A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing
MED12, an RNA Polymerase II Mediator subunit implicated in human cognitive development, is identified as a critical transcriptional coactivator of the amyloid precursor protein (APP) intracellular domain, implicating MED12/Mediator in a broad range of developmental and pathological processes driven by APP signal transduction.
Keywords: Alzheimer disease, amyloid precursor protein, MED12, Mediator
Abstract
Regulated intramembrane proteolysis of the amyloid precursor-protein (APP) produces both a characterstic amyloid-β peptide that contributes to neuritic plaque formation and neurodegeneration in Alzheimer disease and a small APP intracellular domain (AICD) that transcriptionally activates genes implicated in Alzheimer disease pathology. Although the biochemical events leading to amyloidogenic APP processing at the cell membrane have been described in detail, comparably little is known about the mechanistic basis of AICD-dependent gene regulation in the nucleus. In this study, we show that the AICD activates transcription by targeting MED12, an RNA polymerase II transcriptional Mediator subunit that is implicated in human cognitive development. The AICD binds to MED12/Mediator in vitro and in vivo. Disruption of the AICD/MED12 interaction inhibits AICD transactivation potential and expression of AICD target genes. Mediator, in a MED12-dependent manner, occupies only AICD-bound promoter DNA, indicating that the AICD recruits Mediator to activate transcription. These results identify the MED12 interface in Mediator as a crucial transducer of AICD transactivation and a potential therapeutic target in Alzheimer disease.
Introduction
The amyloid precursor protein (APP) and two APP-like proteins—APLP1 and APLP2—comprise a family of type I integral membrane proteins with essential and overlapping roles in brain development and function (Zheng & Koo, 2006; Walsh et al, 2007). Among its family members, APP is notable for its association with Alzheimer disease, a progressive neurodegenerative disorder characterized by early short-term memory deficits and eventual loss of cognitive and executive functions. The pathological characteristics of Alzheimer disease are the presence of extracellular neuritic plaques enriched in amyloid-β peptides and intracellular neurofibrillary tangles comprising hyperphosphorylated tau protein (Mucke, 2009). Amyloid-β production through amyloidogenic processing of APP by membrane-bound β- and γ-secretases results in the simultaneous production of a 50-amino-acid carboxy-terminal fragment corresponding to the APP intracellular domain (AICD; Haass, 2004). APLP1 and APLP2 are proteolytically processed in a mechanistically similar way, although sequence divergence within their amyloid-β regions precludes plaque formation (Scheinfeld et al, 2002; Li & Sudhof, 2004). Notably, the AICD is among the most highly conserved protein regions found in APP family members, suggesting that it is functionally important.
The AICD has been reported to translocate into the nucleus and activate gene transcription (Cao & Sudhof, 2001; Gao & Pimplikar, 2001). Notably, several AICD-target genes have been implicated in cellular processes relevant to Alzheimer disease. For example, neprilysin—a synaptic ectoenzyme that degrades amyloid-β in the extracellular space—helps to maintain the concentration of amyloid-β below the threshold for self-aggregation, whereas p53-mediated apoptosis has been implicated in Alzheimer-disease-associated neuronal death (Pardossi-Piquard et al, 2005; Alves da Costa et al, 2006). More recently, a cohort of AICD-target genes implicated in control of cytoskeletal dynamics, including α2-actin, IGFBP3 and TAGLN, was found to be upregulated in the frontal cortex of Alzheimer disease patients, whereas another AICD-regulated gene, Aquaporin 1, was shown to be upregulated in astrocytes surrounding amyloid-β plaques within the brains of Alzheimer disease patients (Muller et al, 2007; Huysseune et al, 2009). Together, these observations suggest that AICD-dependent nuclear signalling might contribute to the pathophysiology of Alzheimer disease.
