A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing
RAD21L is identified as a new meiosis-specific cohesin with a unique spatiotemporal distribution. RAD21L and REC8 have symmetrical, mutually exclusive localization on the not-yet-synapsed homologues, implying that cohesins could establish a code for homologue recognition.
Keywords: cohesin, meiosis, chromosome segregation, homologue synapsis, centromere
Abstract
We identify a new mammalian cohesin subunit, RAD21-like protein (RAD21L), with sequence similarity to RAD21 and REC8. RAD21L localizes along axial elements in early meiotic prophase, in a manner that is spatiotemporally different to either REC8 or RAD21. Remarkably, RAD21L and REC8 have symmetrical, mutually exclusive localization on the not-yet-synapsed homologues, implying that the cohesin patterning could provide a code for homologue recognition. RAD21 transiently localizes to axial elements after the dissociation of RAD21L and REC8 in late pachytene, a period of recombination repair. Further, we show that the removal of cohesins and synaptonemal complex during late meiotic prophase is promoted by Polo-like kinase 1, which is similar to the mitotic prophase pathway.
Introduction
When chromosomes are replicated in S-phase, sister chromatids are held together by a multi-protein complex—cohesin—consisting of structural maintenance of chromosomes (SMC) subunits (SMC1 and SMC3) and non-SMC subunits (stromal antigen (SA)1/2 and kleisin RAD21/SCC1; Nasmyth & Haering, 2009). In mitotic prophase, a large amount of cohesin dissociates from the chromosome arms, by a process known as the ‘prophase pathway' that depends on the phosphorylation of cohesin by Polo-like kinase 1 (PLK1) during prophase and prometaphase (Hirano, 2000; Hauf et al, 2005; Peters et al, 2008). At the onset of anaphase, residual cohesin along the chromosomes is cleaved by separase, leading to chromosome separation (Nasmyth & Haering, 2009).
The meiotic cell-cycle consists of a single DNA replication followed by two rounds of chromosome segregation (meiosis I and II). The cohesin complex in meiosis differs from that in mitosis, as SCC1/RAD21 is mostly replaced by a meiotic counterpart, REC8 (Watanabe, 2004). In mammalian germ cells, other meiosis-specific cohesin subunits, SA3 and SMC1β, are also expressed (Prieto et al, 2001; Revenkova et al, 2001). During prophase I, sister chromatids are organized into proteinaceous structures of axial elements, on which the synaptonemal complex is assembled to promote homologue synapsis, meiotic recombination and crossover resolution, a process yielding chiasmata between homologues (Baker et al, 1996; Edelmann et al, 1996; Romanienko & Camerini-Otero, 2000; Lipkin et al, 2002; Page & Hawley, 2004). Cohesin, which interacts with the synaptonemal complex components and localizes along axial elements, might act as a basis for synaptonemal complex assembly (Eijpe et al, 2000; Pelttari et al, 2001; Prieto et al, 2001; Revenkova et al, 2001; Lee et al, 2003). In SMC1β-deficient mouse, axial elements are shortened, chromatin loops are extended and homologous synapsis is incomplete (Revenkova et al, 2004; Novak et al, 2008). Thus, cohesin is crucial not only for sister chromatid cohesion, but also for various meiosis-specific chromosomal events. In yeast, meiosis-specific kleisin REC8 is required for several chromosomal events including synaptonemal complex formation, reciprocal recombination, monopolar attachment of sister chromatids and protection of centromeric cohesion (DeVeaux & Smith, 1994; Klein et al, 1999; Parisi et al, 1999; Watanabe & Nurse, 1999). Remarkably, these meiosis-specific properties of chromosomes are not restored by ectopic expression of RAD21 in Rec8 mutant yeast cells, even though sister chromatid cohesion is restored by RAD21-cohesin (Toth et al, 2000; Yokobayashi et al, 2003). Thus, the kleisin subunit of cohesin seems to be important for the meiosis-specific function of the cohesin complex. In mammals, meiosis-specific kleisin REC8 is thought to be important for proper synapsis (Bannister et al, 2004; Xu et al, 2005). Although it has been thought that REC8 is the only meiosis-specific kleisin in mammals, we report the isolation of a novel meiosis-specific kleisin subunit, RAD21L, which together with RAD21 and REC8 might have a spatiotemporally distinct role in the promotion of chromosome dynamics in mammalian meiosis.
