Abstract
Purpose
To test whether eyes with central serous retinopathy (CSR) have elevated retinal flavoprotein fluorescence (FPF) using a novel clinical imaging method.
Methods
Three male patients with unilateral CSR were examined for FPF at 535nm induced by 1ms flashes of 467nm light. FPF was captured with an electron multiplying charged-coupled device (EMCCD) camera with a 512×512 pixel chip. Average intensity (AI) of retinal FPF for each affected eye was compared to the contralateral, unaffected eye and to six age-matched control eyes by analyzing histograms of pixel intensities plotted for each eye.
Results
For each patient, the CSR-affected eye had a significantly greater retinal FPF when compared to the retinal FPF of the unaffected eye. Eyes affected with CSR had retinal FPF values which averaged 98% greater than the retinal FPF of age-matched control eyes.
Conclusions
FPF analysis may be useful for rapidly and non-invasively identifying metabolic tissue stress of central serous retinopathy.
Keywords: autofluorescence, central serous chorioretinopathy, central serous retinopathy, flavoprotein fluorescence, flavoproteins, metabolic stress, mitochondrial dysfunction, retinal stress
1. Introduction
Central serous retinopathy (CSR) is characterized by idiopathic breakdown of the outer blood-retina barrier formed by the retinal pigment epithelium (RPE).1 The etiology of the disease is unknown, but fluorescein and indocyanine green (ICG) angiographic studies have shown that the pathogenesis involves RPE and choriocapillaris dysfunction, as well as choroidal lobular ischaemia and venous congestion.1–3 Optical coherence tomography (OCT) detects neurosensory retinal and RPE detachments, chronic exudates, and cystic changes within the retina.1,4–7 Hypofluorescent or hyperfluorescent fundus autofluorescence is attributed to changes in subretinal and RPE lipofuscin content.1,8–11
Oxidant damage can induce mitochondrial stress and apoptotic cell death in tissues soon after the onset of many retinal diseases, suggesting that measurement of mitochondrial metabolic activity may serve as an early indicator of disease.12–14 Prior to apoptosis, mitochondria exhibit impaired electron transport by energy-generating enzymes in the respiratory chain,15,16 causing increased oxidation of flavoproteins and their autofluorescence.17–19 Flavoprotein fluorescence (FPF) has previously been used to measure impaired mitochondrial metabolism leading to myocardial cell apoptosis in the beating heart during ischemia-reperfusion injury in vivo.20 Recently, we detected abnormal FPF in eyes of humans with diabetes and pseudotumor cerebri,21–23 as well as in a case of bilateral CSR.23 These observations led to the hypothesis that FPF may be elevated in eyes with retinal dysfunction due to CSR. We now present FPF analysis of three unilateral CSR patients in order to further show the utility of FPF as an indicator of CSR-induced retinal metabolic stress.
2. Materials/Methods
Three men, aged 35, 42, and 30 years, with unilateral CSR and no other ophthalmic or systemic disease underwent a single session of retinal FPF analysis on their affected and unaffected eyes at the University of Michigan after routine ophthalmic examination. For each CSR patient, three volunteers, each within 2 years of the patient’s age, were obtained as age-matched controls. All controls underwent funduscopic examinations during routine general eye clinic examinations at the University of Michigan. All control eyes and the unaffected eyes of CSR-patients showed no evidence of any retinal or ocular abnormality, active or inactive. This study was approved by the institutional review board (IRB) at the University of Michigan. Prior to inclusion, each patient signed a written informed consent.
To measure retinal FPF in humans, a fundus camera was modified as previously described with narrow-band excitation and emission filters, a high-sensitivity EMCCD camera, and attached computers with customized software.21–23 After pupillary dilation, four 535nm FPF acquisitions, each induced by a 1ms, 467 nm incident flash, were obtained over a three degree field, centered on the fovea of each eye. Due to the instrument’s depth of focus FPF was captured from all retinal layers. The images were stored as 512 x 512 pixel 16-bit grayscale TIFF files. Histogram curves of pixel intensities for each eye were extracted to yield average intensity (AI) of retinal FPF using a method previously described.21–23 t test and ANOVA were used to compare AI values between the two eyes of each subject. SAS 9.0 software (SAS Institute Inc., Cary, NC) was used for statistical analyses. P-values <0.05 were considered significant.