Little is known about the mechanism by which the AICD activates gene transcription in the nucleus. Established interactions between the AICD, the transcriptional adaptor Fe65 and acetyltransferase Tip60 suggest it is linked to histone modification (Cao & Sudhof, 2001), which is supported by work documenting AICD-dependent histone acetylation on the neprilysin promoter (Belyaev et al, 2009). However, a physical or functional AICD target within the RNA polymerase II general transcription machinery suggesting a direct mechanistic basis for gene regulation has not been reported so far. In this paper, we identify the MED12 subunit within the RNA polymerase II transcriptional Mediator as a direct physical target and functional transducer of the AICD, as well as the intracellular domains of APLP1 and APLP2. Mediator is a conserved multi-subunit signal processor through which regulatory information conveyed by gene-specific transcription factors is transduced to RNA polymerase II in order to alter gene expression programmes controlling cell growth, homeostasis, development and differentiation (Kornberg, 2005; Malik & Roeder, 2005). MED12 is implicated in vertebrate neuronal development, and genetic variation in MED12 is associated with cognitive and behavioural dysfunction in humans (Philibert et al, 2001; Wang et al, 2006; Risheg et al, 2007; Schwartz et al, 2007). Our findings that MED12 mediates the AICD transactivation potential and expression of AICD-target genes identify MED12 as a new component in the APP-dependent nuclear-signalling pathway, and further implicate Mediator in a range of developmental and pathological processes driven by APP signal transduction.
Results
APP/APLP ICDs bind to MED12/Mediator
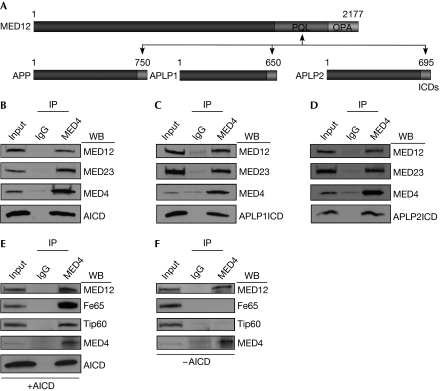
To clarify the role of MED12 in neural-specific transcription control, we processed the MED12 C-terminus (MED12C; amino acids 1,616–2,177) through a yeast two-hybrid screen using a human foetal brain cDNA library (Zhou et al, 2006) and recovered APLP1, a neural-restricted APP-family member (Lorent et al, 1995). To confirm a physical interaction between MED12 and APLP1 and map their reciprocal binding domains, we tested the ability of glutathione S-transferase (GST)–MED12 and GST–APLP1 derivatives to bind to a panel of APLP1 and MED12 truncation fragments, respectively. This analysis identified the principal reciprocal interaction surfaces on each protein which include a proline-, glutamine- and leucine-rich (PQL) domain on MED12 (amino acids 1,616–2,050) and the C-terminal 50 amino acids of APLP1 corresponding to its transcriptionally active intracellular domain (ICD50; Fig 1A; supplementary Figs S1 and S2 online). As the ICDs of all three APP family members are highly conserved, we tested and confirmed that MED12 could also bind to APP and APLP2 ICD50 (Fig 1A; supplementary Figs S1 and S2 online). Thus, MED12 binds specifically through its PQL domain to the transcriptionally active ICDs of all three APP/APLP family members.
Figure 1.
Amyloid precursor protein (APP)/APP-like protein intracellular domains (APLP ICDs) bind to MED12/Mediator. (A) Schematic summary of reciprocal binding domains on APP/APLP proteins and MED12. A proline-, glutamine-, leucine-rich (PQL) domain on MED12 interacts with all three APP/APLP ICDs. Numbers refer to amino-acid coordinates. (B–F) Nuclear extracts from HeLa cells transiently expressing the ICD50 of (B) APP, (C) APLP1, (D) APLP2 or (E) Fe65/Tip60 with or (F) without APP ICD were immunoprecipitated with IgG or antibodies for the core Mediator subunit MED4. Immunoprecipitates were resolved by SDS–PAGE and processed by western blot analysis using the specified antibodies. Input, 10% of nuclear extract used for immunoprecipitation. AICD, amyloid precursor protein intracellular domain; APLP, amyloid precursor protein-like protein; APP, amyloid precursor protein; ICD, intracellular domain; IP, immunoprecipitation; PAGE, polyacrylamide gel electrophoresis; PQL, proline-, glutamine-, leucine-rich; WB, western blot.