Results and Discussion
Identification of the novel cohesin subunit RAD 21L
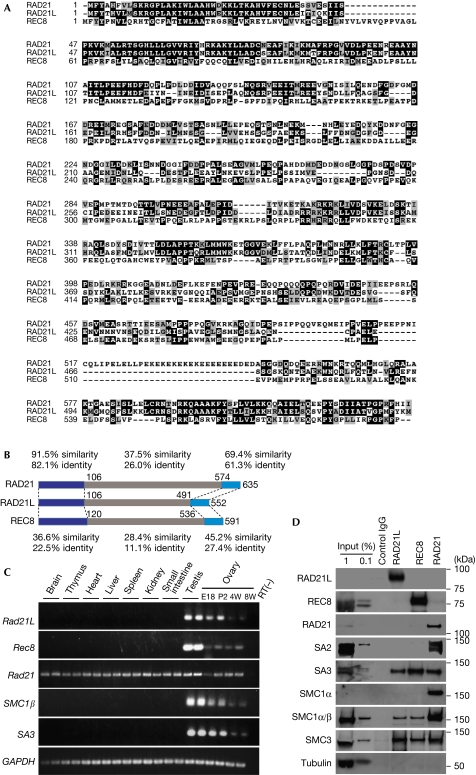
During liquid chromatography mass spectrometry/mass spectrometry analysis of centromere protein (CENP-C)-associated factors immunoprecipitated from mouse testis chromatin, we identified a hypothetical protein LOC668929 (supplementary Fig S1 online). Our BLAST search showed that LOC668929 is similar to kleisin family proteins (RAD21 and REC8) of the cohesin complex (Fig 1A) and this protein is conserved in other vertebrate species (supplementary Fig S2 online). As LOC668929 is more similar to RAD21 than REC8 (Fig 1B), we named LOC668929 as a RAD21-like protein (RAD21L). Reverse transcription–PCR demonstrated that the mRNA of Rad 21L is expressed in both the testis and ovary, similar to other meiosis-specific Rec8, SA3 and SMC1β (Fig 1C). Expression of Rad21L is detectable in the testis by postnatal day 7, approximately at the time when the first wave of meiosis begins (Anderson et al, 2008), indicating that Rad21L is expressed concomitantly with meiotic entry (supplementary Fig S4 online).
Figure 1.
Identification of a new cohesin subunit RAD21L. (A) Amino-acid sequence alignment of mouse RAD21L, RAD21 and REC8. The RAD21L sequence has been submitted to the DDBJ, EMBL and GenBank databases under accession number AB602048. (B) Schematic showing the amino-acid similarity and identity of RAD21L compared with REC8 and RAD21. (C) Tissue specificity of mRNA expression for the indicated genes was analysed by RT–PCR. (D) Immunoprecipitates from spermatocyte extracts obtained using RAD21L, REC8 and RAD21 antibodies or control immunoglobulin G (IgG) were analysed by western blotting using antibodies against the indicated proteins. Input: 1 and 0.1% of whole soluble extract. E, embryonic day; IgG, immunoglobulin G; P, postnatal; RT–PCR, reverse transcription PCR; RT(–), control PCR; W, week.
The core cohesin complex is composed of two SMC proteins (SMC 1 and SMC 3) and two non-SMC subunits (kleisin and SA). In mouse, SMC1α and SA1/SA2 are ubiquitously expressed in somatic cells and in germ cells, whereas their paralogues, SMC1β and SA3, are meiosis specific. To determine whether RAD21L is a subunit of cohesin and identify the subunits with which it forms a complex, we performed immunoprecipitation using specific antibodies recognizing each kleisin subunit, REC8, RAD21 and RAD21L, in testis extracts (Fig 1D). The results indicate that substantial fractions of RAD21L and REC8 form distinct complexes with meiosis-specific subunits SMC1β and SA3, but much less with ubiquitous SMC1α or SA1/SA2, whereas RAD21 associates with both mitotic and meiotic versions of SMC1 and SA. Our data do not exclude the possibility that a subtle fraction of REC8 might also associate with SMC1α, as reported previously (Revenkova et al, 2004). Thus, we conclude that RAD21L is a novel meiosis-specific cohesin subunit, and that at least three different types of cohesin complexes exist in mammalian meiotic cells.
