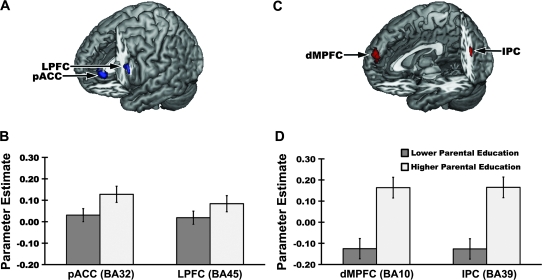
Figure 1.
Higher parental education predicted greater activation of the pACC (BA32) and LPFC (BA45) to stimuli signaling monetary gains (PF) compared with monetary losses (NF) (panel A), along with greater activation of the dMPFC (BA10) and IPC (BA39) to stimuli signaling monetary gains compared with a control condition (panel C). Data shown in panels A and C are clusters where parental education was associated with activation to PF at whole-brain corrected statistical significance thresholds implemented in mixed-effects parametric analyses with covariate control for personal (adult) educational attainment (see Materials and Methods). MNI coordinates for peaks within each labeled cluster and corresponding statistics are as follows: Panel A, pACC (−2, 40, −2, t73 = 4.51, z = 4.22, k = 109) and LPFC (−42, 18, 20, t73 = 4.35, z = 4.09, k = 80); Panel C, dMPFC (0, 60, 26, t73 = 4.55, z = 4.26, k = 167) and IPC (−44, −60, 32, t73 = 5.86, z = 5.29, k = 107). Shown in panels B and D are mean (±standard error) extracted contrast parameter estimates corresponding to BOLD activation values for each of the 4 clusters shown in panels A and C. Participants in the higher parental education group had a biological mother or father who attained a postsecondary or higher degree. Participants in the lower parental education group did not have a biological mother or father who attained a postsecondary or higher degree (see Materials and Methods).
To determine the covariation between parental education and functional connectivity at the group level, individual connectivity maps generated from each of the 4 seeds described above (pACC, LPFC, dMPFC, and IPC) were submitted to GLMs in SPM2. In each GLM, parental education was entered as an explanatory factor of interest, with participants’ own (adult) education entered as a covariate. We specifically tested for covariation between parental education and functional connectivity across individuals in targeted region-of-interest (ROI) analyses that focused on prefrontal and ventral and dorsal striatal regions implicated in reward processing (see Introduction). For these analyses, we maintained a corrected false-positive detection rate of P < 0.05 within the search volumes of all ROIs by employing voxel-wise and combined cluster extent thresholds determined by the spatial smoothness (in FWHM) of each second-level parametric map and 2000 iterative Monte Carlo simulations implemented in AlphaSim (Ward 2000). Detailed information regarding all anatomical masks used for ROI analyses and the statistical thresholds employed are given in Supplementary Figure S1. We emphasize that the connectivity values derived from our analyses do not reflect the strength of the directional (effective) associations between brain regions that can be quantified by more advanced approaches (Friston 1994). Hence, to extend our functional connectivity findings reported below, we executed a focused set of dynamic causal modeling (DCM) analyses described next.
Analysis of Parental Education and Effective Connectivity
The functional connectivity analyses described above and reported below revealed a significant and unique covariation between parental education and the coupling between fMRI BOLD signal activity in prefrontal and striatal regions across our task paradigm, irrespective of the effects of variations in the monetary gain (PF) and loss (NF) conditions. Specifically, lower parental education covaried across participants with a reduced functional connectivity between 1) the dMPFC and ventral striatum and 2) the pACC and orbital regions of the medial and bilateral prefrontal cortex (see Fig. 2). As noted above, these functional connectivity analyses, however, do not allow for inferences about the strength of the directional (effective) connectivity exhibited between these regions, particularly in association with the modulatory influence of processing PF stimuli signaling monetary gains. Accordingly, we executed bilinear DCM within SPM following published guidelines on model development and DCM parameter inference (Friston 1994; Penny et al. 2004; Stephan et al. 2010) (We note that DCM analyses were conducted following the suggestion of an anonymous reviewer who noted the inferential limitations of functional connectivity analyses, particularly with respect to the modulatory influence of the PF condition.). DCM provides for quantitative inferences about the strength of the directional interactions between brain regions, as constrained within the framework of neuroanatomical networks that are specified a priori and that have predefined forward and backward connections between regions. By this approach, the a priori networks we tested were informed and constrained by the functional connectivity findings reported herein. These included a dMPFC and ventral striatum network (two regions) and a pACC–OFC network (4 regions). In DCM, parameter estimates are labeled as “intrinsic” and “modulated.” Intrinsic parameters are taken to reflect connectivity estimates that are independent of the effects of task conditions, whereas modulated parameters are taken to reflect connectivity estimates that are associated with the change in effective connectivity induced by an experimental manipulation or task condition, such as the PF condition of our task paradigm.
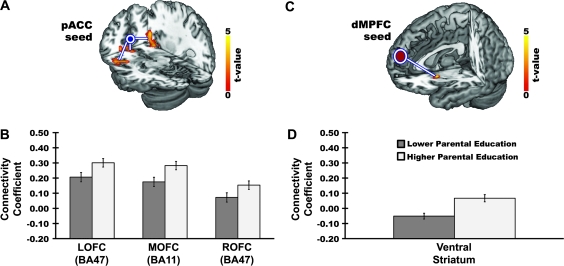
Figure 2.
Higher parental education predicted a greater functional connectivity between the pACC (BA 32) and 3 regions of the OFC, encompassing BA47 of the right and left OFC and BA11 of the medial OFC (panel A). Higher parental education also predicted a greater functional connectivity between the dMPFC (BA10) and the left ventral striatum (panel C). Panels A and C illustrate statistical parametric maps from ROI analyses revealing areas of the OFC (A) and ventral striatum (C) where parental education was associated positively with functional connectivity for each seed shown after covariate control for participants’ own education. Parametric maps are displayed at ROI-volume corrected statistical thresholds (see Materials and Methods). MNI coordinates for peaks within each cluster and corresponding statistics are as follows: Panel A, pACC-lateral orbitofrontal cortex (LOFC) (−40, 30, −14, t73 = 3.42, z = 3.28, k = 113), pACC-medial orbitofrontal cortex (MOFC) (−4, 36, −14, t73 = 3.85, z = 3.66, k = 122), and pACC-right orbitofrontal cortex (ROFC) (34, 32, −14, t73 = 5.14, z = 4.73, k = 295); Panel C, dMPFC-ventral striatum (−22, 10, −2, t73 = 2.87, z = 2.78, k = 49). Panels B and D illustrate the mean (±standard error) extracted connectivity coefficients for the clusters profiled in A and C as a function of the parental education groups described in Figure 1 and in the Materials and Methods.
