Summary
Human immunodeficiency virus-1 (HIV-1) canarypox vaccines are safe but poorly immunogenic. CD40 ligand (CD40L), a member of the tumor necrosis factor superfamily (TNFSF), is a pivotal co-stimulatory molecule for immune responses. To explore whether CD40L can be used as an adjuvant for HIV-1 canarypox vaccine, we constructed recombinant canarypox viruses expressing CD40L. Co-immunization of mice with CD40L expressing canarypox and the canarypox vaccine expressing HIV-1 proteins, vCP1452, augmented HIV-1 specific cytotoxic T lymphocyte (CTL) responses in terms of frequency, polyfunctionality and interleukin (IL)-7 receptor α chain (IL-7Rα, CD127) expression. In addition, CD40L expressed from canarypox virus could significantly augment CD4+ T cell responses against HIV-1 in mice. CD40L expressed from canarypox virus matured human monocyte-derived dendritic cells (MDDCs) in a tumor necrosis factor α (TNF-α) independent manner, which underwent less apoptosis, and could expand ex vivo Epstein-Barr virus (EBV)-specific CTL responses from healthy human individuals and ex vivo HIV-1-specific CTL responses from HIV-1-infected individuals in the presence or absence of CD4+ T cells. Taken together, our results suggest that CD40L incorporation into poxvirus vectors could be used as a strategy to enhance their immunogenicity.
Keywords: cytotoxic T cell, AIDS, canarypox vector
Introduction
Safe and effective vaccines are desperately needed to contain the spread of Human Immunodeficiency Virus Type 1 (HIV-1), which continues to spread globally [1, 2]. Members of the poxvirus family (poxviridae) have, in recent years, received considerable attention for the development of vaccine vectors that can induce humoral and cellular immunity against virus infections as well as immunotherapy for cancer [3]. Several advantages of these vectors for vaccine development include strict cytoplasmic replication that is primarily abortive in human cells, and their good long term safety profile [4].
ALVAC is an attenuated canarypox derived vector that cannot replicate productively in mammalian cells [5, 6]. ALVAC HIV-1 vaccine is a candidate HIV-1 vaccine, which is now in Phase III clinical trials [7, 8] (http://www.iavireport.org/specials/OngoingTrialsofPreventiveHIVVaccines.pdf). Previous studies have shown that ALVAC HIV-1 vaccines are safe [9], and unlike adenovirus vectors, may not be limited by pre-existing anti-vector immunity [10, 11]. However, ALVAC immunogenicity in humans is low, eliciting HIV-1-specific cytotoxic CD8+ T cell (CTL) responses in less than 25% of normal volunteers in clinical studies [12–14], implying that further efforts need to be done to improve the immunogenicity of current canarypox HIV-1 vaccines.
Several members of tumor necrosis factor/tumor necrosis factor receptor superfamily (TNFSF/TNFRSF), including CD40L (CD154)/CD40, have been shown to enhance immunity by promoting expansion and survival of T, B, and dendritic cells (DCs) [15–17]. CD40L-CD40 interaction is considered as the main pathway through which CD4+ T cells provide help for the generation of CD8+ T cell responses and T cell dependent antibody responses [16, 18, 19]. CD40L is a type II membrane protein predominantly expressed on activated CD4+ T cells. CD40 is constitutively expressed on immature DCs and B cells. Engagement of CD40 by CD40L can induce activation and maturation of immature DCs, prevent their apoptosis, and “license” them to prime CD8+ T cell responses. In fact, previous studies showed that CD40 stimulation alone could substitute CD4+ T cells in generating CD8+ T cell responses [20–23]. Stimulation of CD40, mainly through agonistic anti-CD40 antibody, has been used experimentally to enhance immune responses elicited by antigens from bacteria, viruses, and tumors (see reference [24] for review).
We previously reported that soluble recombinant human CD40L protein could enhance ex vivo viral specific CD8+ T cell memory responses in HIV-1-infected and healthy individuals [25–27]. To test whether CD40L can be used as an adjuvant for HIV-1 ALVAC vaccines, we constructed recombinant canarypox viruses expressing CD40L and evaluated their effect on immune responses elicited by HIV-1 ALVAC vaccine in mice. We also studied the effect of CD40L expressed from canarypox on human DC maturation and memory cytotoxic T lymphocytes (CTL) expansion in cells taken from healthy HIV-1-uninfected and HIV-1 infected individuals.
Materials and methods
Construction of recombinant canarypox expressing CD40L
The methods for construction of recombinant canarypox expressing CD40L are as described elsewhere [28, 29]. Briefly, the expression cassettes for mouse membrane form of CD40L, mouse multimeric soluble form of CD40L, SP-D-CD40L, and human membrane form of CD40L were inserted into the C5 locus of canarypox vector, ALVAC II (Sanofi-Pasteur, Toronto, ON, Canada). These viruses are designated vCPmCD40L, vCPmSP-D-CD40L, and vA3131-2, respectively. The recombinant viruses were subjected to six rounds of plaque purification on chicken embryo fibroblasts (CEFs, Charles River Laboratories, Wilmington, MA). The expression of CD40L was analyzed by RT-PCR, flow cytometry, and Western blot. For RT-PCR, CEFs were infected by recombinant viruses or their parental control ALVAC II at 5 plaque forming units (pfu)/cell (MOI) for 3 days. Total RNA was isolated from infected CEFs using Trizol Reagent (Invitrogen, Burlington, ON, Canada) and subjected to RT-PCR with specific primers amplifying the coding sequence of CD40L. The identity of the amplified product was further verified by DNA sequencing. For flow cytometry, human peripheral blood mononuclear cells (PBMCs) were infected with recombinant viruses or ALVAC II at 5 MOI for 24 h and then stained with PE-conjugated anti-mouse or anti-human CD40L (BD Biosciences, Mississauga, ON, Canada). For Western blot of membrane form of CD40L, Hela cells or human monocytic cell line THP-1 were infected with recombinant viruses or ALVAC II at 5–10 MOI for 24–48 h. Infected cells were then harvested and lysed with CelLytic ™-M (Sigma, Oakville, ON, Canada). The cell lysates were loaded on SDS-PAGE gel, electrophoresed, and blotted onto Hybond-P PVDF membrane (GE Healthcare, Piscataway, NJ). The membrane was then blocked and probed with goat anti-mouse CD40L or goat anti-human CD40L antibody (R&D Systems, Inc., Minneapolis, MN), followed by horseradish peroxidase-conjugated anti-goat antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). For Western blot of SP-D-CD40L, the soluble multimeric CD40L, supernatant of Hela cells infected with recombinant virus at 10 MOI for 24 h was collected. Biotinylated anti-mouse CD40L antibody (MR1; BD Biosciences) was bound to streptavidin-coated magnetic beads (Invitrogen). The magnetic beads were incubated with the supernatant and loaded onto SDS-PAGE gel, electrophoresed, and blotted onto Hybond-P PVDF membrane (GE Healthcare). The membrane was then blocked and probed with anti-mouse SP-D (surfactant-associated protein-D) mAb (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) followed by horseradish peroxidase-conjugated goat anti-mouse antibody (Santa Cruz Biotechnology, Inc). The signal was developed on Kodak BioMax MR-1 film (PerkinElmer Life and Analytical Sciences, Woodbridge, ON, Canada) using chemiluminescence.
