Abstract
Background
Industrial food animal production employs many of the same antibiotics or classes of antibiotics that are used in human medicine. These drugs can be administered to food animals in the form of free-choice medicated feeds (FCMF), where animals choose how much feed to consume. Routine administration of these drugs to livestock selects for microorganisms that are resistant to medications critical to the treatment of clinical infections in humans.
Objectives
In this commentary, we discuss the history of medicated feeds, the nature of FCMF use with regard to dose delivery, and U.S. policies that address antimicrobial drug use in food animals.
Discussion
FCMF makes delivering a predictable, accurate, and intended dose difficult. Overdosing can lead to animal toxicity; underdosing or inconsistent dosing can result in a failure to resolve animal diseases and in the development of antimicrobial-resistant microorganisms.
Conclusions
The delivery of antibiotics to food animals for reasons other than the treatment of clinically diagnosed disease, especially via free-choice feeding methods, should be reconsidered.
Keywords: antibiotic resistance, antibiotics, antimicrobials, cow, feed blocks, industrial food animal production, livestock, medicated feed supplements, poultry, swine
Animal feed is a broad term that, for animals produced for human consumption (e.g., dairy and beef cattle, hogs, layer hens, broiler chickens, turkeys), includes a diet that is specific to an animal’s species, age, and production stage, and may vary according to time of year and plant species grown (Forbes 2007). Feeds are often provided to food animals on a “free-choice” basis, which means that animals elect whether or not to eat the feed and how much of it to ingest. Feeds containing medically active ingredients, such as antibiotic and antiparasitic drugs or insecticides, that are fed on a “free-choice” basis, are designated free-choice medicated feeds (FCMF) by the U.S. Food and Drug Administration (FDA 2010h). The FDA has approved 685 drugs for medicated feed, some of which are consumed on a free-choice basis (for product list in a searchable database, see FDA 2010d).
The FDA reported that 13.1 million kg of antimicrobial drugs were sold or distributed for use in food-producing animals in 2009 (FDA 2010i). Many of the same antibiotics or classes of antibiotics used to treat clinical infections in humans are also used in industrial food animal production (IFAP) (McEwen and Fedorka-Cray 2002).
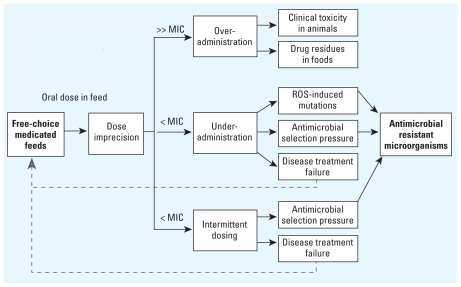
The use of FCMF in food animals has been associated with imprecise drug intake, leading to under- or overadministration of drugs (Figure 1) (Bogan and Marriner 1983; Hall 2000; Toutain et al. 2010). Overadministration of drugs may lead to animal toxicity (Guardabassi and Kruse 2008; Hall 2000) and the presence of drug residues in meat or milk, although this is rare (Burch et al. 2008). Underadministration or inconsistent administration of drugs may lead to animal treatment failure (Burch et al. 2008) or the emergence of antibiotic-resistant strains of microorganisms in food animals (Guardabassi and Kruse 2008; Khachatourians 1998; Lees et al. 2006). Antibiotic-resistant commensal and environmental bacteria can contribute to maintaining or perpetuating a reservoir of resistance genes (Chee-Sanford et al. 2009; Silbergeld et al. 2008; Wright 2007), and these bacteria can share genes for antibiotic resistance with pathogenic bacteria via horizontal gene transfer (Andremont 2000). Multiple resistance genes travel on the same mobile genetic element (e.g., plasmid), allowing one pharmaceutical to select for microorganisms that are resistant to multiple classes of antibiotics (Wright 2007). In addition to natural selection and horizontal gene transfer as mechanisms for resistance, sublethal bactericidal antibiotic use at doses below those expected to provide overt selective pressure induces mutations in bacterial genomes that may confer antibiotic resistance (Kohanski et al. 2010). Humans are exposed to antibiotic-resistant bacteria through many pathways, including direct animal contact (Price et al. 2007a), contact with environmental media, such as soil, water, and air, contaminated with animal waste (Graham et al. 2009), and consumption or handling of contaminated food products from animals raised with antibiotics (FDA 2010e; Johnson et al. 2009).
Figure 1.
Dose imprecision for food animals that consume FCMF and a pathway to antibiotic resistance. Overadministration leads to very high plasma or target tissue levels of antibiotic. Underadministration leads to levels of antibiotic that never reach minimum inhibitory concentrations (MICs). Intermittent dosing leads to levels of antibiotic that fluctuate and periodically dip below MICs for variable periods of time. ROS, reactive oxygen species. Dotted lines indicate positive feedback, potentially driving increased use of FCMF.