RAD21L localizes along the axial elements in meiosis
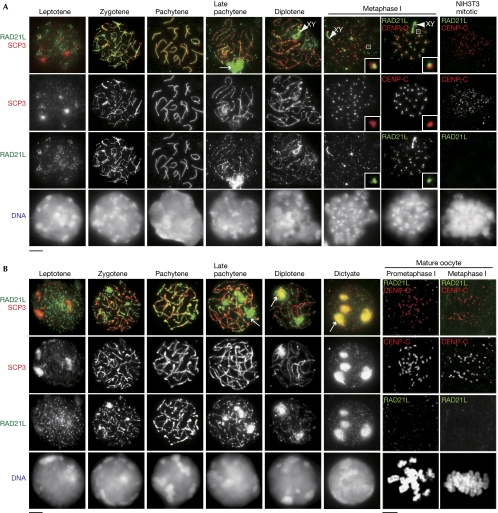
We immunostained RAD21L in spermatocytes with SCP3, a component of synaptonemal complex, whose staining pattern can define meiotic stage (Fig 2A). RAD21L first becomes detectable around the heterochromatic regions in leptotene and localizes along axial elements during zygotene when homologous chromosomes are synapsed. During pachytene, fully synapsed axial elements are strongly labelled with RAD21L. From late pachytene onwards, RAD21L gradually dissociates from axial elements with the production of self-assembled poly-complexes or aggregants in the nucleus. During diplotene, dissociation of RAD21L from axial elements progresses on autosomes, but not on XY chromosomes. At metaphase I, residual RAD21L cohesin remains on the chromosome arm and partly colocalizes with SCP3, suggesting that RAD21L is retained at or close to the centromere regions where SCP3 progressively accumulates at metaphase I (Parra et al, 2004). This observation was further confirmed by colocalization of RAD21L with centromere protein CENP-C (Fig 2A). In a control experiment using NIH3T3 mitotic cells, RAD21L is not detectable (Fig 2A), indicating that our RAD21L antibody specifically recognizes RAD21L, but does not cross-react with RAD21 (supplementary Fig S3 online). The overall localization pattern of RAD21L in spermatocytes seems to be similar to that of REC8 (Eijpe et al, 2003; Lee et al, 2003).
Figure 2.
RAD21L cohesin localizes along axial elements in both spermatocytes and oocytes during meiosis. (A) Chromosome spreads of spermatocytes were stained with RAD21L and SCP3 antibodies. Arrow: RAD21L aggregates. Arrowhead: XY body. NIH3T3 mitotic cells were stained with RAD21L and CENP-C antibodies. (B) Chromosome spreads of fetal oocytes were stained as shown in (A). Oocytes at 2 h (Prometaphase I) and 6 h (Metaphase I) after GVBD were stained with RAD21L and CENP-C antibodies as indicated. Scale bars, 5 μm. CENP-C, centromere protein; GVBD, germinal vesicle breakdown.
Next, we immunostained RAD21L cohesin in oocytes (Fig 2B). In fetal oocytes, RAD21L cohesin is found in leptotene, loaded along the axial element from zygotene until pachytene, and disappears in late pachytene, a pattern similar to that in spermatocytes. From late pachytene until dictyate arrest stage, the dissociated pools of RAD21L and SCP3 produce aggregants, presumably at the nucleoli (Prieto et al, 2004). Although REC8 is detectable around centromeres at prometaphase I or metaphase I in mature oocytes (Lee et al, 2006), RAD21L and RAD21 are not detectable at these stages, suggesting sexual dimorphism of the requirement for cohesins. These observations are consistent with the notion that REC8 cohesin is solely responsible for chromosome arm and centromere cohesion at metaphase I in oocytes (Tachibana-Konwalski et al, 2010).