Mice and vaccination
Six to eight week old female Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were maintained at the Division of Comparative Medicine, Faculty of Medicine, University of Toronto. All animal work was performed following a protocol approved by University of Toronto. Mice were immunized 3 times at 2-week intervals by injecting 100μl solution containing 107 pfu vCP1452 (Sanofi-Pasteur) and 107 pfu ALVAC II or 107 pfu vCP1452 and 107 pfu recombinant viruses expressing CD40L into the quadriceps of both hind limbs (50μl for each). vCP1452 expresses the products of several HIV-1 genes, including HIV-1 MN strain gp120 fused with the gp41 from the HIV-1 LAI strain, HIV-1 LAI strain gag, part of pol, and a nef/pol polypeptide string.
Splenocytes preparation
Six weeks after the last immunization, mice were euthanized and spleens were removed and processed into single-cell suspensions in RPMI-1640 supplemented with 2mM L-glutamine, 10% fetal bovine serum (FBS), 50μM 2-mercaptoethanol, 100 U/ml of penicillin and 100μg/ml streptomycin (Invitrogen).
Interferon gamma (IFN-γ) ELISpot
Polyvinylidene difluoride 96-well plates (MAIPS4510; Millipore, Bedford, MA) were coated with 10μg/mL rat anti-mouse IFN-γ antibody (Cat No. 551309; BD Biosciences) in PBS at 4°C overnight. The plates were then washed 3 times with PBS and blocked with R-10 medium (RPMI-1640 supplemented with 10% fetal bovine serum) for at least 2 h. Splenocytes and P815 cells (mouse mastocytoma cell line) that had been pulsed with or without H-2Kd-resticted immunodominant HIV-1 Gag199–207 peptide AMQMLKETI (Mimotopes Pty Ltd., Clayton Victoria, Australia) were added to the plates and incubated at 37°C for 18 h. The plates were then washed, labeled with biotinylated rat anti-mouse IFN-γ antibody (Cat No. 554410; BD Biosciences), and incubated at room temperature for 2 h with shaking. After additional washes, streptavidin-horseradish peroxidase (Pierce, Rockford, IL) was added to the plates for 1 h at room temperature with shaking. The plates were washed and spots representing individual IFN-γ-producing cells were detected after 5-min color development using AEC substrate (BD Biosciences). IFN-γ spots-forming cells (SFC) were counted using an automated reader (Immunospot II; Cellular Technologies, Cleveland, OH).
Tetramer staining
Splenocytes were stained with FITC-anti-mouse-CD8 mAb, PE- anti-mouse-CD127 mAb (BD Biosciences), and APC-AMQMLKETI/H-2Kd tetramer (NIH tetramer facilities, Atlanta, GA). Splenocytes from naïve mice stained with FITC-anti-mouse-CD8 mAb were used as control for tetramer and CD127 gating. The stained cells were then collected using FACSCalibur flow cytometer (BD Biosciences) and the data were analyzed with FlowJo software (Tree Star, Ashland, OR). FMO (fluorescence minus one) controls were used to determine thresholds for positive staining.
Intracellular cytokine staining
For mice splenocytes, cells were stimulated with 10μg/ml AMQMLKETI in the presence of monensin (or brefeldin A for tumor necrosis factor-α (TNF-α) staining) (BD Biosciences) (and FITC-anti-mouse CD107a mAb (BD Biosciences) for CD107a staining) for 6 h. The cells were then washed and stained with FITC- or PerCP-anti-mouse-CD8 mAb for 30 min at 4°C. After washing, the cells were permeablizd with Cytofix/Cytoperm solution (BD Biosciences) and stained with PE-anti-mouse IFN-γ mAb and PE-Cy7- anti-mouse TNF-α mAb. For human MDDCs, the cells were infected with vA3131-2, ALVAC II, or vCP205 (Sanofi-Pasteur) at 10 MOI, or incubated with medium alone at 37°C for 24 h. vCP205 is an HIV-1 canarypox vaccine that can express HIV-1 MN strain gp120 fused with gp41 from HIV-1 LAI strain, HIV-1 LAI strain gag and part of pol [28]. Monensin was added during the last 6 h incubation. Then the cells were permeablized with Cytofix/Cytoperm solution (BD Biosciences) and stained with APC-mouse anti-human TNF-α mAb and PE-mouse anti-human IL-12 mAb (BD Biosciences). For human cytotoxic T cells (CTL), CTL were incubated with peptide-pulsed or non-peptide pulsed autologous B-lymphoblastoid cell lines (B-LCL) for 6 h in the presence of monensin at 37 °C in 5% CO2. The cells were stained with FITC-mouse anti-human CD8 mAb (BD Biosciences) for 30 min at 4°C. After washing, the cells were permeablizd with Cytofix/Cytoperm solution and stained with PE-mouse anti-human IFN-γ mAb (BD Biosciences). The samples were collected using FACSCalibur flow cytometer (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Splenocytes proliferation and cytokine production
Splenocytes were cultured in triplicate in 96-well round-bottom plates in 200μl media with or without HIV-1 p24 core protein (Protein Sciences, Meriden, CT). After 5 days incubation at 37°C in 5% CO2, 100μl of supernatant was removed for measurement of IFN-γ and IL-4 by ELISA (R&D Systems). 20μl of [3H]thymidine (1μCi/well) was then added to each well and the plates were incubated for an additional 16 h, following which the cells were harvested onto glass fiber filters and counted in scintillant in a beta counter. Proliferation was expressed as a stimulation index (the ratio of the counts per minute obtained with antigen/counts per minute without antigen).
Serum antibody measurements
HIV-1 p24 core protein was coated onto 96-well ELISA plate (Thermo-Electron, Milford, MA). Mice sera at different dilutions were added to the plates and incubated at room temperature for 2 h with shaking. The p24-antibody was then detected using HRP-conjugated goat anti-mouse immunoglobulin (Southern Biotech, Birmingham, AL) and SureBlue TMB Microwell Peroxidase Substrate (KPL, Gaithersburg, MD).
Study subjects
We were interested in studying human memory HIV-1 and Epstein-Barr virus (EBV) specific anti-viral responses. Thus, untreated HIV-1 infected individuals and HIV-1 negative EBV seropositive individuals were recruited. For CTL assays, four untreated, HIV-1-seropositive individuals (Participants #1 to #4) with varying stages of disease progression were studied, whose clinical profiles are depicted in Table 1. Three asymptomatic HIV-1-uninfected individuals (Participants #5 to #7), who were HLA-A*0201 positive and had detectable EBV specific CD8+ T cell IFN-γ responses by ELISpot assay (data not shown), were also studied. Leukopheresis was performed to obtain large amounts of peripheral blood mononuclear cells (PBMCs). Prior to the study, individuals were class I HLA typed and screened for HIV-1 or EBV specific CTL by culturing PBMCs with HLA-restricted HIV-1 or EBV peptides and detecting IFN-γ producing CD8+ T cells by ELISpot assay as previously described [data not shown, and [30]]. HLA restricted epitopes to HIV-1 for individual participants #1-#4 are shown in Table 1. HIV-1 uninfected participants #5-#7 responded to the HLA-A *0201 restricted BMLF1 region of EBV, (GLCLVAML). Informed consent was obtained from participants in accordance with the guidelines for conduct of clinical research at the University of Toronto and St. Michael’s Hospital. All investigational protocols were approved by the University of Toronto and St. Michael’s Hospital institutional review boards.