Use of medicated feed has been demonstrated to introduce residual antimicrobials and their metabolites into the waste streams of animal operations. As much as 75% of administered antibiotics (Chee-Sanford et al. 2009), and considerable fractions of some antiparasitic medications (Lumaret and Errouissi 2002; Wall and Strong 1987), are not absorbed by animals and are eliminated in waste. Some insecticides and antiparasitic drugs included in certain feed supplements are designed to be excreted by animals to control insects attracted to animal droppings (Wall and Strong 1987). These wastes contribute to drug loads in watersheds and in other environmental media that may become available for human or nontarget organism exposure (Arikan et al. 2008; Chee-Sanford et al. 2009; Lumaret and Errouissi 2002). One route for drug exposure to humans is through food crops that take up antibiotics when fertilized with animal manure containing these same antibiotics (Kumar et al. 2005). The human health consequences of exposures to these drugs and their metabolites at environmentally relevant concentrations remain largely uncharacterized.
The FDA regulates medicated feed (FDA 2010a, 2010b) and in doing so separates animal drugs into two types: therapeutic drugs that are used to “diagnose, cure, mitigate, treat, or prevent disease in animals,” and animal production drugs that are intended only for healthy livestock and used to “enhance the production of edible or nonedible products or to increase the efficiency of a particular phase of life” to increase the rate of weight gain, improve feed efficiency, or enhance milk production (Sechen 2006). Both types of drugs, by FDA regulation, can be sold without veterinary prescription as medicated feed products (FDA 2000, 2010c). As part of the FDA approval, all medicated feed products are required to have labels listing active ingredients by medication name and concentration, indications for use, and all other ingredients by weight so that contents can be read and interpreted by workers at animal production facilities. In some cases, a medicated feed is broadly labeled both for disease treatment of sick animals and for disease prevention or growth promotion in healthy animals.
The purpose of this commentary is to discuss the history of medicated feed, the nature of FCMF use, and its role in the development of antimicrobial-resistant microorganisms. We also discuss the past and current legislative efforts to address antimicrobial use in food animals.
A History of Medicated Feed
The rationale for including antibiotics in feed was constructed in the late 1940s and early 1950s when studies began to report a correlation between the use of antibiotics (mainly chlortetracycline and oxytetracycline) in livestock, swine, and poultry with increased rates of animal weight gain (Jones and Ricke 2003). The food animal production industry implemented in-feed antibiotic use in conjunction with high-throughput single-species cultivation to enhance production of grain-fed, feedlot livestock and poultry (Pew Commission on Industrial Farm Animal Production 2008). Antibiotic and antihelminthic additives were included in mineral and other feed supplements beginning in the early 1960s and 1970s, respectively (Hanson 1963). In 1960, the Agricultural and Medical Research Council Committee of Great Britain suggested that antibiotics offered economic advantages to livestock producers by lowering production costs (Kiser 1976).
Concerns were raised as early as the 1950s when researchers noted that antibiotic-resistant bacteria emerged when animals were administered antibiotics (Finland 1956). The potential for adverse human health consequences resulting from low-level exposures to antibiotic residues in meat, milk, and eggs also was identified (Randall 1956). Apprehension regarding the use of human drugs in animals was noted in a 1969 European report, Use of Antibiotics in Animal Husbandry and Veterinary Medicine (Swann 1969). U.S. regulators addressed these concerns in a 1972 report by the FDA Task Force on the Use of Antibiotics in Animal Feeds (Van Houweling and Gainer 1978) and an FDA-proposed policy statement (Edwards 1972). In 1972 and 1973, strong reaction against the proposed FDA policy statement from representatives of the livestock industry, veterinary pharmaceutical manufacturers, and some animal science researchers prompted revision of the final 1974 policy to incorporate consideration of the value of antibiotics in animal feed for increased rate of gain, feed efficiency, and disease control (Gardner 1973; Kiser 1976). Industry groups have continued to influence the regulatory trajectory of policies surrounding the use of antimicrobials in food animal production.
Antimicrobial Dose Delivery by FCMF
Difficulties in ensuring precision in antimicrobial dose delivery, a function of numerous factors, may result in the inability to deliver predictable, uniform, or intended dose levels. These factors, although not mutually exclusive, can be grouped into concerns regarding labeling, veterinary oversight, feed characteristics, the behavior of animal production facility workers, animal behavior, and drug pharmacokinetics.