DCM thus estimates neuronal state changes from observed fMRI signal activity per unit of time in regions of a predefined network by the following equation:
where, dz/dt is an N-dimensional time series vector corresponding to estimated neuronal state “changes” for N regions within a network. In addition, z is an N-dimensional time series vector corresponding to modulatory neuronal activity within network regions, and u is an M-dimensional time series vector corresponding to experimental condition variation. Furthermore, neuronal activity represented by z (presumably generating fMRI signal variation) is estimated by a so-called hemodynamic Balloon expansion model (Friston 1994, 2009). Finally, A corresponds to a matrix, (aij)N × N in which aij represents the strength of the intrinsic (task independent) connectivity from region i to j; Bk corresponds to a matrix, , in which represents the modulatory influence of an experimental condition, k, on the intrinsic connectivity from region i to j, with k = 1 … M (or the total number of experimental conditions); and where C is a matrix, (cik)N × M, in which cik corresponds to the direct input to region i attributable to variation in a given experimental condition k (e.g., processing monetary gains during PF). By this differential equation approach, DCM parameter estimates are thus comprised of the additive contributions of intrinsic connectivity between regions (A), the bilinear effects of a modulatory task condition (Bk), and the direct input into the proposed network (C). For our DCM analyses, we used the same extracted fMRI BOLD signal time series from the same dMPFC and ventral striatum ROIs described above, comprising one network and also from the same pACC and OFC ROIs described above, comprising a second network (coordinates for each region are provided in Fig. 2). For all participants, we used Bayesian selection procedures to compare 3 different model prototypes for each of these 2 networks. The first prototype assumed that the PF condition modulated the strength of the forward (or top-down) effective connectivity from the dMPFC to the ventral striatum and from the pACC to the medial and bilateral OFC regions. The second prototype assumed that the PF condition modulated the strength of the backward (or “bottom-up”) effective connectivity from the ventral striatum to the dMPFC and from the medial and bilateral OFC regions to the pACC. The final prototype assumed that the PF condition modulated both the forward and backward (top-down and bottom-up or bidirectional) effective connectivity between all regions in both networks. Across prototypes, we set the intrinsic connections between regions to be bidirectional, as informed by our functional connectivity findings and by known reciprocal projections between these areas, which have been established in human and animal studies (Ferry et al. 2000; Öngür and Price 2000; Haber 2003; Heimer and Van Hoesen 2006; Haber and Knutson 2010). Finally, to achieve model parsimony (Stephan et al. 2010), we assumed that the modulatory input into these networks was from the dMPFC (first network) and from the pACC (second network), recognizing that activity changes in these cortical regions were themselves driven by multimodal sensory relays (primary inputs). Model prototype comparisons and final selections were informed by considering model complexity and accuracy, as estimated by the natural logarithm of model evidence provided by the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) associated with each prototype for each participant (Penny et al. 2004). More precisely, comparisons between model prototypes i and j were based on the Bayes factor (BF) of the AIC and BIC for models i and j, where BFij is computed as the ratio of the probability (p) of the data (y) given the model (m), 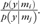 . In this way, model prototype i has been suggested to be superior to model j if BFij > 3 (Penny et al. 2004; Stephan et al. 2010). In comparing model prototypes, we found that the forward (top-down) model prototype was superior to the bidirectional prototype for 93% and 99% of the participants for the first (dMPFC–ventral striatum) and second (pACC-OFC) networks, respectively. Furthermore, the backward (bottom-up) model prototype was superior to the bidirectional model prototype for 97% and 99% of the participants for the first (dMPFC–ventral striatum) and second (pACC-OFC) networks, respectively. Finally, the backward (bottom-up) model prototype was superior to the forward (top-down) model prototype for only 4% (n = 3) of the participants for both the first (dMPFC–ventral striatum) and second (pACC-OFC) networks, respectively. Accordingly, we selected for further analysis the forward (top-down) DCM models for both the first (dMPFC–ventral striatum) and second (pACC-OFC) networks of interest. Importantly, our selection of the top-down model was confirmed at the group level using a Wilcoxon signed-rank sum test procedure, which was comprised of pairwise nonparametric tests of AIC and BIC values (Penny et al. 2004). Hence, in comparing forward, backward, and bidirectional model prototypes, the forward (top-down) model was superior in all comparisons for both networks, all z-values > 4.98, asymptotic Ps < 0.001.
. In this way, model prototype i has been suggested to be superior to model j if BFij > 3 (Penny et al. 2004; Stephan et al. 2010). In comparing model prototypes, we found that the forward (top-down) model prototype was superior to the bidirectional prototype for 93% and 99% of the participants for the first (dMPFC–ventral striatum) and second (pACC-OFC) networks, respectively. Furthermore, the backward (bottom-up) model prototype was superior to the bidirectional model prototype for 97% and 99% of the participants for the first (dMPFC–ventral striatum) and second (pACC-OFC) networks, respectively. Finally, the backward (bottom-up) model prototype was superior to the forward (top-down) model prototype for only 4% (n = 3) of the participants for both the first (dMPFC–ventral striatum) and second (pACC-OFC) networks, respectively. Accordingly, we selected for further analysis the forward (top-down) DCM models for both the first (dMPFC–ventral striatum) and second (pACC-OFC) networks of interest. Importantly, our selection of the top-down model was confirmed at the group level using a Wilcoxon signed-rank sum test procedure, which was comprised of pairwise nonparametric tests of AIC and BIC values (Penny et al. 2004). Hence, in comparing forward, backward, and bidirectional model prototypes, the forward (top-down) model was superior in all comparisons for both networks, all z-values > 4.98, asymptotic Ps < 0.001.
In accordance with our a priori focus on the covariation between parental education and the modulatory influence of processing monetary gains (as induced by the PF condition), statistical testing and corresponding inferences were limited to the DCM effective connectivity parameters reflecting the normalized forward (top-down) dMPFC⇒ventral striatum and pACC⇒OFC pathways defined by the above selection procedures. Thus, we tested whether the strength of the effective connectivity between the above regions, as modulated by the PF condition, covaried with parental education, net potential confounders. For these tests, we executed hierarchical regression analysis described next.