3. Results
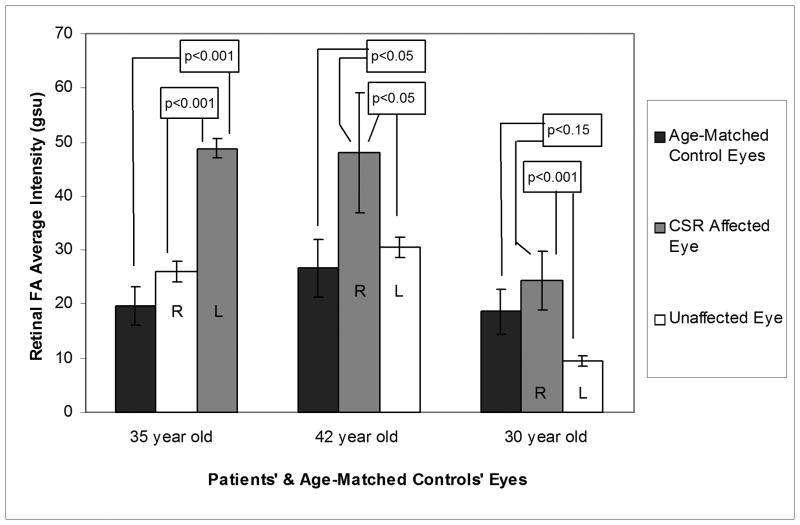
Funduscopic examination, Amsler grid testing, OCT, and fluorescein angiography all showed findings of CSR in each affected eye (Table 1, Figure 1), whereas the unaffected eyes showed no signs of disease. Retinal FPF AI of the affected eyes of patient 1 and patient 2 (35yo and 42yo, Table 1), were statistically greater than those of eyes of three age-matched control subjects (p-value: <0.001 and <0.05, respectively). Retinal FPF AI of the affected eye of patient 3 (30yo, Table 1) was 30% greater than the eyes of age-matched controls, but did not reach statistical significance (p< 0.15) (Figure 2). Importantly, significant asymmetry existed between the affected eye and the unaffected eye of each CSR patient (p-value: <0.001, <0.05, and <0.001) (Table 1).
Table 1.
Patient Characteristics
| Patient Age, y | Symptoms | Visual Acuity/Amsler Grid | Fundus Signs | FPF Average Intensity | FPF Average Intensity (R vs. L)* | OCT/Fluorescein Angiography | Disease Duration |
|---|---|---|---|---|---|---|---|
| 35 | L blurred vision | R: 20/20 L: 20/20/central metamorphopsia |
PED temporal to fovea | R: 26.0±1.8 L: 48.8±1.8 |
p < 0.001 | PED/PED | 10 months |
| 42 | R refractive shift | R: 20/25+1 L: 20/20/paracentral scotoma |
PED inferotemporal to fovea | R: 48.0±11.2 L: 30.5±1.9 |
p < 0.05 | PED/early staining | 1 month |
| 30 | L dark & blurred vision | R: 20/15 L: 20/30-2/central metamorphopsia |
blunted foveal reflex, SRF in macula | R: 9.4±0.9 L: 24.3±5.4 |
p < 0.001 | minimal SRF/leakage | 1 week |
Significant if p = or <0.05
FPF, flavoprotein autofluorescence; OCT, optical coherence tomography, PED, pigment epithelial detachment; SRF, subretinal fluid.