To determine whether APP/APLP ICDs interact with intact Mediator, we immunoprecipitated Mediator from nuclear extracts of HeLa cells transiently expressing APP/APLP ICDs and examined the immunoprecipitates for the presence of each ICD by western blot assay. This analysis revealed specific coimmunoprecipitation of each APP/APLP ICD50 with intact Mediator, confirming their association in mammalian cells (Fig 1B–D). Notably, Fe65 and Tip60—established co-activators of the AICD—were coprecipitated with Mediator in the presence, but not the absence, of coexpressed AICD, demonstrating that Fe65 and Tip60 can form an AICD-dependent multimeric complex with Mediator in mammalian cells (Fig 1E,F).
APP/APLP ICDs functionally interact with MED12
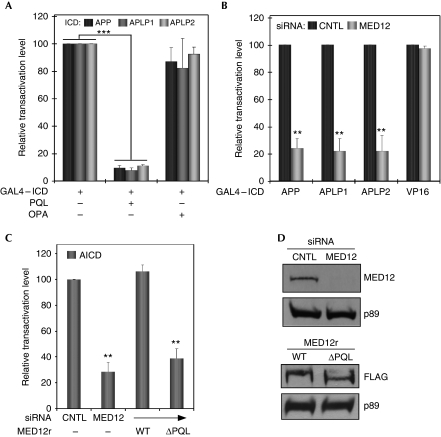
If the APP/APLP ICDs functionally target the MED12 interface in Mediator, their corresponding binding (PQL) domain on MED12 might inhibit their respective transactivation potentials through dominant-negative interference. To test this possibility, we examined the influence of MED12–PQL expression on the transactivation activity of each APP/APLP ICD tethered to the GAL4 DNA-binding domain (Cao & Sudhof, 2001). Ectopic expression of the MED12–PQL domain, but not of its neighbouring MED12–OPA domain, to which APP/APLP ICDs do not bind (supplementary Fig S2 online; data not shown), efficiently inhibited the transcriptional activities of all three APP family ICDs (Fig 2A).
Figure 2.
Amyloid precursor protein (APP)/APP-like protein intracellular domains (APLP ICDs) functionally interact with MED12. (A–C) HeLa cells were transfected with GAL4–APP/APLP ICDs or GAL4–VP16 with either (A) MED12 proline-, glutamine-, leucine-rich (PQL) or OPA domains or (C) siRNA-resistant FLAG-tagged MED12 wild-type or ΔPQL derivatives. (B,C) Cells were transfected with control (CNTL) or MED12-specific siRNAs 48 h before plasmid transfections. Cells collected 36 h after plasmid transfections were processed for luciferase and β-gal activities. Normalized luciferase activities associated with individual GAL4 derivatives were calculated relative to luciferase activities obtained in cells transfected with (A) GAL4 alone or (B,C) GAL4 and CNTL siRNA. For comparison, the relative luciferase activity for each GAL4 derivative was assigned a transactivation level of 100%; its corresponding activity in the presence of (A) the PQL or OPA domains or (B,C) MED12-specific siRNA is expressed relative to this value. Data represent the mean±s.e.m. of at least three independent transfections performed in duplicate. Except where specified (A), asterisks denote statistically significant differences relative to CNTL siRNA (Student's t-test **P<0.005; ***P<0.001). (D) Western blots from representative transient knockdown/rescue assays using the specified antibodies (TFIIH p89 subunit acts as a loading control). AICD, amyloid precursor protein intracellular domain; APLP, amyloid precursor protein-like protein; APP, amyloid precursor protein; CNTL, control; ICD, intracellular domain; PQL, proline-, glutamine-, leucine-rich; siRNA, short-interfering RNA, WT, wild type.