Table 1.
Profiles of participants
| Participant(s) | Years HIV-1-infected | CD4 count (/mm3) | Viral Load (copies/ml) | CD8 Epitope | HAART |
|---|---|---|---|---|---|
| Pt 1 | 11 | 950 | <50 | B27/gag/KRWIILGLNK | no |
| Pt 2 | 5 months | 560 | >500,000 | B8/nef/FLKEKGGL | no |
| Pt 3 | 9 | 820 | 158 | A*0201/gag/SLYNTVATL | no |
| Pt 4 | 2 | 330 | 200,000 | A*0201/gag/SLYNTVATL | no |
| Pt 5–7 | HIV-1 negative | NA | NA | A*0201/EBV/GLCLVAML | NA |
NA=not applicable
HAART= highly active anti-retroviral therapy
Generation of MDDCs
The method for generation of MDDCs has been described previously [26]. Briefly, purified monocytes obtained using Monocyte Negative Isolation Kit (Dynal, Oslo, Norway) were cultured in RPMI-1640 medium supplemented with 10% FBS, 2mM L-glutamine, 25mM HEPES, 100 U/ml of penicillin and 100μg/ml streptomycin in the presence of 50ng/ml human rGM-CSF and 100ng/ml human rIL-4 (PeproTech, Rocky Hill, NJ). After 7 days of culture, more than 50% of the cells were CD1ahigh, MHC-II+, CD80low, and CD14-, which represents an immature DC phenotype. The immature MDDCs (iMDDCs) were then infected with vA3131-2, ALVAC II, or vCP205 (Sanofi-Pasteur) at 10 MOI, or incubated with medium alone at 37°C for 48h. Then the cells were harvested and used as MDDCs.
MDDCs maturation and apoptosis analysis
For maturation analysis, MDDCs were stained with PE-mouse anti-human CD80 or CD83 mAb and FITC-mouse anti-human CD86 mAb (BD Biosciences) and analyzed using flow cytometer. For apoptosis analysis, MDDCs were permeablized with Cytofix/Cytoperm solution, stained with FITC- or PE-mouse anti-human caspase-3 mAb (BD Biosciences), and analyzed using flow cytometry.
Induction of peptide-specific CTL
Peptide-specific CTL were expanded by a modification of a method previously described [26]. Briefly, CD4+ T cell-containing or –depleted PBMCs were cocultured for 10 d with autologous MDDCs that were either pulsed or not pulsed with HLA-restricted epitopes of HIV-1 proteins or EBV proteins. MDDCs were previously infected with vA3131-2 or ALVAC II at 10 MOI, or incubated with medium alone at 37°C for 48h.
Cytotoxicity assay
Autologous B-LCL were labeled by incubating in 100μCi sodium 51Cr chromate and pulsed with the specific peptide at 10μM for 1 h at 37°C. Control B-LCL were either pulsed with an irrelevant peptide or cultured in medium alone. Labeled target cells and serial dilutions of effector cells in triplicate were incubated for 4 h. Supernatants were then collected and analyzed in a microplate scintillation counter (TopCount; Packard Instrument, Meriden, CT). Background chromium release was always<10%. Percentage of lysis was calculated from the formula: 100% × (E-M)/(T-M), in which E is experimental release, M is the release in the presence of R-10 medium, and T is the release in the 5% Triton X-100 detergent. Specific lysis was determined by subtracting the lysis of control targets from the lysis of peptide-pulsed targets.
Statistical analysis
Results are expressed as the mean±standard errors of the mean (SEM). Statistical analysis was performed using Prism 4.0 software (GraphPad Software, San Diego, CA). For murine samples, a two-tailed t test was performed. For human samples, a two-tailed t test for paired samples was performed. P < 0.05 was considered statistically significant.
Results
Construction of ALVAC expressing CD40L and testing adjuvancy with vCP1452 in a murine model
We postulated that incorporating CD40L into ALVAC could be used as an adjuvant for the HIV-1 vaccine vCP1452. A previous study reported that soluble multimeric form of CD40L, SP-D-CD40L, was superior to membrane CD40L in augmenting immunogenicity elicited by HIV-1 DNA vaccine [31]. We thus constructed ALVAC vectors expressing both membrane CD40L (vCPmCD40L) and soluble multimeric CD40L (vCPmSP-D-CD40L). Expression of CD40L by vectors was confirmed by detecting transcripts by RT-PCR, and protein by FACS analysis and Western blot of infected cells (Fig. 1). In order to test whether addition of ALVAC expressing CD40L could boost immunogenicity to the HIV-1 protein expressing ALVAC, vCP1452, Balb/c mice were immunized according to the following schedule: group 1: Naïve unimmunized mice; group 2: 107 pfu of vCP1452 + 107 pfu ALVAC II (empty vector); group 3: 107 pfu of vCP1452 + 107 pfu vCPmCD40L; group 4: 107 pfu of vCP1452 + 107 pfu vCPmSP-D-CD40L. Vaccines were given 3 times at 2 week intervals. Six weeks after the last immunization, mice were sacrificed and spleens and sera were collected for immunological analysis. Additional controls included mice that were immunized with vCPmCD40L or vCPmSP-D-CD40L without vCP1452, in which we did not detect any immune responses above naïve control mice (data not shown). CD8+ T cell immune responses were assessed by IFN-γ-ELISpot after co-culture of splenocytes with P815 cells pulsed with the H-2Kd restricted immunodominant epitope of HIV-1 Gag, AMQMLKETI, intracellular cytokine staining after stimulation with AMQMLKETI, and H-2Kd restricted-AMQMLKETI tetramer staining [32]. Mice immunized with ALVAC expressing either form of CD40L had about two fold increased numbers of CD8+ T cells producing IFN-γ against the HIV-1-Gag epitope compared with HIV-1 vaccine vCP1452 without CD40L (83±16 SFC/million cells versus 177±35 SFC/million cells versus 156±28 SFC/million cells for vCP1452+ALVAC II, vCP1452+vCPmCD40L, vCP1452+vCPmSP-D-CD40L, respectively, P<0.05 for both pairs) (Fig. 2A). The adjuvant effect of CD40L on CD8+ T cells was further demonstrated by tetramer staining (Fig. 2B and C). Six weeks after the last immunization, we could barely detect HIV-1 Gag AMQMLKETI-tetramer positive CD8+ T cells in vCP1452+ALVAC II group while in CD40L groups we could easily detect tetramer positive CD8+ T cells (Fig. 2B and C). Recent studies suggest polyfunctional T cells that produce more than one cytokine, chemokine, and marker of degranulation correlate with virologic control and could be used as an immunological parameter for assessing vaccine efficacy [33–35]. We therefore measured the effect of vCPmCD40L and vCPmSP-D-CD40L on the induction of polyfunctional CD8+ T cells. Splenocytes were assessed for co-staining with IFN-γ and TNF-α mAbs or IFN-γ and CD107a mAbs by flow cytometry (Fig. 2D–2G). Co-immunization with ALVAC expressing CD40L could double the number of polyfunctional CD8+ T cells elicited by the HIV-1 vaccine, vCP1452 (Fig. 2D–2G). Both CD40L groups also had greater frequencies of HIV-1 Gag specific IFN-γ CD8+ T cells (Fig. 2H), which included those that were mono-functional (only expressing IFN-γ, data not shown) or co-expressed other cytokines.