Mutually exclusive localization of RAD21L and REC8
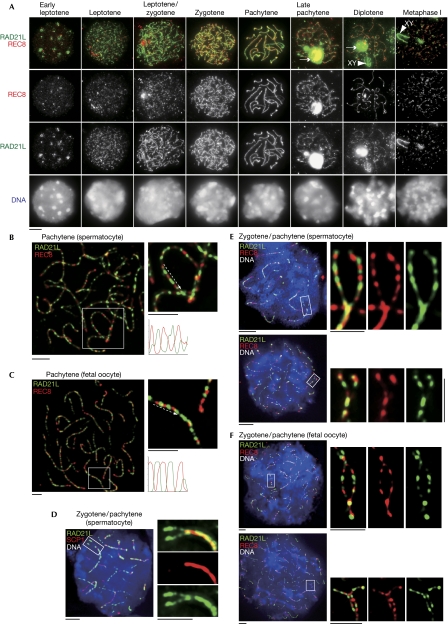
We compared the localizations of REC8 and RAD21L in spermatocytes for differences in their dynamics on chromosomes (Fig 3A). Although both REC8 and RAD21L become detectable in leptotene, RAD21L localizes along the axial elements earlier than REC8 during late leptotene/early zygotene, implying that REC8 and RAD21L might have different roles in the initial axial element formation. The localization of both REC8 and RAD21L along the axial elements reaches a maximum level in pachytene, and then they gradually dissociate in late pachytene (Fig 3A).
Figure 3.
Comparative localization of RAD21L and REC8 cohesins during meiosis. (A) Chromosome spreads of spermatocytes were stained with RAD21L and REC8 antibodies. Arrow: RAD21L aggregates. Arrowhead: XY body. (B,C) Immunostaining of chromosome spreads of (B) pachytene spermatocytes and (C) pachytene fetal oocytes. Magnified images are shown on the right. Line scans of green/red signals along paired chromosomes (dashed arrow) were performed and the plots are shown (bottom right). (D) Immunostaining of chromosome spreads of spermatocytes at zygotene/pachytene transition with RAD21L and SCP1, a component of central element of synaptonemal complex, antibodies. Magnified images of the two not-yet-synapsed homologous chromosomes are shown on the right. (E, F) Immunostaining of chromosome spreads of (E) spermatocytes and (F) fetal oocytes at zygotene/pachytene transition with RAD21L and REC8 antibodies. Magnified images of the two not-yet-synapsed homologous chromosomes are shown on the right. Scale bars, 5 μm.
Magnified views showed mutually exclusive localization rather than colocalization of RAD21L and REC8 along the axial elements during pachytene in spermatocytes and oocytes (Fig 3B,C). Intriguingly, the alternative localization patterns of RAD21L and REC8 on the two not-yet-synaped homologous chromosomes show a symmetry (Fig 3D–F), indicating that RAD21L and REC8 cohesin-enriched regions are already established on each homologue before synapsis. This localization patterns suggest that RAD21L and REC8 might have their intrinsic loading sites on the chromosomes and form distinct cohesin-enriched domains along the axial elements. Although synaptonemal complex formation and synapsis are partly defective in REC8-deficient mice, pairs of homologues are still nonrandomly aligned in close proximity (Bannister et al, 2004; Xu et al, 2005), suggesting that homologue pairing has initiated in the absence of REC8. Our findings raise the possibility that if RAD21L and REC8, including cohesin complexes, interact in a self-selective manner, the pattern of RAD21L and REC8 distribution along the chromosomes might provide a ‘barcode' to facilitate finding the proper pairing partner before the recombination-dependent tight association of homologues. In any case, the residual ability of the REC8-deficient mouse to achieve homologue pairing might depend on the alternative kleisin RAD21L rather than RAD21 (see below).