To further examine the functional interaction between APP/APLP ICDs and MED12/Mediator, we monitored the influence of RNA interference-mediated MED12 suppression on the transactivation activity of the ICD of each APP family member. Depletion of MED12 strongly inhibited the transcriptional activity of each APP/APLP ICD (Fig 2B,D). By contrast, depletion of MED12 had no influence on the transactivational activity of the herpes simplex virus VP16 transactivation domain (Fig 2B), the established targets of which in Mediator are MED17/25 (Ito et al, 1999). Thus, the influence of MED12 knockdown in this context seems to be restricted to the APP-family ICDs. To confirm that MED12 mediates transactivation by the AICD through direct interaction, we assessed the respective abilities of short-interfering RNA (siRNA)-resistant wild-type MED12 (MED12r) and a siRNA-resistant AICD–binding-defective MED12 derivative lacking the PQL domain (MED12r–ΔPQL) to rescue the AICD transactivation in MED12-depleted cells. When expressed at equivalent levels, MED12r, but not MED12r–ΔPQL, elicited efficient rescue of AICD-directed transactivation (Fig 2C,D). This finding precludes the possibility that reduced AICD-driven transactivation elicited by MED12 siRNA derives from a nonspecific ‘off-target' effect, and confirms that the AICD physically interacts with the MED12 interface in Mediator to activate transcription.
MED12 mediates AICD-dependent gene expression
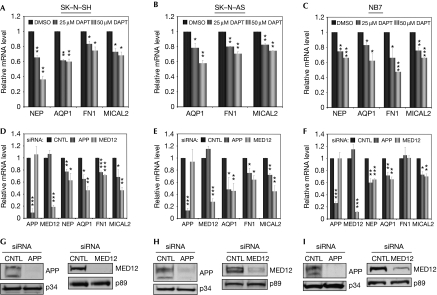
To examine the role of MED12/Mediator as a transducer of AICD-dependent nuclear signalling in a more physiological context, we monitored the effect of MED12 depletion on the expression levels of AICD-regulated genes in three different human neuroblastoma cell lines: SK–N–SH (Tachida et al, 2008), SK–N–AS (Tillement et al, 2006) and NB7 (Belyaev et al, 2009). Of those genes identified as targets of AICD-dependent regulation from previous functional studies (Pardossi-Piquard et al, 2005; Alves da Costa et al, 2006; Muller et al, 2007), we confirmed four as targets of presenilin- and AICD-dependent regulation in neuroblastoma cells (Neprilysin (NEP), Aquaporin 1, Microtubule-associated monoxygenase, calponin and LIM domain containing 2 (MICAL2) and Fibronectin 1). Thus, pharmacological inhibition of γ-secretase with DAPT (Fig 3A–C) or RNAi-mediated APP depletion (Fig 3D–I) significantly reduced the expression levels of NEP, Aquaporin 1, MICAL2 and Fibronectin 1 in at least two out of the three neuroblastoma cell lines examined. Notably, RNAi-mediated MED12 depletion also significantly reduced expression of these four genes in a manner perfectly concordant with their regulation by the AICD, implicating MED12/Mediator in AICD-dependent gene regulation (Fig 3D–I). Finally, of these four genes only MICAL2 was coordinately regulated similarly in HeLa cells (data not shown), suggesting possible neural-cell-type-specific gene regulation by the AICD and MED12/Mediator.
Figure 3.