Hierarchical Regression Procedures
We executed analyses outside of SPM to test whether parental education covaried with BOLD activation values (contrast parameter estimates in Fig. 1) and functional and effective connectivity values (shown in Fig. 2 and Fig. 3) after accounting for several potential confounders. For these analyses, we executed 3-step hierarchical regressions using the Statistical Package for the Social Sciences 18.0 (SPSS) to partition the variance in activation and connectivity values that was accounted for by parental education (entered in step-2 of the models), over-and-above the potential confounders of participants’ own (adult) educational attainment, age, sex, family income, community-level socioeconomic position, alcohol use, depressive symptoms, and self-reported reward responsiveness. We further tested whether 2 additional indicators of parental socioeconomic position (maximum parental occupational grade computed from the Hollingshead scale and perceived familial economic status) entered on step 3 of the hierarchical models could account for any remaining variance in BOLD activation or connectivity values after first accounting for confounders and parental education variables entered on steps 1 and 2, respectively. The additional parental socioeconomic indicators were entered last in the models to protect against multicollinearity effects and because educational attainment is likely to precede and determine occupational opportunities and corresponding levels of income across individuals. In the regressions, the test statistic was the ΔR2 from step 1 to step 2 to step 3 of the models.
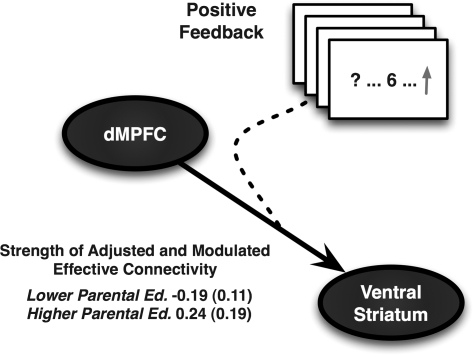
Figure 3.
Higher parental education predicted a comparatively stronger directional (effective) connectivity from the dMPFC (BA10) to the ventral striatum, as modulated by PF stimuli signaling monetary gains. At upper right is an illustration of the PF-modulated feedforward pathway from the dMPFC to the ventral striatum, which was estimated across participants by DCM (see Materials and Methods). At lower left are the normalized, covariate-adjusted, and z-score standardized (±standard error) DCM connectivity coefficients for the dMPFC⇒ventral striatum pathway as a function of the parental education groups (as in Figs 1–2). Variables used for covariate adjustment by multiple regression were participants’ own (adult) educational attainment, age, sex, family income, community-level socioeconomic position, alcohol use, depressive symptoms, and dispositional reward responsiveness (see Materials and Methods).
Results
Parental Education and Corticolimbic Activation
In whole-brain analyses, higher parental education predicted a greater activation of 4 cortical areas to PF stimuli signaling monetary gains, as revealed by contrasts comparing positive to NF (signaling monetary losses) conditions and PF to C conditions (Fig. 1). These areas included BA32 of the pACC, BA45 of the LPFC, BA10 of the dMPFC, and BA39 of the IPC. Notably, these findings were obtained with covariate control for participants’ own (adult) educational attainment. Moreover, in subsequent hierarchical regression analyses including several confounders (adult educational attainment, age, sex, family income, community-level socioeconomic position, alcohol use, depressive symptoms, and reward responsiveness), parental education continued to predict BOLD activation values (cluster-level contrast parameter estimates) when these confounding factors were entered on step 1 of the models (pACC: F1,65 = 23.32, ΔR2 = 24%, P < 0.001; LPFC: F1,65 = 19.92, ΔR2 = 21%, P < 0.001, dMPFC: F1,65 = 30.84, ΔR2 = 29%, P < 0.001; IPC: F1,65 = 25.87, ΔR2 = 27%, P < 0.001). Moreover, neither the maximum occupational status attained by either parent nor the perceived economic status of participants’ families accounted for variation in pACC, LPFC, dMPFC, or IPC BOLD activation values above and beyond that already accounted for by parental educational attainment, all step 3 ΔR2 values < 1.7%, Fs < 1, Ps > 0.44.
In contrast to the above findings for PF activation, parental education did not predict activation to NF, as revealed by the NF > PF and NF > C contrasts. Furthermore, parental education did not predict activation to PF or NF when our analyses were restricted to the ventral or dorsal striatal ROIs defined in Supplementary Figure S1, even when the ROI thresholds were lowered to a lenient Puncorrected < 0.01, k = 0. It is unlikely that the latter null findings resulted from a failure of the fMRI task paradigm to engage the striatum because marked ventral and dorsal striatal activation was revealed to both the PF and NF conditions across all participants in whole-brain main effects analyses employing corrected statistical thresholds (for summaries of the main effects of our task paradigm, see Supplementary Table S4, Figs S2 and S3).
Finally, in whole-brain and ROI analyses, we did not find evidence for “negative” associations between parental education and corticostriatal activation (e.g., regions where individuals in the “lower” parental education group showed “greater” activation to PF or NF). In summary, these findings indicate that higher parental education uniquely predicted greater cortical BOLD activation to PF stimuli signaling monetary gains and not to NF signaling monetary losses.
Parental Education and Corticostriatal Functional Connectivity
As shown in Figure 2, higher parental education predicted a more positive functional connectivity between the pACC and 3 regions of the OFC, namely, 2 bilateral orbitofrontal areas corresponding to BA47 and one ventromedial area corresponding to BA11. As for the activation findings above, these connectivity findings were obtained with covariate control for participants’ own (adult) educational attainment. Moreover, in hierarchical regression analyses including potential confounding factors (adult educational attainment, age, sex, family income, community-level socioeconomic position, alcohol use, depressive symptoms, and reward responsiveness), parental education continued to predict interindividual variation in functional connectivity for each pair of regions when these confounding factors were entered on step 1 of the models (pACC—right BA47: F1,65 = 34.28, ΔR2 = 32%, P < 0.001; pACC—left BA47: F1,65 = 20.31, ΔR2 = 22%, P < 0.001, pACC—medial BA11: F1,65 = 13.71, ΔR2 = 15%, P < 0.001). Finally, neither the maximum occupational status attained by either parent nor the perceived economic status of participants’ families accounted for interindividual variation in pACC functional connectivity above and beyond that already accounted for by parental educational attainment, all step 3 ΔR2 values < 2.1%, Fs < 1, Ps > 0.39.
As also shown in Figure 2, higher parental education predicted a more positive functional connectivity between the dMPFC and the left ventral striatum at the anatomical border of the ventral putamen. Again, in hierarchical regressions including the same potential confounding factors as above, parental education continued to predict variation in dMPFC–ventral striatum connectivity when these confounding factors were entered on step 1 of the models (dMPFC–ventral striatum: F1,65 = 7.02, ΔR2 = 7.4%, P = 0.01). Also as above, neither the occupational status attained by either parent nor the perceived economic status of participants’ families accounted for additional variation in dMPFC–ventral striatum above and beyond that already accounted for by parental educational attainment, step 3 ΔR2 = 0.2%, F < 1, P = 0.92.