Quality control of medicated feed
Requirements for quality control in manufacturing for antibiotics intended for use in animal finished feed products are less rigorous than those for pharmaceuticals intended for parenteral use in livestock or for human use (FDA 2009, 2010f, 2010g). Quality control problems can occur when drugs are combined with other feed ingredients. In an investigation of feeds labeled as nonmedicated, 44% (71 of 161) actually contained antimicrobials, and more than one-third (87 of 247) of feeds labeled as medicated contained undeclared antimicrobials, most notably chlortetracycline, sulfonamides, penicillin, and ionophores (Lynas et al. 1998). Investigators hypothesized that the presence of these unlabeled drugs was a result of cross-contamination in feed production mills. Quality control issues in animal feed content are not limited to those containing medication—a 2008 commercial feed survey by the U.S. Department of Agriculture (USDA) found that nearly 20% (123 of 657) of animal feed products were mislabeled; their product ingredient claims were not substantiated by an independent testing laboratory (USDA 2008).
The undeclared presence of medications in feed supplements results in the unintended delivery of antimicrobials and could compromise the USDA organic certification status of food animal products. Allowance of antibiotic use in organic breeder stock (organic broiler chicks before and on the first day of hatching) as well as in herds used for cattle replacement of organic dairy cattle may undermine the spirit of USDA organic certification.
Animal production facility worker behavior
Because veterinary oversight or prescription is not required to purchase, mix, or administer most medicated feed products (FDA 2000), workers at animal production facilities may be exclusively responsible for the decision to use medicated feed. Certain medicated feeds are labeled to treat specific morbidities, for example, anaplasmosis or Pasteurella pneumonia (FDA 2010d). Such diseases often require clinical diagnosis and laboratory confirmation (Hartwig and Ensley 2010). Allowing workers to purchase medicated feed without a veterinary prescription to treat diseases that require a veterinarian to diagnose may result in drug administration in a manner inconsistent with its intended use.
Animal and herd behavior
The number of animals per feeding location or feed block, feed location within animal production facility, other housing or grazing area characteristics and herd or flock social interactions are factors shown to influence feed intake for ruminants and poultry (Appleby 1998; Bowman and Sowell 1997, 2002; Kincheloe 2004). Interanimal dominance scenarios influence the quantity of feed consumed by ruminants (Bowman and Sowell 2002), and in poultry, pecking order affects medicated feed intake, leading to differences in drug exposure among animals (Toutain et al. 2010). Acceptance of feed increases with animal age and prior experience with feed type (Bowman and Sowell 1997; Kincheloe 2004). A fraction of livestock herds refuse FCMF; these animals were shown to have low serum and plasma concentrations of drugs (Bogan and Marriner 1983). Administering medication via FCMF is not an efficient way to achieve accurate serum and plasma concentrations of drugs across a herd or flock of animals.
Nondomesticated animals
When FCMF are freely available to livestock, they may be available to wildlife living on or near animal production operations. Studies in Europe and the United States have demonstrated that antibiotic-resistant bacteria can be found in feral hogs and other wildlife (Costa et al. 2008; Literak et al. 2010; Ramlachan et al. 2007). Wildlife living on farms are more likely to harbor antibiotic-resistant Escherichia coli than wildlife not living on farms (Kozak et al. 2009). Although exposure to feed may explain transfer of resistant bacteria to wildlife, other possible production-related pathways of exposure exist, including contact with contaminated manure, dust, or water. Wildlife movement from the source farm rarely is restricted and can spread antimicrobial-resistant microorganisms and diseases to other communities or farms (Ramlachan et al. 2007; Ward et al. 2006).
Drug absorption, pharmacokinetics, and pharmacodynamics
After drug consumption, factors that influence absorption and pharmacokinetics determine target organ dose (Lees et al. 2006). Some drugs given orally, such as tetracyclines, may bind to cations (e.g., calcium) that are found in feed, lowering their bioavailability and resulting in serum concentrations below minimum inhibitory concentrations, even in animals dosed at levels for disease treatment (Nielsen and Gyrd-Hansen 1996; Underhill and Danish 1992). Proteins, carbohydrates, and fats present in feeds also may alter absorption of many drugs (Underhill and Danish 1992). Commensal bacteria present in the rumen of cows may chemically alter drugs administered orally (Then 1982). Finally, disease states alter an animal’s ability to absorb and process drugs for systemic delivery (Guardabassi and Kruse 2008). Fever, concurrent organ damage (e.g., hepatic lipidosis in dairy cows), and gastrointestinal disease may influence the final serum concentrations of a drug (Kozak et al. 2009; Underhill and Danish 1992). This is of particular concern when medications in feed supplements are licensed for disease treatment; the animals most in need of a drug may be the ones least likely or able to access doses consistent with disease treatment.
Taken in sum, numerous factors influence the ability to deliver predictable or intended doses of drugs to animals via FCMF. Given the limited oversight, the availability of FCMF without a veterinary prescription, the potential for undeclared drugs, and variability in drug concentrations within and between feeds, unintended (and therefore inappropriate) drug delivery is likely. At a minimum, these factors make predicting the actual delivered dose to any given individual animal nearly impossible and predicting herd averages for drug delivery complicated. Worse, inappropriate or imprecise drug dosing may drive selection for resistant microorganisms (Lees et al. 2006), that affect both veterinary and human medicine.