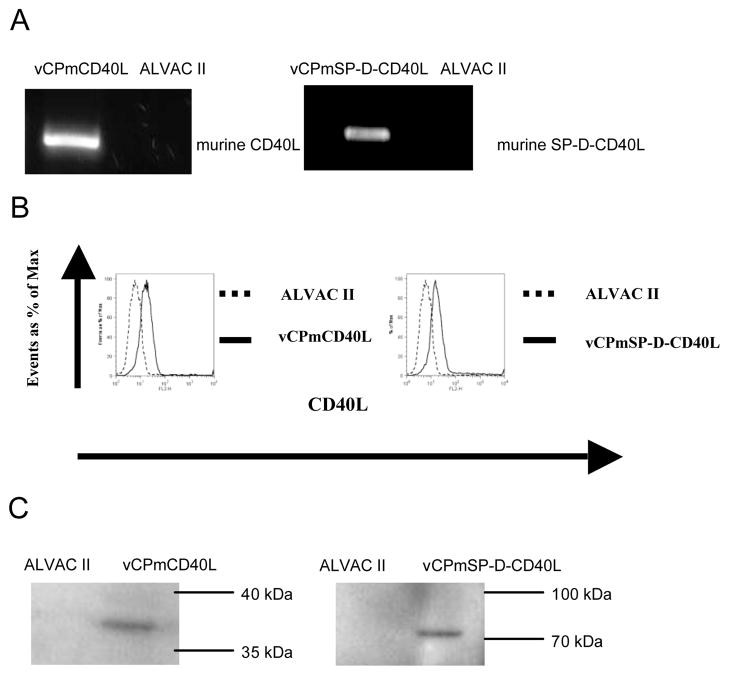
Figure 1.
Expression of murine CD40L by recombinant canarypox vector. Recombinant canarypox viruses vCPmCD40L and vCPmSP-D-CD40L were generated as described in Materials and Methods. (A) RT-PCR. CEF cells were infected by vCPmCD40L, vCPmSP-D-CD40L or its parental control ALVAC II at 5 MOI for 3 days. Total RNA was isolated from infected CEF cells using Trizol Reagent and subjected to RT-PCR with specific primers amplifying the coding sequence of murine CD40L or SP-D-CD40L. The identity of the amplified product was further verified by DNA sequencing (not shown). (B) Flow cytometry. Human PBMCs were infected by vCPmCD40L, vCPmSP-D-CD40L or ALVAC II at 5 MOI for 24 hrs and stained with PE-labeled anti-mouse CD40L mAb. (C) Western blot. Hela cells were infected by vCPmCD40L, vCPmSP-D-CD40L, or ALVAC II at 10 MOI for 24–48hrs. Cell lysates or magnetic beads incubated with supernatant of infected cells were subjected to Western blot and detected with a goat anti-mouse CD40L or anti-mouse SP-D antibody. As expected, the membrane CD40L was about 35–40kD and soluble multimeric form of CD40L was about 70kD.
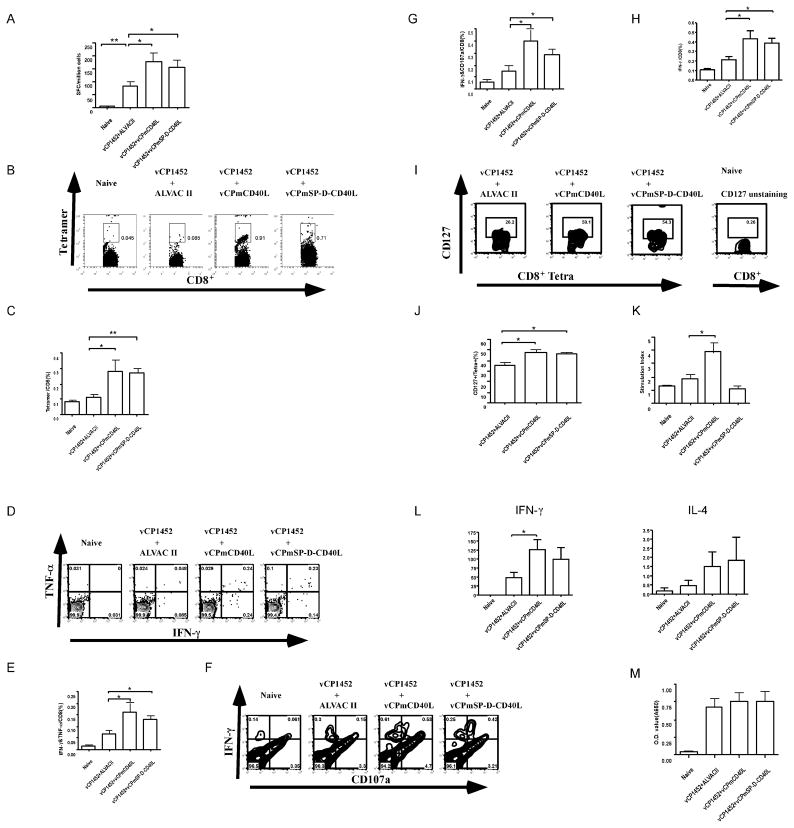
Figure 2.
CD40L expressed by recombinant ALVAC virus boosts the immunogenicity of an HIV-1 canarypox vaccine in mice. Female Balb/c mice of 6–8 wks age were immunized 3 times with an HIV-1 canarypox vaccine, vCP1452, with either of vCPmCD40L or vCPmSP-D-CD40L or ALVAC II. Six weeks after the last immunization, mice were sacrificed and spleens were taken and used for preparation of splenocytes. (A) IFN-γ ELISpot. Splenocytes were stimulated with P815 cells pulsed with an H-2Kd restricted HIV-1 Gag peptide, AMQMLKETI, for 16 hrs. Cells producing IFN-γ were detected and counted as described in Materials and Methods. (B) and (C) MHC-I tetramer analysis. Splenocytes were stained with anti-mouse CD8 mAb and AMQMLKETI/H-2Kd tetramer. (B) shows representative flow cytometry results for each group of mice. Numbers are percentage of tetramer positive cells in CD8+ cells. (C) shows summary of the tetramer data for each group of mice. (D) to (G) Polyfunctional CD8+ T cells analyzed by flow cytometry. Splenocytes were stained with anti-mouse IFN-γ and anti-mouse TNF-α mAbs and co-expressing cells are measured in (D) and (E) or anti-mouse IFN-γ and anti-mouse CD107a mAbs and co-expressing cells are measured in (F) and (G). (D) and (F) show representative flow cytometry results and (E) and (G) show summary of the polyfunctional CD8+ T cell data for each group of mice. (H) CD8+ T cells producing IFN-γ analyzed by flow cytometry. Splenocytes were stained with anti-mouse CD8 and anti-mouse IFN-γ mAbs. (I) and (J) Memory HIV-1-Gag specific CD8+ cells analyzed by flow cytometry. Splenocytes were stained with anti-mouse CD8, anti-mouse CD127 (IL-7Rα) mAbs, and AMQMLKETI/H-2Kd tetramer. In (I) CD8+/Tetramer+ cells are gated and measured by CD127 (y-axis). CD8+ T cells from naïve mice unstained for CD127 was used as control for CD127 gating. (J) shows summary data of CD127 expression in tetramer+ cells. (K) Lymphocyte proliferation. Splenocytes were stimulated with HIV-1 p24 Gag for 6 days and [3H]-thymidine incorporation was measured. (L) Cytokine production. Splenocytes were stimulated with HIV-1 p24 Gag for 5 days. IFN-γ and IL-4 in the supernatant were measured by ELISA. (M) Serum anti-Gag antibody. HIV-1 p24 Gag was used as antigen, and mice serum anti-Gag antibodies were measured by ELISA. Data shown are mean±SEM. n=4–10. *: P<0.05. **: P<0.01.