In contrast to the connectivity findings corresponding to the pACC and dMPFC seeds, we observed no significant associations between parental education and connectivity corresponding to the LPFC and IPC seed regions shown in Figure 1. It is unlikely that this pattern of null results could be attributed to a lack of statistical power or a failure of these 2 regions to show significant patterns of functional connectivity in our task paradigm, as whole-brain random-effects analyses on all participants revealed marked connectivity for all 4 of the seed regions used for parental education analyses (see Supplementary Table S5 and Figs S4–S7). Finally, and as for the activation findings reported above, in whole-brain and ROI analyses, we did not find evidence for negative associations between parental education and functional connectivity patterns across our 4 seed regions (e.g., regions where individuals in the lower parental education group showed greater functional connectivity).
Analyses of Participants’ Own (Adult) Education and Corticostriatal Functionality
We note that participants’ own (adult) educational attainment was used as a nuisance variable (covariate of no interest) in whole-brain and ROI analyses of the associations between parental education and adult fMRI BOLD activation and connectivity. Nonetheless, it could be asked whether higher adult education, itself, was associated with corticostriatal activation or connectivity in overlapping or nearby regions where we observed statistically significant effects for parental education (e.g., those regions illustrated in Figs 1 and 2). In addressing this question, we found no evidence of overlapping regional associations between activation or connectivity with parental and adult educational attainment. Hence, when we employed the same voxel-wise significance and cluster extent thresholds that were employed for the parental education analyses above, there were no suprathreshold positive (or negative) associations between personal education and 1) BOLD activation for any of the task contrasts or 2) connectivity patterns for any of the 4 cortical seeds, which remained the case even when we used the MNI coordinates reported in Figures 1 and 2 for small-volume corrections (for illustration, see Supplementary Fig. S8 for glass brain depictions of whole-brain personal education analyses executed at lenient and uncorrected statistical significance and extent thresholds). Hence, it appears that our observations regarding parental education are unique from the contributions of participants’ own educational attainments.
Parental Education and Effective Connectivity
Higher parental education predicted a stronger directional (effective) connectivity from the dMPFC to the ventral striatum, as modulated by PF stimuli signaling monetary gains. This finding was revealed by a hierarchical regression analysis, which included parental education as a step 2 predictor variable that was entered after the step 1 covariates of participants’ own (adult) educational attainment, age, sex, family income, community-level socioeconomic position, alcohol use, depressive symptoms, and self-reported reward responsiveness, F1,64 = 4.11, ΔR2 = 5.4%, P < 0.05 (see Fig. 3) (An influential outlier case from the lower parental education group, which exceeded 3 SD of the mean modulated dMPFC⇒ventral striatum effective connectivity parameter value was omitted from analysis. Including this case in the model changed the association between parental education and modulated dMPFC⇒ventral striatum effective connectivity to F1,65 = 2.93, ΔR2 = 3.8%, P = 0.09.). Furthermore, neither the maximum occupational status attained by either parent nor the perceived economic status of participants’ families accounted for additional variance in modulated dMPFC⇒ventral striatum effective connectivity above and beyond that already accounted for by parental educational attainment, F < 1, ΔR2 = 0.5%, P = 0.82.
In contrast to the above dMPFC⇒ventral striatum effective connectivity finding, parental education did not predict effective connectivity at a conventional level of statistical significance from the pACC to the medial or bilateral orbitofrontal cortices, as modulated by PF stimuli signaling monetary gains and after covariate control for age, sex, family income, community-level socioeconomic position, alcohol use, depressive symptoms, and self-reported reward responsiveness, Fs < 3.2, step 2 ΔR2 values < 4.4%, Ps ≥ 0.08. Furthermore, pACC⇒OFC effective connectivity changes, as modulated by PF, also did not covary significantly with parental occupation or reported family economic status when these variables were added to step 3 of the hierarchical models, Fs < 1.7, step 3 ΔR2 values < 4.6%, Ps > 0.19. In aggregate, these findings indicate that the covariation between parental education and the connectivity between pACC and OFC regions did not appear to be modulated by the PF task condition at a conventional level of statistical significance.
Discussion
Midlife adults whose parents attained a lower level of education than those of other study participants showed reduced activation to PF stimuli signaling monetary gains in 4 cortical areas: the pACC, dMPFC, LPFC, and IPC. Also among adults whose parents attained a lower level of education, 2 prefrontal areas in particular—the pACC and dMPFC—showed a reduced functional connectivity with areas of the OFC and ventral striatum. Furthermore, adults with less educated parents exhibited a reduced strength in the directional or effective connectivity from the dMPFC to the ventral striatum, as modulated by PF stimuli signaling monetary gains. Finally, other indicators of parental socioeconomic position (occupation, perceived family economic status) did not explain additional variation in corticostriatal activation or connectivity after first accounting for potential confounders and parental education. Critically, none of the findings regarding parental education were explained by our participants’ own socioeconomic position (as reflected by their education, income, and community of residence) or other factors plausibly related to alterations in reward processing and corticostriatal functionality. All together, these findings suggest that a presumptive indicator of early socioeconomic disadvantage, lesser parental educational attainment, is associated with individual differences in the activation and connectivity of corticostriatal brain areas in adulthood. As such and in recognition of the study limitations noted below, these findings may provide insight into the preclinical and neurobiological pathways linking early socioeconomic disadvantage to latent risk for the development of later adult health outcomes that are associated with the expression of reward-related behaviors.