RAD21 transiently localizes on axial elements
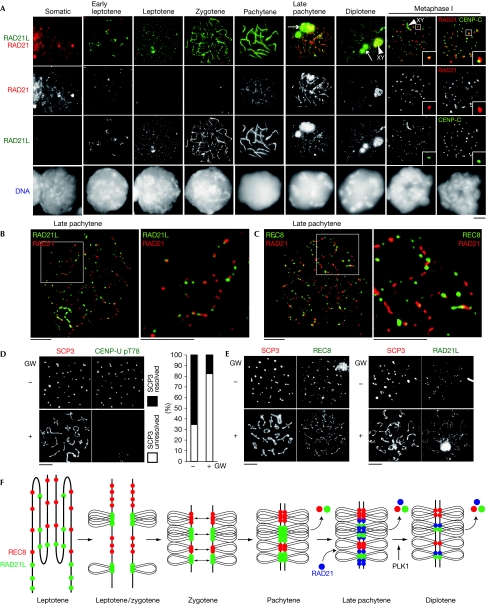
We comparatively examined the localizations of RAD21 and RAD21L by using immunostaining (Fig 4A). As the amino-acid sequences of RAD21 and RAD21L are similar in the amino-terminal and carboxy-terminal regions, we carefully prepared antibodies to avoid cross-reaction (supplementary Fig S3 online). Although RAD21 is detectable in mitotic cells, it disappears in nuclei from early leptotene until zygotene, whereas RAD21L appears along axial elements. RAD21 transiently reappears on axial elements in late pachytene when REC8 and RAD21L start to dissociate (Figs 3A and 4A). Crucially, RAD21 rarely colocalizes with either RAD21L or REC8 (Fig 4B,C), suggesting that RAD21 replaces RAD21L and REC8 or localizes at different sites to have a specialized role. From diplotene onward, RAD21 dissociates from axial elements and finally remains around the centromeres at metaphase I, in agreement with the results of a previous study (Parra et al, 2004). As RAD21 emerges on axial elements during pachytene when crossover resolution progresses (Baker et al, 1996; Edelmann et al, 1996; Lipkin et al, 2002), loading of RAD21-type cohesin might be promoted by DNA-damage repair-dependent mechanisms (Potts et al, 2006; Ström et al, 2007; Ünal et al, 2007). By contrast, RAD21L- and REC8-type cohesins becomes detectable on chromatin in early leptotene (Fig 3A), suggesting that these cohesins are involved in canonical sister chromatid cohesion; established coupling with pre-meiotic DNA replication.
Figure 4.
RAD21 cohesin transiently localizes on axial element during late meiotic prophase. (A) Chromosome spreads of spermatocytes were stained with RAD21L and RAD21 antibodies. Metaphase I spermatocytes (far right) were stained with CENP-C and RAD21 antibodies. Arrow: RAD21L aggregates. Arrowhead: XY body. (B) Late pachytene spermatocytes were stained with RAD21L and RAD21 antibodies or (C) REC8 and RAD21 antibodies. Magnified images are shown on the right. (D) Chromosome spreads of diakinesis/metaphase I cells with or without GW843682X (GW) were stained with SCP3 and CENP-U pT78 antibodies. The staining patterns were classified into two categories and quantified (n=32, 39 for cells with and without GW, respectively). (E) Chromosome spreads of diakinesis/metaphase I cells with or without GW were stained with SCP3 and REC8 or RAD21L antibodies. (F) Schematic illustration of the localization and dissociation of RAD21L, REC8 and RAD21 cohesins along the axial element during meiotic prophase. Scale bars, 5 μm. PLK1, Polo-like kinase 1.
It has been proposed previously that RAD21 is involved in sister chromatid cohesion and localizes along the axial elements throughout all stages of prophase until metaphase I (Parra et al, 2004; Xu et al, 2004), but our analysis does not detect RAD21, at least in zygotene. One reason for this discrepancy might be the cross-reactivity of RAD21 antibodies with RAD21L in previous studies (Parra et al, 2004; Xu et al, 2004; supplementary Fig S3 online). In REC8-deficient mice, sister chromatid cohesion and axial element formation are still preserved, suggesting that a kleisin other than REC8 might be integral to meiotic sister chromatid cohesion (Bannister et al, 2004; Xu et al, 2005). Our results indicate that this alternative kleisin is RAD21L, not than RAD21.