MED12 mediates amyloid precursor protein intracellular domain (AICD)-dependent target gene expression. (A–C) RNA from SK–N–SH, SK–N–AS or NB7 cells treated with 0, 25 or 50 μM DAPT or (D–F) control (CNTL), APP- or MED12-specific siRNAs was subjected to RT–qPCR. mRNA levels for the indicated genes were normalized to RPL19 mRNA and expressed relative to their corresponding mRNA levels in (A–C) DMSO-treated or (D–F) control siRNA-treated cells. Data represent the mean±s.e.m. of at least three independent experiments performed in duplicate. Asterisks denote statistically significant differences relative to (A–C) DMSO or (D–F) CNTL siRNA (Student's t-test *P<0.05; **P<0.005; ***P<0.001). Note that NEP expression in SK–N–AS cells was below the limit of reliable detection and therefore omitted from analysis. (G–I) Western blots from representative transient knockdown assays using the specified antibodies (TFIIE p34 and TFIIH p89 subunits serve as loading controls). APP, amyloid precursor protein; CNTL, control; DMSO, dimethylsulphoxide; NEP, neprilysin; RT–qPCR, reverse transcription–quantitative PCR; siRNA, short-interfering RNA.
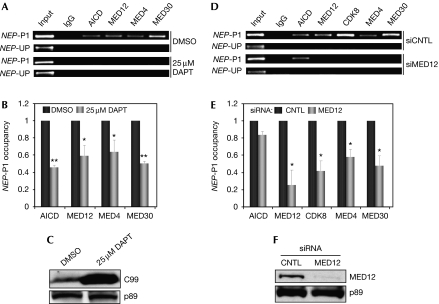
To explore the mechanistic relationship between the AICD and MED12/Mediator, we used chromatin immunoprecipitation (ChIP) to monitor their interdependence for occupancy of the NEP promoter. Depletion of AICD levels in NB7 cells through DAPT inhibition of γ-secretase activity significantly reduced NEP promoter occupancy by the AICD, as expected, as well as by MED12/Mediator (Fig 4A–C). By contrast, RNAi-mediated MED12 depletion reduced occupancy of the NEP promoter by Mediator, but not by the AICD, suggesting that the AICD recruits Mediator to the NEP promoter in a MED12-dependent manner (Fig 4D–F).
Figure 4.
Mediator is recruited to the amyloid precursor protein intracellular domain-bound NEP promoter in a MED12-dependent manner. Soluble chromatin from NB7 cells treated with (A,B) 0 or 25 μM DAPT or (D,E) control or MED12-specific siRNA was subjected to immunoprecipitation using the indicated antibodies. Immunoprecipitated chromatin was analysed by (A,D) semiquantitative or (B,E) quantitative PCR using primers specific for NEP promoter 1 (NEP-P1) or upstream (NEP-UP) sequences. In (B,E) data represent the mean±s.e.m. of at least three independent experiments performed in triplicate. Asterisks denote statistically significant differences in NEP-P1 occupancy for each protein, relative to its occupancy in either (B) DMSO-treated or (E) CNTL siRNA-treated cells. (Student's t-test *P<0.05; **P<0.005). (C,F) Western blots from representative enzyme inhibition and transient knockdown ChIP assays using the specified antibodies. AICD, amyloid precursor protein intracellular domain; C99, amyloid precursor protein carboxy-terminal fragment and γ-secretase substrate; ChIP, chromatin immunoprecipitation; CNTL, control; DMSO, dimethylsulphoxide; NEP, neprilysin; siRNA, short-interfering RNA.
Discussion
Despite increasing evidence for support a nuclear function for the AICD in gene regulation, none of the more than 20 previously reported AICD interaction partners represent a direct link to the RNA polymerase II general transcription apparatus. It has thus been difficult to determine how the AICD might activate transcription. Our identification of MED12/Mediator as a direct physical and functional target of the AICD suggests a simple solution to this problem. We propose a model in which promoter-bound AICD recruits Mediator through its MED12 interface to stimulate the assembly, activation and/or regeneration of transcription complexes on its target genes (Malik & Roeder, 2005). Alternatively, or additionally, Mediator might facilitate the imposition of an AICD-driven transcriptionally permissive chromatin environment (Ding et al, 2008).