Our first set of findings indicates that adults from lower parental educational backgrounds show reduced activation to monetary gains (signaled by PF) in cortical areas implicated in several reward-related processes, including regulating and suppressing impulsive decisions (pACC); representing abstract or secondary rewards (dMPFC); maintaining reward-related information in working memory (LPFC); and computing the probability of future reward-related outcomes (IPC) (for reviews, see London et al. 2000; Rolls 2000; Schultz et al. 2000; Martin-Soelch et al. 2001; Kringelbach and Rolls 2004; Volkow et al. 2004; Bechara 2005; Fareri et al. 2008; Ernst and Fudge 2009; Geier and Luna 2009; Haber and Knutson 2010). While we cannot specify the precise processes engaged by our task paradigm, it would appear at minimum that adults from lower parental educational backgrounds show a reduced cortical response to reward-related stimuli. Speculatively, this could suggest that aspects of early socioeconomic disadvantage are associated with lasting developmental alterations in cortical (and possibly executive) aspects of reward processing. We note here that the corticostriatal activation patterns observed across all of our participants agree with those observed in prior studies employing variants of the same task, particularly those in which each trial has signaled monetary feedback in event-related designs (e.g., Delgado et al. 2000; for summaries, see Supplementary Table S4 and Figs S2 and S3). The corticostriatal activation patterns we observed also accord with those observed using other incentive-based paradigms involving reward-related stimuli (e.g., Breiter and Rosen 1999; Knutson and Cooper 2005). However, unlike previous studies, each trial in our paradigm was not associated with a specific monetary outcome (i.e., gaining or losing a specified amount of money). Rather, each trial provided PF or NF, which reflected the “accuracy” of the participant’s guess that would determine a later monetary reward. Thus, our paradigm limits our ability to identify patterns of activation associated with trial-by-trial monetary outcomes, which might be related more closely to interindividual variation in parental education. Furthermore, our blocked paradigm precludes an analysis of corticostriatal activity uniquely associated with “anticipatory” and “outcome” processes engaged during each trial. Thus, employing event-related paradigms that better allow for the dissociation of anticipation and outcome processes may help further evaluate the relation between parental education and specific aspects of reward-related corticostriatal functionality in adulthood. Furthermore, we note that the PF condition also provided potentially reinforcing feedback about achievement, possibly engaging processes associated with differential sensitivity to success. Thus, another potential limitation of this study is that we did not obtain subjective ratings at the time of neuroimaging that would have allowed us to better determine the meaning of the task to our participants, particularly with respect to individual differences in sensitivity to achievement success. Hence, the absence of such ratings leaves open the possibility that our findings may partly reflect a reduced sensitivity to achievement success among those from lower parental educational backgrounds, which could be investigated through different experimental and assessment paradigms. Finally, we recognize that the associations between parental education and corticostriatal activation could reflect a nonspecific association between early socioeconomic circumstances and the processing (both anticipation and feedback) of any type of motivationally salient stimulus (cf., Zink et al. 2003, 2004). However, we note that parental education only predicted cortical activation in response to PF conditions (associated with monetary gains) and not to NF conditions (associated with monetary losses). Hence, lower parental education does not appear to be linked to diffuse “hyporesponsiveness” to salient environmental stimuli.
Our second set of findings indicates that individuals from lower parental educational backgrounds show a reduced functional connectivity between the pACC and both medial and lateral areas of the OFC as well as between the dMPFC and ventral striatum. It is noteworthy that electrophysiological and neuroimaging studies in children suggest that early socioeconomic disadvantage is associated with developmental alterations in prefrontal areas, which have been speculated to affect the maturation of top-down or executive control functions implicated in incentive learning and impulse control (Casey et al. 2005; Galvan et al. 2006; Fareri et al. 2008; Ernst and Fudge 2009; Geier and Luna 2009; Hackman and Farah 2009). It is possible that our findings extend this evidence in children, insofar as an indicator of early socioeconomic disadvantage, lower parental education, predicted alterations in prefrontal connectivity in midlife adults. It is also noteworthy that the anterior cingulate cortex is critically involved in reward processing and adaptive decision making (Bechara 2005; Haber and Knutson 2010), with some evidence from adults to suggest that regions of the pACC may be especially sensitive to rewarding stimuli, such as monetary gains (Fujiwara et al. 2009). Furthermore, there is recent evidence that the pACC shows marked developmental plasticity in its functional connectivity. For example, Kelly et al. (2009) have recently provided in vivo neuroimaging evidence for a progressive maturation of pACC task independent or intrinsic functional connectivity from childhood to early adulthood, reflective of a developmental transition from more diffuse to focal patterns of connectivity, particularly between pACC and OFC regions. Kelly et al. (2009) interpreted this developmental transition in pACC functional connectivity to reflect the maturation of self-regulatory behavioral processes supported by the pACC and its networked regions. Indeed, the pACC and OFC are densely networked with one another and have been described in terms of an organizational orbitomedial network important for reward-related information processing and associated behaviors, including tracking the relative value of primary and secondary rewards as well as integrating multimodal reward-related information to guide decisions and suppress maladaptive actions (Öngür and Price 2000; Rolls 2000).
In addition to differences in pACC-OFC connectivity patterns between adults from lower and higher parental educational backgrounds, we observed comparable differences in the functional connectivity between the dMPFC and ventral striatum. The dMPFC appears to play an important role in top-down regulatory functions as well as in representing distant or future abstract (secondary) rewards (Ochsner and Gross 2005; Haber and Knutson 2010). Furthermore, the ventral striatum is critically involved in reward functions, including the encoding of reward prediction errors and processing reward uncertainty (Schultz et al. 2000; Haber and Knutson 2010). Recent developmental studies also suggest that the ventral striatum is involved in vulnerability to risk-taking and addictive behaviors (Casey et al. 2005; Fareri et al. 2008; Ernst and Fudge 2009; Geier and Luna 2009; Geier et al. 2010). Thus, it is plausible that early environmental or family influences engendered by aspects of socioeconomic disadvantage might influence the development, functionality, and connectivity of corticostriatal areas, resulting in the later emergence of disadvantageous health behaviors. For example, aspects of early disadvantage might affect myelination patterns, dendritic arborization processes, or other developmental outcomes leading to an ineffective top-down regulation of limbic or forebrain circuits driving reward processing in later life. In turn, such developmental alterations affecting connectivity or circuit signaling may relate to risky behaviors (e.g., cigarette smoking, impulsive choices, etc.) and/or lifestyle habits favoring immediate rewards over future health consequences. In this regard, it is noteworthy that individual differences in delay discounting, reflecting one’s relative tendency to chose small and immediate rewards versus large and delayed rewards, have been associated with both parental education (Sweitzer et al. 2008) and corticostriatal activation to the same guessing task used in the present study (Hariri et al. 2006). Furthermore, findings from DCM effective connectivity analyses in the present study appear to agree with the notion that so-called top-down or forward directional cortical control over striatal activity is associated with parental education in particular, net the influence of potential confounders. Hence, during the PF condition associated with monetary gains, those adults from higher parental educational backgrounds showed a stronger effective connectivity along a dMPFC to ventral striatal pathway (Fig. 3). Although intriguing, we note that the developmental, as well as intergenerational and prospective, lifespan measurements and study designs needed to formally test such speculations are largely impractical at the present time.