PLK1 promotes dissociation of cohesin and SC
The meiotic cohesins together with the synaptonemal complex component SCP3 mostly, but not entirely, dissociate from axial elements from late prophase until metaphase I. As PLK1 is crucial for the prophase dissociation of cohesin from chromosome arms in mitosis (Losada et al, 2002; Sumara et al, 2002), we examined the possibility that the removal of meiotic cohesins in late meiotic prophase depends on PLK1. In vitro cultures of spermatocytes were treated with or without PLK1 inhibitor GW843682X and induced to undergo metaphase I by the addition of okadaic acid. Cells at diakinesis/metaphase I were immunostained for SCP3 and CENP-U pT78, a substrate of PLK1 (Kang et al, 2006). In control cells, SCP3 is lost from the chromosome arm regions, instead accumulating at centromeres with a reduction in axial element length. However, in CENP-U pT78-negative cells, SCP3 is still mostly retained along the axial elements with less compaction (Fig 4D). In a comparative experiment using REC8 and RAD21L antibodies, we found that both REC8 and RAD21L are retained more along the axial elements than control cells (Fig 4E). We cannot exclude the possibility that inhibition of PLK1 under this condition might indirectly delay the progression into metaphase I. However, these results indicate that PLK1 might promote the dissociation of SCP3 and cohesin from axial elements in late meiotic prophase, presumably to facilitate chromosome condensation. This observation is similar to that in budding yeast meiosis, where PLK/CDC5 drives the exit from pachytene and synaptonemal complex breakdown (Sourirajan & Lichten, 2008) while removing a subpopulation of cohesin from chromosomes before the onset of anaphase I (Yu & Koshland, 2005). Thus, our results indicate that PLK1-dependent synaptonemal complex disassembly is conserved in yeast and animal meiosis. It has been shown that meiotic cohesin associates with synaptonemal complex components (Eijpe et al, 2000), and that, REC8 is required for full assembly of synaptonemal complex (Xu et al, 2005). As synaptonemal complex disassembly roughly coincides with cohesin dissociation (Figs 2 and 4E), it is possible that the PLK1 targets responsible for synaptonemal complex disassembly might be cohesin complexes. Whereas the PLK1 target remains to be identified, this possibility seems appealing if synaptonemal complex disassembly is related to the prophase pathway in mammalian mitosis, in which cohesin SA2 is the crucial target of PLK1 (Hauf et al, 2005).
In summary, our results show that mammalian germ cells express at least three distinct types of cohesin complex, each of which includes REC8, RAD21L or RAD21, and might have spatiotemporally different divisions of labour for the progression of meiosis (Fig 4F). Their distinct dynamics are provided mainly by the kleisin subunit. These meiotic cohesin complexes are assumed to function not only in sister chromatid cohesion, but also in specialized chromosomal events in meiotic progression such as axial element formation, homologue pairing and the assembly/disassembly of synaptonemal complex. This notion is consistent with evidence from Caenorhabditis elegans meiosis that REC8 and its paralogues COH3 and COH4 cohesins are required not only for sister chromatid cohesion, but also for axial element formation and synaptonemal complex assembly in distinct ways (Severson et al, 2009). The distribution patterns of cohesins might facilitate the twisting of two homologues for pairing during early prophase so that the axial elements on the homologues are progressively coiled around each other, as suggested in maize where AFD1/REC8 shows a bilaterally symmetrical pattern on the paired axes (Wang et al, 2009). The cohesin barcode model might also be applicable to organisms such as yeast, which express single meiosis-specific kleisin REC8, because REC8 localizes along the chromatin, forming a uniquely spaced distribution (Lengronne et al, 2004; Kim et al, 2010). Further studies including the generation of RAD21L-knockout and RAD21L/REC8-double-knockout mice will show the crucial functions of meiosis-specific cohesins in mammals.