An effective CTL based vaccine should theoretically induce long-lasting memory CD8+ T cells that correlate with IL-7Rα (CD127) expression [36]. Co-immunizing with CD40L increased the frequency of Gag AMQMLKETI-tetramer positive CD8+ T cells that expressed CD127 (Fig. 2I and J). We then studied the effects of CD40L on other aspects of adaptive immune response. To assess CD4+ T cell immune responses we examined proliferative responses and cytokine production to p24 HIV-1 Gag antigen. Similarly to CD8+ T cell responses, co-immunization with ALVAC expressing membrane CD40L increased splenocyte proliferation to HIV-1 Gag protein, as well as IFN-γ production within supernatants of stimulated cells (Fig. 2K and L). Of note however, was that the multimeric soluble form of CD40L could not enhance CD4+ T cell responses. HIV-1 Gag induced IFN-γ but not IL-4 was significantly increased in the vCP1452+CD40L groups compared with the vCP1452+ALVAC II group (Fig 2L), indicating a Th1 rather than a Th2 response was augmented by CD40L. In addition, we detected HIV-1 Gag-antibody in sera of vaccinated mice and found both membrane form and soluble multimeric form of CD40L could not boost HIV-1 humoral immune response elicited by vCP1452 (Fig. 2M), which is consistent with CD40L tending to polarize toward a Th1 response. Taken together, our results demonstrate that CD40L expressed from canarypox vector could augment the cellular immune response of an HIV-1 canarypox vaccine. We found no significant differences in the immunogenicity of CD8+ T cell responses if the CD40L was expressed as a membrane or secreted multimeric form in the canarypox vector.
Canarypox vector-expressed CD40L can activate and mature human MDDCs in vitro
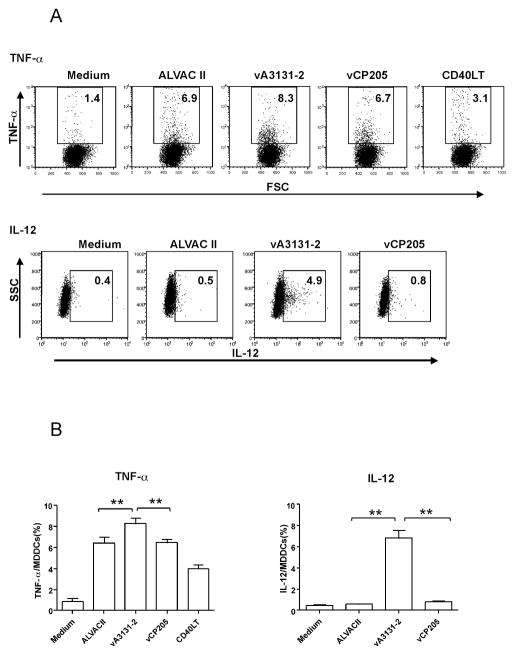
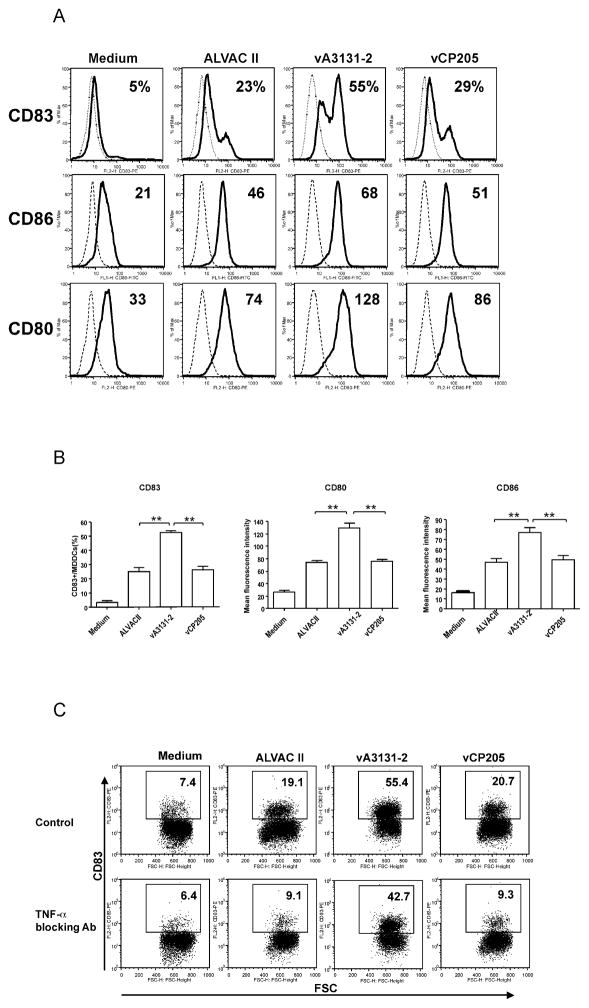
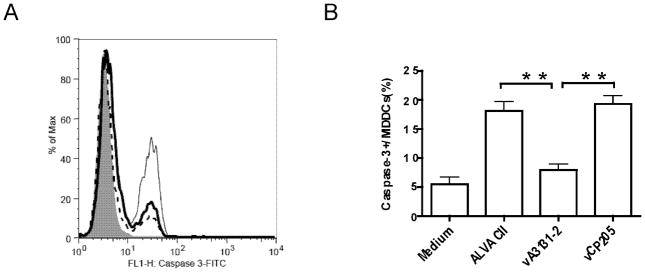
The above murine data suggests CD40L might be used as an adjuvant for the current HIV-1 ALVAC vaccine. As a first step for possible clinical application, we first studied the effect of canarypox vector-expressed CD40L on human immune responses in vitro. A recombinant canarypox vector expressing a membrane form of human CD40L, vA3131-2, was constructed and expression of human CD40L in infected cells was verified by flow cytometry and Western blot (data not shown). Previous studies show that CD40L could activate and mature human monocyte derived dendritic cells, which then can expand immune responses [26, 37, 38]. Therefore, we first studied the effect of CD40L expressing from the canarypox vector on human immature monocyte derived dendritic cells (iMDDCs). iMDDCs generated from HIV-1 infected individuals with detectable HIV-1 specific CD8+ T cell responses were infected by vA3131-2 (expressing human CD40L), its parental control ALVAC II, or vCP205, an HIV-1 canarypox vaccine expressing HIV-1 gag, env, and pol. Maturation and activation of iMDDCs were then assessed by cytokine production and expression of surface markers using flow cytometry. As shown in Fig. 3 and 4, both ALVAC II and vCP205 infection of iMDDCs upregulated the production of TNF-α and IL-12 and expression of DC maturation markers, CD83, CD86, and CD80, indicating that the canarypox vector itself could activate human MDDCs, as was previously shown [39]. However, vA3131-2 (ALVAC expressing CD40L) infection always led to significantly higher production of the cytokines, particularly IL-12, and expression of the surface markers CD83, CD80 and CD86 (P<0.01 compared with ALVAC II or vCP205). Ignatius et al. [39] previously showed that canarypox vectors mature MDDCs by enhancing TNF-α production and inducing MDDCs apoptosis. However, the activation and maturation of iMDDCs infected by vA3131-2 was minimally affected by adding TNF-α blocking Ab into the culture medium, whereas anti-TNF-α greatly reduced the activation and maturation of iMDDCs infected by ALVAC II and vCP205 (Fig 4C). Furthermore, using caspase-3 as an apoptosis marker, we found parental ALVAC II and vCP205 induced apoptosis of MDDCs, while the CD40L-expressing vector prevented ALVAC vector-induced apoptosis (Fig. 5). Therefore, the activation and maturation of iMDDCs by vA3131-2 is mainly due to CD40L expressed from the canarypox vector. Canarypox vector itself can also moderately activate and mature iMDDCs.