We acknowledge that indicators of socioeconomic position are often viewed in epidemiological studies as reflecting aspects of the social and material environments that can hierarchically stratify employment, occupational, and educational experiences and opportunities across individuals (Gianaros and Manuck 2010). Indicators of socioeconomic position, however, have also been associated with measures of intellectual ability that are partly attributable to heritable influences (Rowe et al. 1999; Manuck et al. 2004). Indeed, in this sample, we found a moderate association between participants’ own (adult) educational attainment and IQ, as measured by neuropsychological test performance on the vocabulary and matrix reasoning subtests of the WASI (r = 0.37, P < 0.005). Accordingly, it is plausible that the associations between parental education and measures of corticostriatal functionality could reflect the influence of correlated—possibly heritable—variation in intellectual ability. However, we found no significant difference in intellectual ability (WASI scores) between study participants from the higher and lower parental education groups (Supplementary Table S1). Furthermore, we found no significant associations between intellectual ability and the patterns of corticostriatal activation or connectivity depicted in Figures 1–3 (all rs < 0.18, Ps > 0.12). Also, we note that our task paradigm placed minimal cognitive demands on study participants. Thus, putatively heritable variation in intellectual ability associated with socioeconomic position would not appear to explain our findings.
Our inferences regarding parental education and corticostriatal activation and connectivity are also restricted by limitations other than those noted for our blocked fMRI task paradigm. First, the relation between retrospective reports of parental education and corticostriatal functionality might be confounded by recall (memory) biases or interindividual variation in reward sensitivity. However, adults are relatively accurate in reporting aspects of their parents’ socioeconomic position, particularly their educational attainment. When validated against historical employment records, for example, adults’ recall of parental socioeconomic indicators are generally accurate, even 50 years after childhood (e.g., adult reports of paternal occupation show an 83% agreement with employment records) (Berney and Blane 1997). Furthermore, adult twins show an approximate 81% agreement in reporting their parents’ educational attainment (Krieger et al. 1998). Thus, to the extent that recall or memory biases influenced our observations, we believe that these biases would have contributed largely to measurement error. Additionally, and with respect to confounding variation in reward sensitivity, we note that we accounted for several demographic attributes, a salient reward-related health behavior (alcohol use), self-reported symptoms of depression, and dispositional reward responsiveness. Second, we did not “prospectively” assess early maltreatment, neglect, or other severe adverse early life experiences at the level of the family, which could be more prevalent among lower socioeconomic households (Evans 2004) and plausibly influence the development and functioning of cortical and striatal circuits. However, we found no evidence suggesting that early adversity or trauma could fully account for our findings. More specifically, as part of the structured clinical interview to screen for DSM-IV Axis-I psychiatric disorders, 47 of our participants were evaluated for traumatic experiences (the reason for this reduced sample size is that assessments for posttraumatic stress disorder were added midway through the AHAB study protocol). In our sample, only one individual reported being neglected as a child, 4 reported being abused, and one reported prior combat experience. Importantly, we found no differences in the frequency of individuals reporting these adverse experiences between our parental education groups (all Ps > 0.47 by Fisher’s Exact Test). Furthermore, we found no association between a composite indicator of parental supervision and indicators of parental education in this sample (see Supplementary Tables S2 and S3). Moreover, to the extent that even subclinical forms of early adversity or stress exposures are predictive of adult negative emotionality and sensitivity to life stress (e.g., Heim and Nemeroff 1999), then one would expect that these emotion and stress-related factors would have covaried with parental education (assuming that lower parental education represents a surrogate marker of early life stress). This expectation, however, was not confirmed in our sample: depressive symptoms and reported levels of stress and stress exposures did not covary with parental education (Supplementary Table S2). Third, we recognize that our participants were relatively well educated, largely ethnically homogenous, and had parents who were generally well educated. These participant characteristics constrain extrapolations to more representative populations with greater ethnic and socioeconomic diversity, as well as diversity along other dimensions of early life disadvantage (e.g., being of minority status and maturing in impoverished communities), which should be examined in future work. Fourth, due to the cross-sectional nature of our study and the retrospective assessment of parental education, which reflected a diffuse developmental period (“childhood and adolescence”), we cannot make inferences regarding the possible effects of upward and downward shifts in familial social mobility during critical or sensitive developmental periods.
In view of the above, an important question raised by the present findings pertains to the early life experiences that may link broad and multidimensional constructs like parental socioeconomic position to corticostriatal development and functionality. There are at least 2 complementary perspectives from which this question could be addressed: a family stress perspective and a cultural-enrichment perspective. From a stress perspective, families of relative socioeconomic disadvantage are thought to experience heightened levels of life stress that are engendered by the uncertainties of future economic and occupational security, by a greater likelihood of residing in disadvantaged communities, and by restricted access to material and social resources and goods—among many other factors (Kessler and Cleary 1980; Elder et al. 1985; McLeod and Kessler 1990; Evans 2004; Taylor 2010; Matthews and Gallo 2011). Importantly, under some circumstances, these sources of life stress may increase risk for marital discord, harsh or withdrawn parenting styles, disrupted and unpredictable family routines (e.g., family dining and sleeping schedules), and conflict-laden family relationships that may adversely affect early life development (McLoyd 1990; Brody et al. 1994; Conger and Elder 1994; Conger et al. 2002). In extension, such stressful family experiences in early life could plausibly upregulate the expression of stress hormones, particularly peripheral and central glucocorticoids, thereby altering the assembly, plasticity, and long-term functioning of cell groups and brain circuits that are important for cognitive functioning and for processing salient environmental stimuli, such as primary or secondary environmental rewards (Lupien et al. 2009; McEwen and Gianaros 2010). In support of this notion, lower parental socioeconomic position has been associated with an accelerated 2-year rise in the glucocorticoid, cortisol, among children—presumably through stress-related pathways (Chen et al. 2010). Furthermore, dopaminergic functionality induced by a methylphenidate challenge (Engert et al. 2009) and dopamine release within the striatum (Pruessner et al. 2004) have been shown to covary with forms of early life stress and parenting practices that could plausibly track a socioeconomic gradient. We note, however, that in our sample, we found no significant associations between parental education and indicators of early family cohesion, conflict, or organization, as assessed by the FES (Supplementary Table S3). Thus, it would appear that a family stress perspective may not fully account for the present observations perhaps due to the limited variation in other sources of disadvantage experienced in this sample as noted above.