Policy Considerations
U.S. federal legislation
Elimination of antibiotics for growth promotion and feed efficiency in IFAP in the United States has been discussed since the 1970s. The latest antibiotics bill was reintroduced by Congresswoman Louise Slaughter (D-NY) in 2009 as the Preservation of Antibiotics for Medical Treatment Act (PAMTA; H.R.1549/S. 619, 2009). PAMTA would withdraw federal approval for use of certain drugs as feed or water additives in food animal production if they are used for growth promotion, feed efficiency, or weight gain, and for disease prevention in the absence of any clinical sign of disease in an animal. A former head of the FDA, Donald Kennedy, called for Congress to pass PAMTA (Kennedy 2010). Passage of the bill would not affect the use of insecticides (e.g., tetrachlorvinphos or methoprene) or antiparasitic drugs (e.g., thiabendazole) in medicated feeds that are fed for therapeutic uses, or antiparasitic drugs for nontherapeutic uses. Although regulating the use of antiparasitics and insecticides is relevant to the ecology of drug resistance, these drugs are more appropriate for oral dosing using herd health management methods. Some evidence supports efficacy of antiparasitics, insecticides, and certain antibiotics in particular species via feed supplement administration (Blagburn et al. 1987; McBride et al. 2008).
Although PAMTA begins to address these concerns, more effort is needed to reform the conventional (nonorganic) U.S. dairy, beef cattle, hog, and poultry industries. In Denmark, swine and poultry productivity stabilized and, in some cases, increased after bans on nontherapeutic uses of certain antibiotics during the late 1990s (Aarestrup 2009; Lawson et al. 2008). Denmark monitors the veterinary use of antimicrobial drugs for IFAP through VETSTAT, a transparent data reporting system that tracks compliance of legislation, helps veterinarians work with animal production facility workers, and provides data for research on veterinary drugs (Stege et al. 2003). In the United States, no federal requirements currently exist for reporting animal antimicrobial drug use by animal production facility staff or veterinarians, although in a historic move, the FDA began reporting annual antimicrobial drug distribution and sales summary data in 2010 as required by the 2008 reauthorization of the Animal Drug User Fee Act (FDA 2010i). If these data are made publically available in a timely fashion and usable format, then an assessment of the extent of antibiotic use in IFAP can be conducted. Although reporting itself will not mitigate antimicrobial resistance risks, understanding the extent, nature, and distribution of antimicrobial use in IFAP will strengthen the impetus for policy interventions aimed at eliminating unnecessary administration of antimicrobials.
U.S. federal regulatory agency involvement
Human cases of fluoroquinolone-resistant, food-borne Campylobacter increased after the licensing of fluoroquinolones for use in poultry during the 1990s (Gupta et al. 2004; Nelson et al. 2007). As a result, the FDA withdrew approval for fluoroquinolone use in water to treat diseases in poultry in 2005 in response to concerns over agricultural drivers of resistant infections in humans (Gupta et al. 2004; Nelson et al. 2007). Even after the fluoroquinolone ban, bacterial resistance persisted in poultry products (Price et al. 2007b), highlighting the importance of early action to remove nontherapeutic use of antibiotics in livestock. In 2010, the FDA issued a draft guidance over concerns with antimicrobial use, stating that “the overall weight of evidence available to date supports the conclusion that using medically important antimicrobial drugs for production purposes is not in the interest of protecting and promoting the public health” (FDA 2010j). However, as guidance, the FDA document is not binding or enforceable.
Conclusion
Delivering antibiotics to food animals for reasons other than treatment of clinically diagnosed disease, especially via free-choice feeding methods, poses an unnecessary public health risk. Mounting evidence suggests the use of antibiotics in food animal production contributes to a considerable fraction of antibiotic-resistant infections in humans (Silbergeld et al. 2008). The increasing number of antimicrobial-resistant infections and their costs in the United States, estimated to be $400 million to $5 billion per year in 1998 (Institute of Medicine 1998) and $16.6 to $26 billion per year in 2009 (Roberts et al. 2009), are of growing concern. Methicillin-resistant Staphylococcus aureus (MRSA) infections acquired outside of hospitals (i.e., community-associated MRSA) have seen a 33% annual increase from 1999 to 2006 (Klein et al. 2009). Although not all community-associated MRSA infections originate from IFAP, certain human cases have been associated with the production of swine, poultry, and dairy cattle in Europe (Broens et al. 2008), and the potential for similar exposure pathways exists in the United States. Human exposures to antibiotic- resistant Campylobacter, Salmonella, and other resistant bacteria via food animal products are also of concern (Angulo et al. 2004; Kassenborg et al. 2004; Price et al. 2005).