Similarly to APP, MED12 is expressed in both developing and adult vertebrate tissues (Rocha et al, 2010). Nevertheless, early developmental phenotypes of MED12 knockout mice support a gene-specific, as opposed to a ubiquitous, function for MED12 in RNA polymerase II transcription. For example, although MED12-deficient mouse embryos die before completing gastrulation, they nevertheless develop further than those lacking the core Mediator subunits required for RNA polymerase II activity (Tudor et al, 1999; Rocha et al, 2010). Furthermore, MED12 hypomorphic mutant embryos support unperturbed development of many embryonic structures, processes and marker genes (Rocha et al, 2010). The broad distribution of MED12 might therefore endow Mediator with a unique interface through which it might be selectively recruited by diverse gene-specific transcription factors, including the AICD.
Notably, among the 33 subunits that comprise Mediator, MED12 is uniquely implicated in vertebrate neural development and disease. In zebrafish, MED12 has been shown to be required for proper development of the brain and neural crest, among other organs, where it is important in the production of monoaminergic neurons and cranial sensory ganglia (Wang et al, 2006). In humans, polymorphisms in the Xq13 gene encoding MED12 have been linked with neuropsychiatric illnesses including schizophrenia and pyschosis, and MED12 mutations have been shown to cause two X-linked mental retardation disorders: FG syndrome and Lujan syndrome (Philibert et al, 2001; Risheg et al, 2007; Schwartz et al, 2007). Our identification of MED12 as a direct ingress in Mediator for AICD-dependent nuclear signalling suggests a possible link between the biological function of APP in brain development and disease and its role in transcriptional regulation. Finally, because genetic variation in MED12 is causally linked with cognitive and behavioural dysfunction in humans, it will be interesting to determine whether polymorphisms in MED12 might also predispose certain individuals to Alzheimer disease.
Methods
Antibodies. CDK8 (sc-1521), TFIIHp89 (sc-293), TFIIEβ (sc-238), Santa Cruz Biotechnology; MED12 (A300-774A), Bethyl Laboratories; MED23 (551175), BD pharmingen; FLAG (F3165), Sigma; APP/APLP1/APLP2 (171610/171615/171616), Calbiochem/EMD Biosciences. Rabbit MED4 polyclonal antibodies were produced and purified as described (Kim et al, 2006).
Cell culture. SK–N-–SH and SK–N–AS cell lines were kindly provided by Drs Veronica Galvan and Luis Penalva (University of Texas Health Science Center at San Antonio, Texas, USA), respectively, and cultured in DMEM, 10% FBS (Hyclone). NB7 cells, a gift from Dr Jill Lahti (St Jude Children's Research Hospital, Memphis, Tennessee, USA) were cultured as described (Belyaev et al, 2009). HeLa cells were cultured as described (Kim et al, 2006).
Plasmids. pMst-AICD, pMst-APLP1ICD and pMst-APLP2ICD for transient reporter assays were generated by PCR-based sub-cloning of APP/APLP ICD-encoding cDNA sequences into the pMst vector (from Dr Thomas Südhof). pCMV5-Fe65 for transient reporter assays, pM-Fe65 and pM-hTip60 for immunoprecipitations were gifts from Dr Thomas Südhof. Plasmids pGAL4-VP16, pG5E1B-luc, pACT-β-galactosidase and p3xFLAG-CMV-hMED12r wild type and ΔPQL for transient reporter assays have been described (Kim et al, 2006; Zhou et al, 2006; Ding et al, 2008).
Transfection, reporter assays and RNAi. Cells were transfected with plasmid DNA using FuGENE 6 (Roche Applied Science); activities of luciferase (Promega Corporation) and β-galactosidase (Applied Biosystems) were determined as described previously (Zhou et al, 2006; Ding et al, 2008). For RNAi, cells were transfected with MED12-specific (M-009092-00), APP-specific (M-003731-00) or control (D-001210-01-05) siRNAs (Dharmacon; 20–40 nM) using Transit SiQuest (Mirus Bio Corporation).