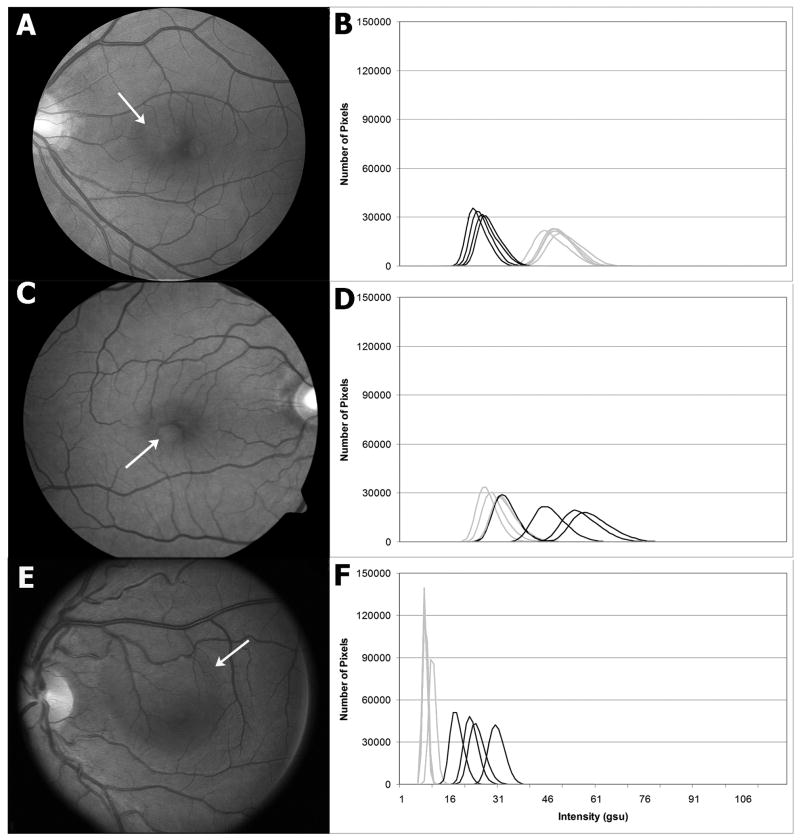
Fig. 1.
Fundus photographs of the affected eyes (A,C,E) and FPF histograms of the affected and unaffected eyes (B,D,F (right, black; left, grey)) of three patients with unilateral CSR. Present were a PED temporal to the left fovea (A), a PED inferotemporal to the right fovea (C), and a blunted foveal reflex with subretinal fluid in the left macula (E). The histograms showed significantly elevated FPF in each CSR-affected eye compared to the contralateral unaffected eye.
Fig. 2.
Bar graphs of retinal FPF AI of the affected and unaffected eyes of three patients with unilateral CSR and the average retinal FPF AI of control volunteers. Age-matched control FPF AI for each patient was obtained from six eyes of three volunteers, all within 1–2 years of the patient’s age.
We found the shot-to-shot reproducibility of our FPF results for control eyes to be very reproducible (Figure 1), including when comparing intersession variability for controls, as we have previously published.22 Diseased eyes often demonstrate higher shot-to-shot FPF variability than controls, probably from slight variations in fixation together with variability in disease-induced cellular stress at different points in the retina.
4. Discussion
Autofluorescence detection of oxidized flavoproteins is distinct from previously used methods to detect fundus autofluorescence, which is primarily due to lipofuscin. As previously described, we minimized the contribution of lipofuscin or lens autofluorescence to our signal by choosing young patients, comparing their affected eye to their unaffected eye, and using a very narrow emission band at the FPF maximum, which excludes most of the emission intensity of lipofuscin, in order to obtain maximal metabolic contrast.21
Conventional fundus autofluorescence, induced by 488nm light, shows hypofluorescence at the focal sites of leakage several weeks after onset in CSR-affected eyes.9,10 The hypofluorescence is presumably due to either reduced lipofuscin from mechanical disruption of the RPE,10 or blockage of RPE lipofuscin by accumulating subretinal fluid.9 Some reports in acute and chronic CSR describe hypofluorescence and hyperfluorescence surrounding the original point of leakage;8–10 hyperfluorescence is attributed to accumulation of lipofuscin pigment in surviving RPE cells,8,9 whereas hypofluorescence in chronic CSR lesions is thought to result from reduced RPE metabolic activity as photoreceptors are lost8 or from progressive RPE cell loss.
Retinal FPF AI was elevated significantly in each CSR-affected eye, regardless of disease duration, when compared to the FPF AI of the contralateral unaffected eye. It was also greater than in age-matched control eyes. Our ability to detect elevated FPF in CSR as early as one week after disease onset contrasts with alterations in conventional fundus autofluorescence that do not develop until several weeks after disease onset,9,10 indicating that our FPF signal is probably not due to lipofuscin, but is the result of impaired mitochondrial metabolic activity. Thus, FPF may be beneficial in the early diagnosis of CSR when retinal metabolic activity is compromised, but before substantial cell loss, presumably from apoptosis, occurs.