Methods
Immunostaining of chromosome spreads from spermatocytes and fetal oocytes and the culture of okadaic acid-induced metaphase I spermatocytes were performed as described (Tanno et al, 2010). Culture and immunostaining of mature oocytes were performed as described (Lee et al, 2008). All methodological details referring to antibodies, reagents, preparation of testis extracts, immunoprecipitation, RT–PCR and mass spectrometry are presented in the supplementary information online. Animal experiments were approved by the Institutional Animal Care and Use Committee (Permission number 20001).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank J. Lee and A. Nambu for reagents and technical advice and S. Kakuta, M. Kadoki, R. Hayashi, I. Takada and the Gotoh laboratory for materials. We also thank P. Carlton, A. Sato and all the members of our laboratory for their valuable support and discussion. This work was supported in part by The Uehara Memorial Foundation, The Sumitomo Foundation, a Grant-in-Aid for Young Scientists (to K.I.), the Global Center of Excellence (COE) Program (Integrative Life Science Based on the Study of Biosignalling Mechanisms), Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and a Grant-in-Aid for Specially Promoted Research from the MEXT, Japan (to Y.W.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC (2008) Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA 105: 14976–14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SM et al. (1996) Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet 13: 336–342 [DOI] [PubMed] [Google Scholar]
- Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC (2004) Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 40: 184–194 [DOI] [PubMed] [Google Scholar]
- DeVeaux LC, Smith GR (1994) Region-specific activators of meiotic recombination in Schizosaccharomyces pombe. Genes Dev 8: 203–210 [DOI] [PubMed] [Google Scholar]
- Edelmann W et al. (1996) Meiotic pachytene arrest in MLH1-deficient mice. Cell 85: 1125–1134 [DOI] [PubMed] [Google Scholar]
- Eijpe M, Heyting C, Gross B, Jessberger R (2000) Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci 113: 673–682 [DOI] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C (2003) Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1β and SMC3. J Cell Biol 160: 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM (2005) Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol 3: e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T (2000) Chromosome cohesion, condensation, and separation. Annu Rev Biochem 69: 115–144 [DOI] [PubMed] [Google Scholar]
- Kang YH et al. (2006) Self-regulated Plk1 recruitment to kinetochores by the Plk1–PBIP1 interaction is critical for proper chromosome segregation. Mol Cell 24: 409–422 [DOI] [PubMed] [Google Scholar]
- Kim KP, Weiner BM, Zhang L, Jordan A, Dekker J, Kleckner N (2010) Sister cohesion and structural axis components mediate homolog bias of meiotic recombination. Cell 143: 924–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Mahr P, Galova M, Buonomo SB, Michaelis C, Nairz K, Nasmyth K (1999) A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98: 91–103 [DOI] [PubMed] [Google Scholar]
- Lee J, Iwai T, Yokota T, Yamashita M (2003) Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci 116: 2781–2790 [DOI] [PubMed] [Google Scholar]
- Lee J, Okada K, Ogushi S, Miyano T, Miyake M, Yamashita M (2006) Loss of Rec8 from chromosome arm and centromere region is required for homologous chromosome separation and sister chromatid separation, respectively, in mammalian meiosis. Cell Cycle 5: 1448–1455 [DOI] [PubMed] [Google Scholar]
- Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y (2008) Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 10: 42–52 [DOI] [PubMed] [Google Scholar]
- Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly GP, Itoh T, Watanabe Y, Shirahige K, Uhlmann F (2004) Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430: 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin SM et al. (2002) Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet 31: 385–390 [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T (2002) Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev 16: 3004–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43: 525–558 [DOI] [PubMed] [Google Scholar]
- Novak I, Wang H, Revenkova E, Jessberger R, Scherthan H, Höög C (2008) Cohesin Smc1β determines meiotic chromatin axis loop organization. J Cell Biol 180: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SL, Hawley RS (2004) The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 20: 525–558 [DOI] [PubMed] [Google Scholar]