Quantitative real-time reverse transcription–PCR. RNA extracted from cells 48 h after addition of DAPT (Calbiochem/EMD Biosciences) or 72 h after siRNA transfections was reverse-transcribed using oligo (dT) and superscript iii (invitrogen). Quantitative PCR was performed using a 7900HT fast real-time PCR system (Applied Biosystems) and Absolute SYBR Green ROX mix (ABgene). For each sample, the amplification plot and the corresponding dissociation curves were examined. Primer sequences are available on request.
Immunoprecipitations and ChIP. HeLa cells were collected 48 h after transfection with or without pCS3+H6T7-ICDs (APP/APLP), pM-Fe65 and pM-Tip60. Nuclear extracts—prepared as described previously (Ding et al, 2008)—were adjusted using 200 mM KCl and 0.2% NP-40, followed by incubation for 12 h at 4°C with 3 μg of either MED4-specific rabbit polyclonal antibody or rabbit IgG. Immune complexes recovered by incubation with protein A-sepharose (20 μl) for 2 h at 4°C were washed five times for 5 min each with 0.3-M KCl D buffer (0.2% NP-40), eluted in Laemmli sample buffer and resolved on 4–20% tris–glycine gradient gels (Lonza) for immunoblot analysis. Chromatin immunoprecipitation was performed as described previously (Ding et al, 2008) from NB7 cells treated with 0 or 25-μM DAPT or control, APP, or MED12-specific siRNAs using 3 μg of control or specific antibodies. Purified DNA was analysed by conventional or real-time PCR. Primer sequences have been described previously (Belyaev et al, 2009).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank T. Südhof, J. Lahti, V. Galvan and L. Penalva for reagents. This work was supported by grant MH085320 from the National Institute of Mental Health (TGB).
Footnotes
The authors declare that they have no conflict of interest.
References
- Alves da Costa C, Sunyach C, Pardossi-Piquard R, Sevalle J, Vincent B, Boyer N, Kawarai T, Girardot N, St George-Hyslop P, Checler F (2006) Presenilin-dependent γ-secretase-mediated control of p53-associated cell death in Alzheimer's disease. J Neurosci 26: 6377–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev ND, Nalivaeva NN, Makova NZ, Turner AJ (2009) Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep 10: 94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Sudhof TC (2001) A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293: 115–120 [DOI] [PubMed] [Google Scholar]
- Ding N et al. (2008) Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol Cell 31: 347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Pimplikar SW (2001) The γ-secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc Natl Acad Sci USA 98: 14979–14984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C (2004) Take five—BACE and the γ-secretase quartet conduct Alzheimer's amyloid β-peptide generation. EMBO J 23: 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysseune S, Kienlen-Campard P, Hebert S, Tasiaux B, Leroy K, Devuyst O, Brion JP, De Strooper B, Octave JN (2009) Epigenetic control of aquaporin 1 expression by the amyloid precursor protein. FASEB J 23: 4158–4167 [DOI] [PubMed] [Google Scholar]
- Ito M, Yuan CX, Malik S, Gu W, Fondell JD, Yamamura S, Fu ZY, Zhang X, Qin J, Roeder RG (1999) Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol Cell 3: 361–370 [DOI] [PubMed] [Google Scholar]
- Kim S, Xu X, Hecht A, Boyer TG (2006) Mediator is a transducer of Wnt/β-catenin signaling. J Biol Chem 281: 14066–14075 [DOI] [PubMed] [Google Scholar]
- Kornberg RD (2005) Mediator and the mechanism of transcriptional activation. Trends Biochem Sci 30: 235–239 [DOI] [PubMed] [Google Scholar]