To our knowledge, no direct evidence of metabolic stress in CSR exists. However, photoreceptor apoptosis occurs in human24,25 and experimental retinal detachment.26,27 In the latter, apoptosis caused by subretinal injection of sodium hyaluronate, is mitochondrial-dependent.26 At its onset, the apoptotic response generates ceramide27 which permeabilizes the mitochondrial outer membrane pore in the presence of Ca2+,28,29 causing a drop in electrochemical potential and oxidation of mitochondrial flavoproteins.17–19 In animals, specific inhibition of the ceramide pathway protects from retinal detachment-induced apoptosis.27 Our in vitro studies on RPE cells23 and neural retina (not shown) show that anti-oxidants and ceramide inhibition reduce apoptosis and the FPF signal induced by H2O2 and ceramide, respectively. Taken together, these data support the contention that CSR may induce metabolic stress that can be detected by non-invasive FPF analysis. In our CSR patients, neurosensory or RPE serous detachments precluded our ability to distinguish the FPF contributions due to the primary stress of CSR on cellular metabolism from that caused by secondary detachments. However, we have reported elevated FPF in both eyes of a patient with bilateral CSR lacking subretinal or sub-RPE fluid,23 suggesting that elevated FPF in CSR is at least partially due to its primary stress on cells.
In each eye with CSR, the serous detachments involved the central three degree field that was analyzed and thus the detached retina contributed to the FPF signal. A serous detachment is a structural alteration due to disease. It is likely that metabolic alterations involve retina beyond the visibly detached area where subretinal fluid may be slight. In addition, although we did test shot-to-shot reproducibility, we did not test intersession reproducibility in a longitudinal fashion, as we only evaluated patients at one session. Further studies will be needed to test these parameters of FPF in CSR.
This study demonstrated two characteristics of FPF that appear to indicate disease: 1) elevated retinal FPF AI in a patient’s eye compared to that of age-matched control eyes (Figure 2), and 2) significantly increased retinal FPF AI of a patient’s eye compared to that of their contralateral eye (Figures 1 & 2). This asymmetry is not present in any age-matched control volunteer, indicating that asymmetry between patients’ eyes may be a characteristic of disease, as we have previously shown for pseudotumor cerebri.21 This can be true even when retinal FPF AI of an eye is not statistically different from the retinal FPF AI of eyes of age-matched control volunteers (Figure 2), as in patient 3. This study suggests that rapid, non-invasive FPF analysis of the human retina is feasible for detecting CSR-induced retinal dysfunction.
Acknowledgments
Funding/Support: This study was supported by grants EY09441 and EY007003 from the National Eye Institute, National Institutes of Health, and by a Research to Prevent Blindness Senior Scientific Investigator Award (Dr. Victor Elner).
Footnotes
Financial Disclosure: Drs. Victor Elner and Howard Petty have a financial interest in the presented material by having founded OcuSciences, Inc., to commercialize this technology.
References
- 1.Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86:126–45. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 2.Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994;14:231–42. doi: 10.1097/00006982-199414030-00008. [DOI] [PubMed] [Google Scholar]
- 3.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous retinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 4.Mitarai K, Gomi F, Tano Y. Three-dimensional optical coherence tomographic findings in central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2006;244:1415–20. doi: 10.1007/s00417-006-0277-7. [DOI] [PubMed] [Google Scholar]
- 5.Ojima Y, Hangai M, Sasahara M, Gotoh N, Inoue R, Yasuno Y, Makita S, Yatagai T, Tsujikawa A, Yoshimura N. Three-dimensional imaging of the foveal photoreceptor layer in central serous chorioretinopathy using high-speed optical coherence tomography. Ophthalmology. 2007;114:2197–207. doi: 10.1016/j.ophtha.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Moschos M, Brouzas D, Koutsandrea C, Stefanos B, Loukianou H, Papantonis F, Moschos M. Assessment of central serous chorioretinopathy by optical coherence tomography and multifocal electroretinography. Ophthalmologica. 2007;221:292–8. doi: 10.1159/000104758. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto H, Gomi F, Wakabayashi T, Sawa M, Tsujikawa M, Tano Y. Morphologic changes in acute central serous chorioretinopathy evaluated by fourier-domain optical coherence tomography. Ophthalmology. 2008;115:1494–500. doi: 10.1016/j.ophtha.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 8.von Ruckmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol. 2002;133:780–6. doi: 10.1016/s0002-9394(02)01428-9. [DOI] [PubMed] [Google Scholar]