A cultural-enrichment perspective may also provide a another useful framework for interpreting the present findings, insofar as prior research has shown socioeconomic differences in so-called cultural-related childrearing repertoires. For example, Lareau (2003) has found that parents of higher socioeconomic position engage in “concerted cultivation” childrearing efforts by providing stimulating learning activities and social interactions. As parents, they reliably report strong motivations to promote their children’s social and cognitive development. In relative contrast, parents of lower socioeconomic backgrounds tend on average to report that child development is a process that unfolds naturally, and they are more likely to report that it is not necessary to do more than provide basic supports, including food, shelter, and comforts. These differential cultural repertoires are likely to translate into differences in the everyday environments of young children, with children from more advantaged backgrounds experiencing more cognitively stimulating and socially enriching home and school environments that provide for optimal neurocognitive developmental trajectories in children and adolescents (Evans 2004; Hackman and Farah 2009; Raizada and Kishiyama 2010; Matthews and Gallo 2011). Some “post hoc” observations from ancillary analyses suggested that such familial and cultural differences may exist in this sample. Specifically, we found that the most reliable correlate of parental socioeconomic position in this sample was the cultural–intellectual orientation of our participants’ families, which was measured by the FES (Supplementary Table S3). It is possible that this dimensional measure of cultural–intellectual orientation may reflect aspects of how cognitively stimulating or enriching the participants’ early household environments may have been, as it assesses the degree to which their families 1) discussed political and social problems, 2) went to lectures, plays, or concerts, 3) showed interest in cultural activities, 4) had intellectual discussions, and 5) enjoyed music, art, and literature. From a translational perspective, we speculate that cognitively stimulating family environments associated with socioeconomic differences in cultural repertoires related to child rearing might plausibly affect brain development and long-term functionality through similar experience-dependent mechanisms that have been delineated in animal models of early environmental enrichment and neuroplasticity. These mechanisms include positive and trophic effects on cell proliferation and density, synaptogenesis and synaptic organization, and dendritic branching patterns in cortical and subcortical cell groups that are important for a range of cognitive, social, and emotional behavioral processes (DiPietro 2000; Chapillon et al. 2002; Markham and Greenough 2004; Sullivan et al. 2006). However, because we cannot establish the causal direction of association between parental socioeconomic position and our FES measures, we are cautious in interpreting these post hoc observations. In extension, we are hesitant to rest study conclusions on their meaning until they are replicated in future, ideally longitudinal, studies.
Conclusions
To summarize, this study provides evidence that an indicator of early socioeconomic disadvantage, lower parental education, is associated with alterations in adult corticostriatal functionality elicited by PF signaling monetary reward. These findings could not be accounted for by interindividual variation in multilevel indicators of adult socioeconomic position or other confounding factors linked to reward processing. As such, adult alterations in reward-related corticostriatal functionality may represent facets of a neurobiological pathway potentially linking early disadvantage with latent risk for later adult health outcomes. More precisely, socioeconomic variation in parental resources, early environmental exposures, access to cognitively stimulating and enriching opportunities, and other factors during childhood and adolescence have been speculated to affect the maturation and plasticity of cortical and striatal brain circuitries that are important for self-regulatory behaviors that influence health throughout life (Shonkoff and Phillips 2000; Hackman and Farah 2009; Shonkoff et al. 2009). Indirect support for this speculation has been provided by epidemiological evidence that indicators of early socioeconomic disadvantage, including lower parental education, are associated with disadvantageous or maladaptive health behaviors as well as impulsive decision making. The present study builds on this evidence and encourages further work on the multilevel and likely multidimensional factors that proximally link early socioeconomic conditions with corticostriatal development and adult health.
Funding
National Heart Lung and Blood Institute (HL) and the National Institute of Mental Health (MH) at the National Institutes of Health (grants P01 HL40962 and RO1 HL065137 to S.B.M., grant K01 MH072837 to A.R.H., and grant K01 MH070616 and R01 HL089850 to P.J.G.).
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/
Acknowledgments
Sarah Abramowitch assisted in study execution. We thank Dr Charles Bradberry for his constructive comments on this manuscript. Conflict of Interest: None declared.
Appendix
Socioeconomic position reflects one's relative access to social and material resources. Indicators of socioeconomic position are typically quantified in metrics of educational attainment, occupational status, and income, which can be measured among individuals, families, communities, and higher levels of social organization (see Materials and Methods and as well as Discussion). In this context, early socioeconomic disadvantage refers to one's circumstances of childhood and adolescence (as reflected by parental socioeconomic position). In this sense, early socioeconomic disadvantage thus corresponds to the life opportunities and developmental experiences engendered by relative differences in the socioeconomic position and circumstances of one's family. Thus, relative increments in parental socioeconomic position may correspond to increased familial access to social, material, and other beneficial resources and opportunities as well as enriched cognitive and household environments (Shonkoff et al. 2009).
References
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Batty GD, Lewars H, Emslie C, Benzeval M, Hunt K. Problem drinking and exceeding guidelines for 'sensible' alcohol consumption in Scottish men: associations with life course socioeconomic disadvantage in a population-based cohort study. BMC Public Health. 2008;8:302. doi: 10.1186/1471-2458-8-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- Berney LR, Blane DB. Collecting retrospective data: accuracy of recall after 50 years judged against historical records. Soc Sci Med. 1997;45:1519–1525. doi: 10.1016/s0277-9536(97)00088-9. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Stoneman Z, Flor D, McCrary C, Hastings L, Conyers O. Financial resources, parent psychological functioning, parent co-care giving, and early adolescent competence in rural two-parent African-American families. Child Dev. 1994;65:590–605. [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev Psychobiol. 2002;41:373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21:31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nat Neurosci. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Elder GH., Jr . Families in troubled times: adapting to change in rural America. New York: Aldine de Gruyter; 1994. [Google Scholar]
- Conger RD, McLoyd VC, Wallace LE, Sun Y, Simons RL, Brody GH. Economic pressure in African-American families: a replication and extension of the family stress model. Dev Psychol. 2002;38:179–193. [PubMed] [Google Scholar]
- D'Angiulli A, Herdman A, Stapells D, Hertzman C. Children's event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22:293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex. 2004;14:1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- DiPietro JA. Baby and the brain: advances in child development. Annu Rev Public Health. 2000;21:455–471. doi: 10.1146/annurev.publhealth.21.1.455. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GH, Jr, Nguyen TV, Caspi A. Linking family hardship to children's lives. Child Dev. 1985;56:361–375. [PubMed] [Google Scholar]