Figure 3.
CD40L expressed by recombinant ALVAC virus enhances TNF-α and IL-12 production of human MDDCs. Human immature MDDCs were infected with recombinant ALVAC virus expressing human CD40L called vA3131-2, an ALVAC-HIV vaccine called vCP205, or a parental control, ALVAC II at an MOI of 10, or incubated with medium alone, or with CD40L trimer (2 μg/ml) as a positive control at 37°C for 24 h. The production of TNF-α and IL-12 was determined by intracellular staining flow cytometry. (A) Data are taken from the HIV-1-infected participant #1 and are representative of experiments with MDDCs derived from two HIV-1-infected and two HIV-1-uninfected individuals. The numbers in each gate represent the percentage of TNF-α or IL-12-positive MDDCs in total MDDCs. (B) Pooled data from all participants are shown. Data shown are mean±SEM. **: P<0.01.
Figure 4.
CD40L expressed by recombinant ALVAC virus promotes human MDDCs maturation independent of TNF-α secretion. Immature MDDCs were infected with recombinant ALVAC virus expressing human CD40L, vA3131-2; an ALVAC-HIV vaccine, vCP205; or a parental control, ALVAC II at an MOI of 10, or incubated with medium alone at 37°C for 48 h. Expression of surface molecules was analyzed by flow cytometry. (A) Numbers in CD83 row represent percentage of CD83 positive cells in total MDDCs and numbers in CD86 and CD80 rows represent the mean fluorescence intensity of specific staining (histogram with solid line) subtracted from the value of background staining with matched isotype control mouse mAbs (dotted histogram). Data shown are from HIV-1-infected participant #1 and representative of six experiments performed with MDDCs obtained from three HIV-1-uninfected blood donors and three HIV-1-infected individuals. (B) Pooled data from all six participants studied are shown. Data shown are mean±SEM. **: P<0.01. (C) Immature human MDDCs were infected or not (medium) with vA3131-2 (CD40L expressing), vCP205, or ALVAC II at an MOI of 10 and then incubated in the presence of a blocking anti-human TNF-α Ab or the control isotype immunoglobulin (20 μg/mL). After 24 h incubation, MDDCs were collected and stained for CD83 expression to monitor DC maturation. Numbers in each dot plot represent percentage of CD83 positive cells in total MDDCs. Expression of CD83 on MDDCs treated with isotype immunoglobulin is similar to that on MDDCs treated with medium. Increasing the concentration of blocking anti-human TNF-α Ab up to 100 μg/mL did not further reduce the CD83 expression in all conditions (data not shown). The results represent one of five experiments.
Figure 5.
Effects of ALVAC recombinant vA3131-2 expressing human CD40L on apoptosis of DCs. Immature MDDCs were infected or not (medium control) with either parental ALVAC II, ALVAC recombinant vA3131-2 expressing human CD40L, or an HIV canarypox vaccine vCP205 at an MOI of 10 at 37°C for 48 h. Infected cells were harvested and early apoptotic marker caspase-3 was determined by intracellular staining and flow cytometry using FITC- or PE-conjugated mAb against human caspase-3. (A) MDDCs were intracellularly stained for caspase-3 expression to detect early apoptotic MDDCs. The level of caspase-3 expression (x axis) is shown for each of the conditions. Background staining with FITC-conjugated isotype control Ab is shown by a solid gray histogram; medium-treated MDDCs are shown by a histogram with a dotted line, MDDCs infected with vA3131-2 are represented with a histogram with a dark line, and MDDCs infected with parental ALVAC II are represented with a histogram with a light line. The results represent one of five experiments from two HIV-1-uninfected and three HIV-1-infected donors. (B) Pooled data from all participants are shown. Data shown are mean±SEM. **: P<0.01.
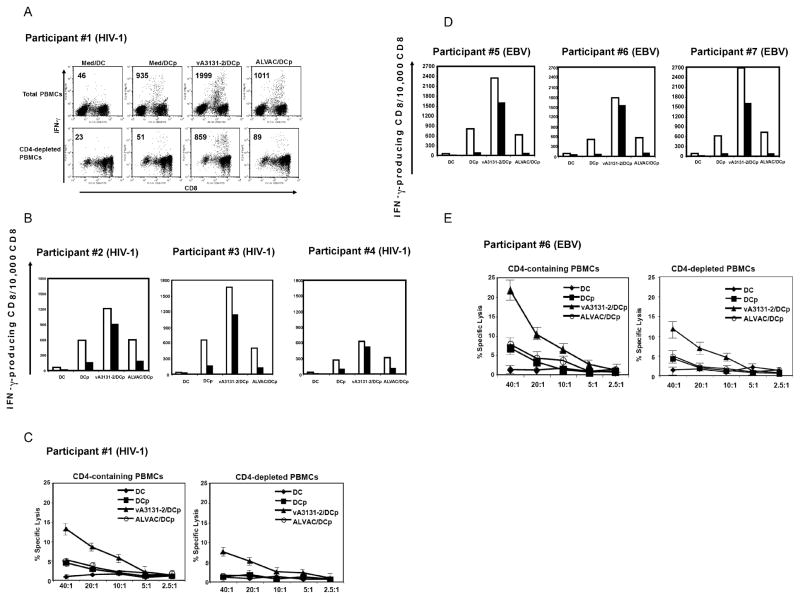
iMDDCs infected by vA3131-2 (ALVAC expressing human CD40L) can expand HIV-1- and EBV-specific memory CTL in vitro in both CD4+ T cell-containing and –depleted conditions
To determine whether CD40L expressed from canarypox vector can expand virus-specific memory CTL responses, we used an in vitro co-culture method in which peptide-pulsed MDDCs stimulate CD8+ T cells in the absence of exogenous cytokines, as previously described [26]. In this co-culture system, expansion of CD8+ T cells is highly dependent on the presence of CD4+ T cells providing help. We also performed experiments in both CD4+ T cell-containing and –depleted conditions to determine whether CD40L expressed from canarypox vector could completely substitute for CD4+ T cell help of CTL responses. MDDCs from 4 HIV-1 infected individuals (Table 1) were infected by vA3131-2 or the control parental canarypox vector, ALAVC II for 48 h, and then pulsed with an HLA class I-restricted HIV-1-specific epitope, and cocultured with autologous PBMCs in CD4+ T cell-containing or –depleted conditions. After 10 days of co-culture, CTL effector activity was assessed by intracellular IFN-γ production and direct cytolysis of peptide-pulsed targets after exposure to peptide-pulsed autologous B-LCL or T cell-depleted PBMCs. Representative experiments measuring CTL by intracellular IFN-γ flow cytometry from subject 1 and the other 3 subjects are illustrated in Fig. 6A and B, respectively. Fig. 6C shows representative chromium release assay results from subject 1. iMDDCs infected by vA3131-2 (expressing CD40L) markedly enhanced HIV-1 specific memory CTL responses when compared with medium-treated iMDDCs or control ALVAC II-infected iMDDCs. In addition, vA3131-2 could replace CD4+ T cell help and enhanced CTL responses to levels observed in the presence of help (Fig. 6A–6C). To determine whether CD40L expressed from canarypox vector could also boost CTL responses against other viruses, iMDDCs from 3 HIV-1-uninfected individuals who had previously demonstrated an HLA-A*0201-restricted response to the EBV epitope of BMLF1 (lytic cycle Ag) (Table 1) were pulsed with peptide and infected by vA3131-2 and co-cultured with CD4+ T cell- containing or –depleted autologous PBMCs for 10 days. EBV-specific CTL responses were measured by intracellular IFN-γ production and direct cytolysis of peptide-pulsed targets after exposure to peptide-pulsed autologous B-LCL or T cell-depleted PBMCs. Similar to HIV-1-infected individuals, iMDDCs infected by vA3131-2 could substantially enhance EBV specific CTL responses and CD40L expressed from vA3131-2 could fully to partially substitute for CD4+ T cell help in our experiments (Fig. 6D and E).