- 9.Framme C, Walter A, Gabler B, Roider J, Sachs HG, Gabel VP. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand. 2005;83:161–7. doi: 10.1111/j.1600-0420.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 10.Eandi CM, Ober M, Iranmanesh R, Peiretti E, Yannuzzi LA. Acute central serous chorioretinopathy and fundus autofluorescence. Retina. 2005;25:989–93. doi: 10.1097/00006982-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Spaide RF, Klancnik JM., Jr Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology. 2005;112:825–33. doi: 10.1016/j.ophtha.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Durnaief JL, Dentchev T, Ying G-S, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–42. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 13.Podesta F, Romeo G, Liu WH, et al. Bax is increased in the retina of diabetic subjects and in association with pericyte apoptosis in vivo and in vitro. Am J Pathol. 2000;156:1025–32. doi: 10.1016/S0002-9440(10)64970-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–5. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 15.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Rad Biol Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Ning X, Baoyu Q, Yuzhen L, Shuli S, Reed E, Li QQ. Neuro-optic cell apoptosis and microangiopathy in KKAY mouse retina. Int J Mol Med. 2004;13:87–92. [PubMed] [Google Scholar]
- 17.Benson RC, Meyer RA, Zaruba ME, McKhann GM. Cellular autofluorescence is it due to flavins? J Histochem Cytochem. 1979;27:44–48. doi: 10.1177/27.1.438504. [DOI] [PubMed] [Google Scholar]
- 18.Kindzelskii A, Petty HR. Fluorescence spectroscopic detection of mitochondrial flavoprotein redox oscillations and transient reduction of the NADPH oxidase-associated flavoprotein in leukocytes. Eur Biophys J. 2004;33:291–299. doi: 10.1007/s00249-003-0361-4. [DOI] [PubMed] [Google Scholar]
- 19.Reinert KC, Dunbar RL, Wangcai G, Gang C, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol. 2004;92:199–211. doi: 10.1152/jn.01275.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ranji M, Kanemoto S, Matsubara M, Grosso MA, Gorman JH, Gorman RD, Jaggard DL, Chance B. Fluorescence spectroscopy and imaging of myocardial apoptosis. J Biomed Optics. 2006;11:064036-1–064036-4. doi: 10.1117/1.2400701. [DOI] [PubMed] [Google Scholar]
- 21.Elner VM, Park S, Cornblath W, Hackel R, Petty HR. Flavoprotein autofluorescence detection of early ocular dysfunction. Arch Ophthalmol. 2008;126:259–260. doi: 10.1001/archophthalmol.2007.44. [DOI] [PubMed] [Google Scholar]
- 22.Field MG, Elner VM, Puro DG, Feuerman JM, Musch DC, Pop-Busui R, Hackel R, Heckenlively JR, Petty HR. Rapid, non-invasive detection of diabetes-induced retinal metabolic stress. Arch Ophthalmol. 2008;126:934–8. doi: 10.1001/archopht.126.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elner SG, Elner VM, Field MG, Park S, Heckenlively JR, Petty HR. Retinal flavoprotein autofluorescence as a measure of retinal health. Trans Am Ophthalmol Soc. 2008;106:215–24. [PMC free article] [PubMed] [Google Scholar]
- 24.Arroyo JG, Yang L, Bula D, Chen DF. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol. 2005;139:605–10. doi: 10.1016/j.ajo.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in human. Arch Ophthalmol. 1995;113:880–6. doi: 10.1001/archopht.1995.01100070054025. [DOI] [PubMed] [Google Scholar]
- 26.Hisatomi T, Sakamoto T, Goto Y, et al. Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr Eye Res. 2002;24:161–72. doi: 10.1076/ceyr.24.3.161.8305. [DOI] [PubMed] [Google Scholar]
- 27.Ranty ML, Carpentier S, Cournot M, Rico-Lattes I, Malecase F, Levade T, Delisle MB, Quintyn JC. Ceramide production associated with retinal apoptosis after retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2008 Oct 29; doi: 10.1007/s00417-008-0957-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–61. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J. 2001;20:4107–21. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]