Instead of farming practices that employ free-choice oral herd or flock dosing with medicated feed, appropriate individual therapeutic antimicrobial administration by injection should be pursued to treat clinically diagnosed disease in food animals. Therapeutic treatment by injection will achieve more predictable plasma drug levels, enhancing opportunities for disease control (Baggot 2007; Lees et al. 2006). In cases where individual treatment is difficult, such as with large poultry operations, improving husbandry and hygiene at production facilities are preferred over the administration of oral therapeutic antimicrobial with veterinary supervision, and all are preferable to FCMF without veterinary supervision. For prevention of diseases in a herd or flock, vaccinations (Redding and Weiner 2009), reduced stress (Dantzer and Mormède 1983), and improved welfare (Broom 2006) are approaches that can help replace antimicrobials.
Footnotes
M.F.D. is supported by the Johns Hopkins Sommer Scholarship and Center for a Livable Future Fellowship.
References
- Aarestrup F. Meeting with a Congress Delegation on the Danish Experience with Stop for Nontherapeutic Use of Antimicrobials. 2009. [[accessed 17 February 2010].]. Available: http://www.louise.house.gov/images/stories/attachments/2009.10.01.pamta.pdf.
- Andremont A. Effect of antibiotics on bacterial resistance ecology: role of the digestive tract [in French] Med Mal Infect. 2000;30(3):S178–S184. [Google Scholar]
- Angulo FJ, Baker NL, Olsen SJ, Anderson A, Barrett TJ. Antimicrobial use in agriculture: controlling the transfer of antimicrobial resistance to humans. Semin Pediatr Infect Dis. 2004;15(2):78–85. doi: 10.1053/j.spid.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Appleby MC. Modification of laying hen cages to improve behavior. Poult Sci. 1998;77(12):1828–1832. doi: 10.1093/ps/77.12.1828. [DOI] [PubMed] [Google Scholar]
- Arikan O, Rice C, Codling E. Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination. 2008;226(1–3):121–133. [Google Scholar]
- Baggot J. Principles of antimicrobial drug bioavailability and disposition. In: Giguere S, Prescott J, Baggot J, Walker R, Dowling P, editors. Antimicrobial Therapy in Veterinary Medicine. 4. Portland, OR: Blackwell Publishing; 2007. pp. 45–80. [Google Scholar]
- Blagburn BL, Hanrahan LA, Hendrix CM, Lindsay DS. Efficacy of fenbendazole-medicated feed blocks against gastrointestinal nematode infections in calves. Am J Vet Res. 1987;48(6):1017–1019. [PubMed] [Google Scholar]
- Bogan JA, Marriner SE. Uptake of fenbendazole by grazing sheep with access to feed blocks containing fenbendazole. Brit Vet J. 1983;139(3):223–227. doi: 10.1016/s0007-1935(17)30488-8. [DOI] [PubMed] [Google Scholar]
- Bowman JG, Sowell BF. Delivery method and supplement consumption by grazing ruminants: a review. J Anim Sci. 1997;75(2):543–550. doi: 10.2527/1997.752543x. [DOI] [PubMed] [Google Scholar]
- Bowman JGP, Sowell BF University of Contestado. Self-fed supplements for beef cattle on grasslands. First Virtual Global Conference on Organic Beef Cattle Production; 2 September–15 October, 2002; Brazil: Embrapa; 2002. pp. 1–8. Available: http://www.cpap.embrapa.br/agencia/congressovirtual/pdf/ingles/03en02.pdf [accessed: 14 October 2010]. [Google Scholar]
- Broens EM, van Clef BAGL, Graat EAM, Kluytmans JAJW. Transmission of methicillin-resistant Staphylococcus aureus from food production animals to humans: a review. CAB Rev Perspect Agric Vet Sci Nutr Nat Res. 2008;3(95):1–12. [Google Scholar]
- Broom DM. Behaviour and welfare in relation to pathology. Appl Animal Behav Sci. 2006;97(1):73–83. [Google Scholar]
- Burch DGS, Duran CO, Aarestrup FM. Guidelines for antimicrobial use in swine. In: Guardabassi L, Jensen LB, Kruse H, editors. Guide to Antimicrobial Use in Animals. Ames, IA: Blackwel; 2008. pp. 102–125. [Google Scholar]
- Chee-Sanford JC, Mackie RI, Koike S, Krapac IG, Lin YF, Yannarell AC, et al. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J Environ Qual. 2009;38(3):1086–1108. doi: 10.2134/jeq2008.0128. [DOI] [PubMed] [Google Scholar]