Figure 6.
CD40L expressed by recombinant ALVAC virus enhances specific antiviral CTL activity. CD4+ T cell-containing or -depleted PBMCs from four HIV-1-infected individuals and three HIV-1-uninfected individuals were cocultured with autologous MDDCs that were either pulsed or not with HLA-restricted epitopes of HIV-1 proteins (for HIV-1 infected individuals) or EBV proteins (for HIV-1-uninfected individuals). MDDCs were previously infected or not (medium) with parental ALVAC II or ALVAC recombinant vA3131-2 expressing human CD40L at an MOI of 10 for 48 h. On day 10, specific CTL activity was assessed by intracellular flow cytometric analysis of IFN-γproducing CD8+ T cells and 51Cr release assay. (A) Representative intracellular IFN-γ flow cytometric data obtained from HIV-1-positive participant #1. (B) Summary data from intracellular IFN-γ flow cytometry of HIV-1-positive participant #2–#4 are graphically depicted. Open bars represent CD4+ T cell-containing condition and dark bar represent CD4+ T cell -depleted conditions. (C) A representative 51Cr release assay result from HIV-1-positive participant #1 is shown. Similar results were obtained with HIV-1-positive participant #2 (data not shown). (D) Summary data from intracellular IFN-γ flow cytometric analysis of EBV-positive participant #5–#7 are graphically depicted. Open bars represent CD4+ T cell -containing condition and dark bars represent CD4+ T cell -depleted conditions. (E) A representative 51Cr release assay result from EBV-positive participant #6 is shown. Similar results were obtained with EBV-positive participant #5 (data not shown). DC, MDDCs not pulsed with peptide; DCp, MDDCs pulsed with peptide; vA3131-2/DCp, vA3131-2-infected MDDCs pulsed with peptide; ALVAC/DCp, parental ALVAC II-infected MDDCs pulsed with peptide.
Discussion
Recently, a phase 2b trial of an adenovirus-based vaccine aiming to induce cellular immune responses against HIV-1 failed to provide protection from HIV-1 infection, nor did it reduce HIV-1 viral load in infected individuals[40]. This failure highlights the challenges facing HIV-1 vaccine development, which calls for new systematic approaches, including novel antigen design, new vaccine vectors, and novel adjuvants[1, 2, 41]. Members of the poxvirus family, especially attenuated poxviruses, like modified vaccinia virus Ankara (MVA), NYVAC, and ALVAC, have been used as vectors for HIV-1 vaccines. However, the immunogenicity of these vaccines in humans is also generally low to modest[42, 43], implying that further efforts need to be done to improve the immunogenicity of current poxvirus vector HIV-1 vaccines.
Our results demonstrate that CD40L expressed from canarypox vector can boost cellular immunogenicity of HIV-1 canarypox vaccine in mice and enhance CD8+ T cell memory responses in both HIV-1-infected and HIV-1-uninfected individuals. Recent advances in immunology have shown that several types of molecules may be used as novel adjuvants to enhance immunogenicity of vaccines, such as cytokines, chemokines, Toll-like receptor ligands, and some costimulatory molecules [44–47]. Among these molecules, CD40L is attractive since it is one of the main mechanisms by which CD4+ T cells provide help to CTL and B cells [16, 18, 19, 31]. The CD4+T-cell-replacement activity of CD40L in our DC co-culture experiments is especially intriguing in the setting of development of a therapeutic vaccine for HIV-1. One advantage of canarypox vector is its high-profiled safety, which has been demonstrated in both animal studies and clinical trials [5, 9, 29, 48]. Another advantage of canarypox vector is that as a DNA virus that can only abortively infect mammalian cells, the target gene will be expressed transiently, which may be especially important for the expression of costimulatory molecules, such as CD40L, since long-term presence of costimulatory molecules might lead to autoimmunity or immunosuppression[49–51]. In our current study, we found no discernible signs of adverse effects of CD40L-expressing canarypox vector when used in mice. In contrast to the work by Stone et al. [31] who demonstrated that multimeric secreted CD40L was more potent than membrane expressed CD40L in a DNA vaccine, we found no differences in adjuvant activity when comparing both forms in the context of canarypox virus vectors. These differences highlight the complexity of transferring similar immune enhancement strategies between different vaccine vectors. Similar findings have also been observed by others. For example, Kovarik et al. found IL-12 could enhance Th1 responses to measles hemagglutinin DNA vaccine but not to ALVAC vaccine expressing the same antigen[52]. Furthermore, other factors, such as antigens and delivery forms of adjuvants, have all been reported to affect the adjuvancy effect[53, 54]. Therefore, different vaccines may need different adjuvants to achieve best vaccination results.
Recent studies suggest that measuring multiple phenotype parameters simultaneously may better reflect the quality of CD8+ T cells [33–35, 55]. The addition of canarypox expressing CD40L had not only increased specific CD8+ T cell responses qualitatively but also improved the quality of CD8+ T cells as exhibited by more specific polyfunctional CD8+ T cells and potentially more long lived memory CD8+ T cells as determined by IL-7Rα staining. Some previous reports suggested that CD40L is also important for enhancing CD4+ T cell responses [56–58]. Our data are consistent with these reports in that the CD40L regimens enhanced splenocyte proliferation and Th1 cytokine production to vaccine antigens, however, only when CD40L was expressed as a membrane molecule in canarypox vector. Surprisingly, our vaccination strategy had no effect on humoral immunity indicating that expressing the TNFSF molecule CD40L in the context of a canarypox virus based vector does not affect B cell responses. This is consistent with a previous report showing a lack of effect of CD40L DNA vector in humoral immunity [31].