- Parisi S, McKay MJ, Molnar M, Thompson MA, van der Spek PJ, van Drunen-Schoenmaker E, Kanaar R, Lehmann E, Hoeijmakers JH, Kohli J (1999) Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol Cell Biol 19: 3515–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MT, Viera A, Gómez R, Page J, Benavente R, Santos JL, Rufas JS, Suja JA (2004) Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci 117: 1221–1234 [DOI] [PubMed] [Google Scholar]
- Pelttari J et al. (2001) A meiotic chromosomal core consisting of cohesin complex proteins recruits DNA recombination proteins and promotes synapsis in the absence of an axial element in mammalian meiotic cells. Mol Cell Biol 21: 5667–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Tedeschi A, Schmitz J (2008) The cohesin complex and its roles in chromosome biology. Genes Dev 22: 3089–3114 [DOI] [PubMed] [Google Scholar]
- Potts PR, Porteus MH, Yu H (2006) Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. EMBO J 26: 3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I, Suja JA, Pezzi N, Kremer L, Martínez-A C, Rufas JS, Barbero JL (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 3: 761–766 [DOI] [PubMed] [Google Scholar]
- Prieto I, Tease C, Pezzi N, Buesa JM, Ortega S, Kremer L, Martínez A, Martínez-A C, Hultén MA, Barbero JL (2004) Cohesin component dynamics during meiotic prophase I in mammalian oocytes. Chromosome Res 12: 197–213 [DOI] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting C, Gross B, Jessberger R (2001) Novel meiosis-specific isoform of mammalian SMC1. Mol Cell Biol 21: 6984–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R (2004) Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol 6: 555–562 [DOI] [PubMed] [Google Scholar]
- Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6: 975–987 [DOI] [PubMed] [Google Scholar]
- Severson AF, Ling L, van Zuylen V, Meyer BJ (2009) The axial element protein HTP-3 promotes cohesin loading and meiotic axis assembly in C. elegans to implement the meiotic program of chromosome segregation. Genes Dev 23: 1763–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourirajan A, Lichten M (2008) Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev 22: 2627–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ström L, Karlsson C, Lindroos HB, Wedahl S, Katou Y, Shirahige K, Sjögren C (2007) Postreplicative formation of cohesion is required for repair and induced by a single DNA break. Science 317: 242–245 [DOI] [PubMed] [Google Scholar]
- Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM (2002) The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell 9: 515–525 [DOI] [PubMed] [Google Scholar]
- Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K (2010) Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 24: 2505–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno Y, Kitajima TS, Honda T, Ando Y, Ishiguro K, Watanabe Y (2010) Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev 24: 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A, Rabitsch KP, Gálová M, Schleiffer A, Buonomo SB, Nasmyth K (2000) Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologues during meiosis I. Cell 103: 1155–1168 [DOI] [PubMed] [Google Scholar]
- Ünal E, Heidinger-Pauli JM, Koshland D (2007) DNA double-strand breaks trigger genome-wide sister-chromatid cohesion through Eco1 (Ctf7). Science 317: 245–248 [DOI] [PubMed] [Google Scholar]
- Wang CR, Carlton PM, Golubovskaya IN, Cande WZ (2009) Interlock formation and coiling of meiotic chromosome axes during synapsis. Genetics 183: 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y (2004) Modifying sister chromatid cohesion for meiosis. J Cell Sci 117: 4017–4023 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nurse P (1999) Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400: 461–464 [DOI] [PubMed] [Google Scholar]
- Xu H, Beasley M, Verschoor S, Inselman A, Handel MA, McKay MJ (2004) A new role for the mitotic RAD21/SCC1 cohesin in meiotic chromosome cohesion and segregation in the mouse. EMBO Rep 5: 378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ (2005) Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 8: 949–961 [DOI] [PubMed] [Google Scholar]
- Yokobayashi S, Yamamoto M, Watanabe Y (2003) Cohesins determine the attachment manner of kinetochores to spindle microtubules at meiosis I in fission yeast. Mol Cell Biol 23: 3965–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HG, Koshland D (2005) Chromosome morphogenesis: condensin-dependent cohesin removal during meiosis. Cell 123: 397–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.