- Costa D, Poeta P, Saenz Y, Vinue L, Coelho AC, Matos M, et al. Mechanisms of antibiotic resistance in Escherichia coli isolates recovered from wild animals. Microb Drug Resist. 2008;14(1):71–77. doi: 10.1089/mdr.2008.0795. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Mormède P. Stress in farm animals: a need for reevaluation. J Anim Sci. 1983;57(1):6–18. doi: 10.2527/jas1983.5716. [DOI] [PubMed] [Google Scholar]
- Edwards CC. Antibiotic and sulfonamide drugs in animal feeds proposed statement of policy. Fed Reg. 1972;37:2444–2445. [Google Scholar]
- FDA (U.S. Food and Drug Administration) [[accessed 19 August 2010].];Animal Drug Availability Act; Veterinary Feed Directive. 2000 21CFR510:514–558. Available: http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=2000_register&docid=00-31151-filed. [Google Scholar]
- FDA (U.S. Food and Drug Administration) Approved Animal Drug Products. 2009. [[accessed 14 December 2009].]. Available: http://www.accessdata.fda.gov/scripts/AnimalDrugsAtFDA/
- FDA (U.S. Food and Drug Administration) Current Good Manufacturing Practices for Finished Pharmaceuticals. 2010a. p. 21CFR211. [Google Scholar]
- FDA (U.S. Food and Drug Administration) Current Good Manufacturing Practices for Medicated Feeds. 2010b. p. 21CFR225. [Google Scholar]
- FDA (U.S. Food and Drug Administration) Electronic Animal Drug Product Listing Directory. 2010c. [[accessed 14 October 2010].]. Available: http://www.fda.gov/downloads/ForIndustry/DataStandards/StructuredProductLabeling/UCM201356.zip.
- FDA (U.S. Food and Drug Administration) FDA Approved Animal Drug Products. 2010d. [[accessed 19 August 2010].]. Available: http://www.accessdata.fda.gov/scripts/animaldrugsatfda/index.cfm?gb=1&showtype=adv.
- FDA (U.S. Food and Drug Administration) National Antimicrobial Resistance Monitoring System—Enteric Bacteria (NARMS): 2007 Executive Report. Rockville, MD: FDA; 2010e. [Google Scholar]
- FDA (U.S. Food and Drug Administration) New Animal Drugs for Use in Animal Feeds. 2010f. p. 21CFR558. [Google Scholar]
- FDA (U.S. Food and Drug Administration) Oral Dosage Form New Animal Drugs. 2010g. p. 21CFR520. [Google Scholar]
- FDA (U.S. Food and Drug Administration) Requirements for Free-Choice Medicated Animal Feed. 2010h. p. 21CFR510.
- FDA (U.S. Food and Drug Administration) Summary Report on Antimicrobials Sold or Distributed for Use in Food-producing Animals, 2009. 2010i. [[accessed 13 December 2010].]. Available: http://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM231851.pdf.
- FDA (U.S. Food and Drug Administration) The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals. Draft Guidance 209. 2010j. [[accessed 11 August 2010].]. Available: http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM216936.pdf.
- Finland M. First International Conference on Antibiotics in Agriculture. Publication 397. Washington, DC: National Academy of Science, National Research Council; 1956. Emergence of resistant strains in chronic intake of antibiotics; pp. 233–258. [Google Scholar]
- Forbes JM. Voluntary Food Intake and Diet Selection in Farm Animals. 2nd ed. London: CABI; 2007. [Google Scholar]
- Gardner S. Statement of policy and interpretation regarding animal drugs and medicated feeds. Fed Reg. 1973;38:9811–9814. [Google Scholar]
- Graham JP, Evans SL, Price LB, Silbergeld EK. Fate of antimicrobial-resistant enterococci and staphylococci and resistance determinants in stored poultry litter. Environ Res. 2009;109(6):682–689. doi: 10.1016/j.envres.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Guardabassi L, Kruse H. Principles of prudent and rational use of antimicrobials in animals. In: Guardabassi L, Jensen LB, Kruse H, editors. Guide to Antimicrobial Use in Animals. Ames, IA: Blackwell; 2008. pp. 1–12. [Google Scholar]
- Gupta A, Nelson JM, Barrett TJ, Tauxe RV, Rossiter SP, Friedman CR, et al. Antimicrobial resistance among Campylobacter strains, United States, 1997–2001. Emerg Infect Dis. 2004;10(6):1102–1109. doi: 10.3201/eid1006.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JO. Ionophore use and toxicosis in cattle. Vet Clin N Am. 2000;16(3):497–509. vii. doi: 10.1016/s0749-0720(15)30083-9. [DOI] [PubMed] [Google Scholar]
- Hanson LE. Feed additives, rationale for medicated feeds. J Agric Food Chem. 1963;11(5):365–367. [Google Scholar]
- Hartwig NR, Ensley DT. Anaplasmosis. 2010. [[accessed 19 August 2010].]. Available: http://www.iabeef.org/Content/anaplasmosis.aspx.