- Li Q, Sudhof TC (2004) Cleavage of amyloid-β precursor protein and amyloid-β precursor-like protein by BACE 1. J Biol Chem 279: 10542–10550 [DOI] [PubMed] [Google Scholar]
- Lorent K, Overbergh L, Moechars D, De Strooper B, Van Leuven F, Van den Berghe H (1995) Expression in mouse embryos and in adult mouse brain of three members of the amyloid precursor protein family, of the α-2-macroglobulin receptor/low density lipoprotein receptor-related protein and of its ligands apolipoprotein E, lipoprotein lipase, α-2-macroglobulin and the 40,000 molecular weight receptor-associated protein. Neuroscience 65: 1009–1025 [DOI] [PubMed] [Google Scholar]
- Malik S, Roeder RG (2005) Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem Sci 30: 256–263 [DOI] [PubMed] [Google Scholar]
- Mucke L (2009) Neuroscience: Alzheimer's disease. Nature 461: 895–897 [DOI] [PubMed] [Google Scholar]
- Muller T, Concannon CG, Ward MW, Walsh CM, Tirniceriu AL, Tribl F, Kogel D, Prehn JH, Egensperger R (2007) Modulation of gene expression and cytoskeletal dynamics by the amyloid precursor protein intracellular domain (AICD). Mol Biol Cell 18: 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardossi-Piquard R et al. (2005) Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of βAPP and APLP. Neuron 46: 541–554 [DOI] [PubMed] [Google Scholar]
- Philibert RA, Sandhu HK, Hutton AM, Wang Z, Arndt S, Andreasen NC, Crowe R, Wassink TH (2001) Population-based association analyses of the HOPA12bp polymorphism for schizophrenia and hypothyroidism. Am J Med Genet 105: 130–134 [PubMed] [Google Scholar]
- Risheg H et al. (2007) A recurrent mutation in MED12 leading to R961W causes Opitz-Kaveggia syndrome. Nat Genet 39: 451–453 [DOI] [PubMed] [Google Scholar]
- Rocha PP, Scholze M, Bleiss W, Schrewe H (2010) Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development 137: 2723–2731 [DOI] [PubMed] [Google Scholar]
- Scheinfeld MH, Ghersi E, Laky K, Fowlkes BJ, D'Adamio L (2002) Processing of β-amyloid precursor-like protein-1 and -2 by γ-secretase regulates transcription. J Biol Chem 277: 44195–44201 [DOI] [PubMed] [Google Scholar]
- Schwartz CE et al. (2007) The original Lujan Syndrome Family has a novel missense mutation (p.N1007S) in the MED12 gene. J Med Genet 16: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachida Y et al. (2008) Interleukin-1 β up-regulates TACE to enhance α-cleavage of APP in neurons: resulting decrease in Abeta production. J Neurochem 104: 1387–1393 [DOI] [PubMed] [Google Scholar]
- Tillement L, Lecanu L, Yao W, Greeson J, Papadopoulos V (2006) The spirostenol (22R, 25R)-20α-spirost-5-en-3β-yl hexanoate blocks mitochondrial uptake of Abeta in neuronal cells and prevents Abeta-induced impairment of mitochondrial function. Steroids 71: 725–735 [DOI] [PubMed] [Google Scholar]
- Tudor M, Murray PJ, Onufryk C, Jaenisch R, Young RA (1999) Ubiquitous expression and embryonic requirement for RNA polymerase II coactivator subunit Srb7 in mice. Genes Dev 13: 2365–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Minogue AM, Sala Frigerio C, Fadeeva JV, Wasco W, Selkoe DJ (2007) The APP family of proteins: similarities and differences. Biochem Soc Trans 35: 416–420 [DOI] [PubMed] [Google Scholar]
- Wang X, Yang N, Uno E, Roeder RG, Guo S (2006) A subunit of the mediator complex regulates vertebrate neuronal development. Proc Natl Acad Sci USA 103: 17284–17289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Koo EH (2006) The amyloid precursor protein: beyond amyloid. Mol Neurodegener 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Kim S, Ishii S, Boyer TG (2006) Mediator modulates Gli3-dependent Sonic hedgehog signaling. Mol Cell Biol 26: 8667–8682 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.