- Engert V, Joober R, Meaney MJ, Hellhammer DH, Pruessner JC. Behavioral response to methylphenidate challenge: influence of early life parental care. Dev Psychobiol. 2009;51:408–416. doi: 10.1002/dev.20380. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Martin LN, Delgado MR. Reward-related processing in the human brain: developmental considerations. Dev Psychopathol. 2008;20:1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Boden JM, Jenkin G. Childhood social disadvantage and smoking in adulthood: results of a 25-year longitudinal study. Addiction. 2007;102:475–482. doi: 10.1111/j.1360-0443.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, research version, non-patient edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui K. Segregated and integrated coding of reward and punishment in the cingulate cortex. J Neurophysiol. 2009;101:3284–3293. doi: 10.1152/jn.90909.2008. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. The maturation of incentive processing and cognitive control. Pharmacol Biochem Behav. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB. Neurobiological pathways linking socioeconomic position and health. Psychosom Med. 2010;72:450–461. doi: 10.1097/PSY.0b013e3181e1a23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social class and mental illness: a community study. New York: John Wiley & Sons; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B, Kubzansky LD, Cohen S, Weiss S, Wright RJ. A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. Int J Epidemiol. 2004;33:271–278. doi: 10.1093/ije/dyh003. [DOI] [PubMed] [Google Scholar]
- Jentsch J, Roth R, Taylor J. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Williams DR, Schwartz JE, Matthews KA, Kiefe CI, Seeman TE. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: the CARDIA study (USA) Soc Sci Med. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Cleary PD. Social class and psychological distress. Am Sociol Rev. 1980;45:463–478. [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. J Cogn Neurosci. 2009;21:1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Krieger N, Okamoto A, Selby JV. Adult female twins' recall of childhood social class and father's education: a validation study for public health research. Am J Epidemiol. 1998;147:704–708. doi: 10.1093/oxfordjournals.aje.a009512. [DOI] [PubMed] [Google Scholar]
- Kringelbach M, Rolls E. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neurophysiology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lareau A. Unequal childhoods: class, race, and family life. Berkeley (CA): University of California Press; 2003. [Google Scholar]
- Lawlor DA, Ebrahim S, Davey Smith G. Adverse socioeconomic position across the lifecourse increases coronary heart disease risk cumulatively: findings from the British women's heart and health study. J Epidemiol Community Health. 2005;59:785–793. doi: 10.1136/jech.2004.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Bleil ME, Petersen KL, Flory JD, Mann JJ, Ferrell RE, Muldoon MF. The socio-economic status of communities predicts variation in brain serotonergic responsivity. Psychol Med. 2005;35:519–528. doi: 10.1017/s0033291704003757. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Muldoon MF. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;29:651–668. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Kunig G, Magyar S, Mino A, Schultz W. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res Brain Res Rev. 2001;36:139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- Masterman DL, Cummings JL. Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. J Psychopharmacol. 1997;11:107–114. doi: 10.1177/026988119701100203. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC Forthcoming 2011. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. doi: 10.1146/annurev.psych.031809.130711. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. J Health Soc Behav. 1990;31:162–172. [PubMed] [Google Scholar]
- McLoyd VC. The impact of economic hardship on Black families and children: psychological distress, parenting, and socioemotional development. Child Dev. 1990;61:311–346. doi: 10.1111/j.1467-8624.1990.tb02781.x. [DOI] [PubMed] [Google Scholar]
- Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. Am J Epidemiol. 2007;166:966–974. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia 'projections' to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Family environment scale manual. Palo Alto (CA): Consulting Psychologists Press; 1981. [Google Scholar]
- Noble KG, Farah MJ, McCandliss BD. Socioeconomic background modulates cognition-achievement relationships in reading. Cogn Dev. 2006;21:349–368. doi: 10.1016/j.cogdev.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev Sci. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Bleil ME, McCaffery J, Mackey RH, Sutton-Tyrrell K, Muldoon MF, Manuck SB. Community socioeconomic status is associated with carotid artery atherosclerosis in untreated, hypertensive men. Am J Hypertens. 2006;19:560–566. doi: 10.1016/j.amjhyper.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, Manuck SB. Parental education is related to C-reactive protein among female middle-aged community volunteers. Brain Behav Immun. 2009;23:677–683. doi: 10.1016/j.bbi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, McClearn GE, Pedersen NL, Nesselroade JR, Bergeman CS. Genetic influence on childhood family environment perceived retrospectively from the last half of the life span. Dev Psychol. 1988;24:738–745. [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur J Epidemiol. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, Moffitt TE. Association between children's experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Graham H, Due P, Hallqvist J, Joung I, Kuh D, Lynch J. The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: an international comparison. Int J Epidemiol. 2005;34:335–344. doi: 10.1093/ije/dyh394. [DOI] [PubMed] [Google Scholar]
- Power C, Hertzman C. Social and biological pathways linking early life and adult disease. Br Med Bull. 1997;53:210–221. doi: 10.1093/oxfordjournals.bmb.a011601. [DOI] [PubMed] [Google Scholar]
- Power C, Hypponen E, Smith GD. Socioeconomic position in childhood and early adult life and risk of mortality: a prospective study of the mothers of the 1958 British birth cohort. Am J Public Health. 2005;95:1396–1402. doi: 10.2105/AJPH.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RD, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010;4:3. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RD, Richards TL, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40:1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rowe D, Veserdal W, Rodgers J. Herrnstein's syllogism: genetic and shared environmental influences on IQ, education, and income. Intelligence. 1999;26:405–423. [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. J Am Med Assoc. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. Washington (DC): National Academy Press; 2000. [PubMed] [Google Scholar]
- Singh-Manoux A, Richards M, Marmot M. Socioeconomic position across the lifecourse: how does it relate to cognitive function in mid-life? Ann Epidemiol. 2005;15:572–578. doi: 10.1016/j.annepidem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. Neuroimage. 2010;49:3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Wilson DA, Feldon J, Yee BK, Meyer U, Richter-Levin G, Avi A, Michael T, Gruss M, Bock J, et al. The International Society for Developmental Psychobiology annual meeting symposium: impact of early life experiences on brain and behavioral development. Dev Psychobiol. 2006;48:583–602. doi: 10.1002/dev.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tob Res. 2008;10:1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Inaugural article: mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Turrell G, Lynch JW, Leite C, Raghunathan T, Kaplan GA. Socioeconomic disadvantage in childhood and across the life course and all-cause mortality and physical function in adulthood: evidence from the Alameda County Study. J Epidemiol Community Health. 2007;61:723–730. doi: 10.1136/jech.2006.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data: AFNI 3dDeconvolve documentation. Milwaukee (WI): Medical College of Wisconsin; 2000. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. San Antonio (TX): Psychological Corporation; 1997. [Google Scholar]
- Wilkinson LS. The nature of interactions involving prefrontal and striatal dopamine systems. J Psychopharmacol. 1997;11:143–150. doi: 10.1177/026988119701100207. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B, Braus DF, Buchel C. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci U S A. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.