- H.R. 1549/S 619. Preservation of Antibiotics for Medical Treatment, 111th Congress (2009–2010).2009. [Google Scholar]
- Institute of Medicine. Workshop Report. Washington, DC: National Academies Press; 1998. Antimicrobial Drug Resistance: Issues and Options. [PubMed] [Google Scholar]
- Johnson JR, McCabe JS, White DG, Johnston B, Kuskowski MA, McDermott P. Molecular analysis of Escherichia coli from retail meats (2002–2004) from the United States National Antimicrobial Resistance Monitoring System. Clin Infect Dis. 2009;49(2):195–201. doi: 10.1086/599830. [DOI] [PubMed] [Google Scholar]
- Jones FT, Ricke SC. Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult Sci. 2003;82(4):613–617. doi: 10.1093/ps/82.4.613. [DOI] [PubMed] [Google Scholar]
- Kassenborg HD, Smith KE, Vugia DJ, Rabatsky-Ehr T, Bates MR, Carter MA, et al. Fluoroquinolone-resistant Campylobacter infections: eating poultry outside of the home and foreign travel are risk factors. Clin Infect Dis. 2004;38(suppl 3):S279–S284. doi: 10.1086/381597. [DOI] [PubMed] [Google Scholar]
- Kennedy D. Cows on drugs [Editorial] New York Times; 2010. Apr 17, [Google Scholar]
- Khachatourians GG. Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. CMAJ. 1998;159(9):1129–1136. [PMC free article] [PubMed] [Google Scholar]
- Kincheloe JJ. Variation in Supplement Intake by Grazing Beef Cows [Master’s Thesis] Bozeman, MO: Montana State University; 2004. [Google Scholar]
- Kiser JS. A perspective on the use of antibiotics in animal feed. J Anim Sci. 1976;42(4):1058–1072. doi: 10.2527/jas1976.4241058x. [DOI] [PubMed] [Google Scholar]
- Klein E, Smith D, Laxminarayan R. Community-associated methicillin-resistant Staphylococcus aureus in outpatients, United States, 1999–2006. Emerg Infect Dis. 2009;15(12):1925–1930. doi: 10.3201/eid1512.081341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski M, DePristo M, Collins J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell. 2010;37(3):311–320. doi: 10.1016/j.molcel.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak G, Boerlin P, Janecko N, Reid-Smith R, Jardine C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl Environ Microbiol. 2009;75(3):559–566. doi: 10.1128/AEM.01821-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Gupta SC, Baidoo SK, Chander Y, Rosen CJ. Antibiotics uptake by plants from soil fertilized with animal manure. J Environ Qual. 2005;34:2082–2085. doi: 10.2134/jeq2005.0026. [DOI] [PubMed] [Google Scholar]
- Lawson L, Jensen V, Otto L. The economics of use and non-use of antimicrobial growth promoters: the case of Danish broiler production. J Int Farm Manag. 2008;4(2):1–13. [Google Scholar]
- Lees P, Concordet D, Aliabadi FS, Toutain P. Drug selection and optimization of dosage schedules to minimize antimicrobial resistance. In: Aarestrup F, editor. Antimicrobial Resistance in Bacteria of Animal Origin. Washington, DC: ASM Press; 2006. pp. 49–72. [Google Scholar]
- Literak I, Dolejska M, Radimersky T, Klimes J, Friedman M, Aarestrup FM, et al. Antimicrobial-resistant faecal Escherichia coli in wild mammals in central Europe: multiresistant Escherichia coli producing extended-spectrum beta-lactamases in wild boars. J Appl Microbiol. 2010;108(5):1702–1711. doi: 10.1111/j.1365-2672.2009.04572.x. [DOI] [PubMed] [Google Scholar]
- Lumaret J-P, Errouissi F. Use of anthelmintics in herbivores and evaluation of risks for the non target fauna of pastures. Vet Res. 2002;33(5):547–562. doi: 10.1051/vetres:2002038. [DOI] [PubMed] [Google Scholar]
- Lynas L, Currie D, McCaughey W, McEvoy J, Kennedy D. Contamination of animal feedingstuffs with undeclared antimicrobial additives. Food Addit Contam. 1998;15(2):162–170. doi: 10.1080/02652039809374626. [DOI] [PubMed] [Google Scholar]
- McBride D, Key N, Mathews KH. Subtherapeutic antibiotics and productivity in U.S. hog production. Rev Agric Econ. 2008;30(2):270–288. [Google Scholar]
- McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34(suppl 3):S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- Nelson JM, Chiller TM, Powers JH, Angulo FJ. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin Infect Dis. 2007;44(7):977–980. doi: 10.1086/512369. [DOI] [PubMed] [Google Scholar]
- Nielsen P, Gyrd-Hansen N. Bioavailability of oxytetracycline, tetracycline and chlortetracycline after oral administration to fed and fasted pigs. J Vet Parmacol Ther. 1996;9(4):305–311. doi: 10.1111/j.1365-2885.1996.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Pew Commission on Industrial Farm Animal Production. Putting Meat on the Table: Industrial Farming Animal Production in America. Baltimore, MD: Pew Charitable Trusts and Johns Hopkins Bloomberg School of Public Health; 2008. [Google Scholar]
- Price L, Graham J, Lackey L, Roess A, Vailes R, Silbergeld E. Elevated risk of carrying gentamicin-resistant Escherichia coli among U.S. poultry workers. Environ Health Perspect. 2007a;115:1738–1742. doi: 10.1289/ehp.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LB, Johnson E, Vailes R, Silbergeld E. Fluoroquinolone-resistant Campylobacter isolates from conventional and antibiotic-free chicken products. Environ Health Perspect. 2005;113:557–560. doi: 10.1289/ehp.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LB, Lackey LG, Vailes R, Silbergeld E. The persistence of fluoroquinolone-resistant Campylobacter in poultry production. Environ Health Perspect. 2007b;115:1035–1039. doi: 10.1289/ehp.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlachan N, Anderson RC, Andrews K, Laban G, Nisbet DJ. Characterization of an antibiotic resistant Clostridium hathewayi strain from a continuous-flow exclusion chemostat culture derived from the cecal contents of a feral pig. Anaerobe. 2007;13(3–4):153–160. doi: 10.1016/j.anaerobe.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Randall WA. First International Conference on Antibiotics in Agriculture. Publication 397. Washington, DC: National Academy of Science, National Research Council; 1956. Antibiotic residues; pp. 259–263. [Google Scholar]
- Redding L, Weiner DB. DNA vaccines in veterinary use. Expert Rev Vaccines. 2009;8(9):1251–1276. doi: 10.1586/erv.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RR, Hota B, Ahmad I, Scott RN, Foster S, Abbasi F, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49(8):1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- Sechen S. The review of animal production drugs by FDA. [[accessed 17 March 2010].];FDA Vet Newsl. 2006 11(1) Available: http://www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/ucm093574.htm. [Google Scholar]
- Silbergeld EK, Davis M, Leibler JH, Peterson AE. One reservoir: redefining the community origins of antimicrobial-resistant infections. Med Clin North Am. 2008;92(6):1391–1407. xi. doi: 10.1016/j.mcna.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Stege H, Bager F, Jacobsen E, Thougaard A. VETSTAT—the Danish system for surveillance of the veterinary use of drugs for production animals. Prev Vet Med. 2003;57(3):105–115. doi: 10.1016/s0167-5877(02)00233-7. [DOI] [PubMed] [Google Scholar]
- Swann M. Use of Antibiotics in Animal Husbandry and Veterinary Medicine. London: Stationery Office; 1969. [Google Scholar]
- Then R. Mechanisms of resistance to trimethoprim, the sulfonamides, and trimethoprim-sulfamethoxazole. Rev Infect Dis. 1982;4(2):261–269. doi: 10.1093/clinids/4.2.261. [DOI] [PubMed] [Google Scholar]
- Toutain PL, Ferran A, Bousquet-Melou A. Species differences in pharmacokinetics and pharmacodynamics. In: Cunningham F, Elliott J, Lees P, editors. Handbook of Experimental Pharmacology, Vol 199, Comparative and Veterinary Pharmacology. Berlin: Springer; 2010. pp. 19–48. [DOI] [PubMed] [Google Scholar]
- Underhill LA, Danish MA. Subtherapeutic use of antibiotics in food-producing animals. Clin Res Reg Affairs. 1992;9(3):187–195. [Google Scholar]
- USDA (U.S. Department of Agriculture) Annual Report on Commercial Feeds and Animal Remedies. Washington, DC: U.S. Department of Agriculture; 2008. [Google Scholar]
- Van Houweling CD, Gainer JH. Public health concerns relative to the use of subtherapeutic levels of antibiotics in animal feed. J Anim Sci. 1978;46:1413–1424. doi: 10.2527/jas1978.4651413x. [DOI] [PubMed] [Google Scholar]
- Wall R, Strong L. Environmental consequences of treating cattle with the antiparasitic drug ivermectin. Nature. 1987;327(6121):418–421. doi: 10.1038/327418a0. [DOI] [PubMed] [Google Scholar]
- Ward AI, Tolhurst BA, Delahay RJ. Farm husbandry and the risks of disease transmission between wild and domestic mammals: a brief review focusing on bovine tuberculosis in badgers and cattle. Animal Sci. 2006;82(06):767–773. [Google Scholar]
- Wright GD. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat Rev Microbiol. 2007;5(3):175–186. doi: 10.1038/nrmicro1614. [DOI] [PubMed] [Google Scholar]