We also show that CD40L expressing canarypox vectors can mature human DCs in a co-culture system and expand HIV-1 specific CTL and EBV specific CTL from HIV-1 infected and uninfected individuals, respectively. Consistent with previous results using exogenous CD40L trimer [26, 37, 38], we found CD40L expressed from canarypox vector could activate and mature human MDDCs to a greater extent than that of the parental control virus or a clinically used HIV-1 canarypox vaccine, vCP205. ALVAC virus has previously been shown to activate and mature DCs via ingestion of apoptotic DCs and TNF-α secretion induced by ALVAC infection [39, 59, 60]. This was verified in our experiments, however, ALVAC expressing CD40L primarily matured DCs independently of TNF-α, supporting a direct effect through CD40L expression on DCs. Furthermore, whereas parental ALVAC and HIV-1 expressing vCP205 induced apoptosis, CD40L-expressing vectors prevented ALVAC vector-induced apoptosis. This latter effect may be important for prolonging DC stimulation of T cells, given that canarypox virus vectors have such a transient expression in vivo. The effects of CD40L expressing canarypox on human DCs were translated in the ability of these DC to expand virus specific memory CTL from HIV-1 infected and uninfected individuals, in the presence or absence of CD4 help. The latter finding is particularly important, given that HIV-1 infected individuals with low CD4 counts respond poorly to vaccinations [61]. Such a strategy could be used to improve immunogenicity of vaccines in HIV-1 infected individuals, as well as in the context of therapeutic vaccination against HIV-1 in HIV-1 infected individuals. Of equal importance, is that, the currently tested HIV-1 expressing canarypox vector, ALVAC-vCP1452, may be significantly improved in terms of immunogenicity, if given with CD40L expressing ALVACs.
Therapeutic vaccination against HIV-1 may be beneficial to HIV-1 infected individuals by helping restore cellular immune responses and extend interruption of highly active anti-retroviral therapy (HAART)[62]. However, two requirements have to be fulfilled by therapeutic vaccines in this scenario: they must be both safe in the setting of immune compromise and secondly must be able to provide ‘help’ to defective CTL responses given the already poor function of CD4+ T cells in HIV-1 infected individuals. In this regard, our results presented here imply canarypox HIV-1 vaccines, which have been shown to be safe in HIV-1 infected individuals[63], plus canarypox vectors expressing CD40L that can provide ‘help’ to CTL, may be a promising therapeutic vaccination strategy against HIV-1 infection.
Acknowledgments
This research is supported by OHTN, CIHR, and Aventis-UTRP. MO received salary support from the OHTN and CIHR. JL is the recipient of OHTN Postdoctoral Fellowship.
References
- 1.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006 Dec;6(12):930–9. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 2.Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Engl J Med. 2007 May 17;356(20):2073–81. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 3.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11341–8. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fries LF, Tartaglia J, Taylor J, Kauffman EK, Meignier B, Paoletti E, et al. Human safety and immunogenicity of a canarypox-rabies glycoprotein recombinant vaccine: an alternative poxvirus vector system. Vaccine. 1996 Apr;14(5):428–34. doi: 10.1016/0264-410x(95)00171-v. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia J, Perkus ME, Taylor J, Norton EK, Audonnet JC, Cox WI, et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992 May;188(1):217–32. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- 6.Cox WI, Tartaglia J, Paoletti E. Induction of cytotoxic T lymphocytes by recombinant canarypox (ALVAC) and attenuated vaccinia (NYVAC) viruses expressing the HIV-1 envelope glycoprotein. Virology. 1993 Aug;195(2):845–50. doi: 10.1006/viro.1993.1442. [DOI] [PubMed] [Google Scholar]
- 7.Pitisuttithum P. HIV-1 prophylactic vaccine trials in Thailand. Curr HIV Res. 2005 Jan;3(1):17–30. doi: 10.2174/1570162052772933. [DOI] [PubMed] [Google Scholar]
- 8.Marovich MA. ALVAC-HIV vaccines: clinical trial experience focusing on progress in vaccine development. Expert Rev Vaccines. 2004 Aug;3(4 Suppl):S99–104. doi: 10.1586/14760584.3.4.s99. [DOI] [PubMed] [Google Scholar]
- 9.de Bruyn G, Rossini AJ, Chiu YL, Holman D, Elizaga ML, Frey SE, et al. Safety profile of recombinant canarypox HIV vaccines. Vaccine. 2004 Jan 26;22(5–6):704–13. doi: 10.1016/j.vaccine.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Liniger M, Zuniga A, Naim HY. Use of viral vectors for the development of vaccines. Expert Rev Vaccines. 2007 Apr;6(2):255–66. doi: 10.1586/14760584.6.2.255. [DOI] [PubMed] [Google Scholar]
- 11.Clements-Mann ML, Weinhold K, Matthews TJ, Graham BS, Gorse GJ, Keefer MC, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998 May;177(5):1230–46. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 12.Cleghorn F, Pape JW, Schechter M, Bartholomew C, Sanchez J, Jack N, et al. Lessons from a multisite international trial in the Caribbean and South America of an HIV-1 Canarypox vaccine (ALVAC-HIV vCP1452) with or without boosting with MN rgp120. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):222–30. doi: 10.1097/QAI.0b013e318149297d. [DOI] [PubMed] [Google Scholar]
- 13.Thongcharoen P, Suriyanon V, Paris RM, Khamboonruang C, de Souza MS, Ratto-Kim S, et al. A Phase 1/2 Comparative Vaccine Trial of the Safety: ALVAC-HIV (vCP1521) Prime With Oligomeric gp160 (92TH023/LAI-DID) or Bivalent gp120 (CM235/SF2) Boost. J Acquir Immune Defic Syndr. 2007 Jul 5; doi: 10.1097/QAI.0b013e3181354bd7. [DOI] [PubMed] [Google Scholar]
- 14.Goepfert PA, Horton H, McElrath MJ, Gurunathan S, Ferrari G, Tomaras GD, et al. High-dose recombinant Canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. J Infect Dis. 2005 Oct 1;192(7):1249–59. doi: 10.1086/432915. [DOI] [PubMed] [Google Scholar]
- 15.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 16.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 17.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004 Jun;4(6):420–31. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 18.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 19.Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003 Jun–Aug;14(3–4):297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 20.Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000 Jan 15;164(2):725–32. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 21.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998 Jun 4;393(6684):478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 22.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998 Jun 4;393(6684):474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 23.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998 Jun 4;393(6684):480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 24.Barr TA, Carlring J, Heath AW. Co-stimulatory agonists as immunological adjuvants. Vaccine. 2006 Apr 24;24(17):3399–407. doi: 10.1016/j.vaccine.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006 Feb 15;176(4):2486–95. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- 26.Yu Q, Gu JX, Kovacs C, Freedman J, Thomas EK, Ostrowski MA. Cooperation of TNF family members CD40 ligand, receptor activator of NF-kappa B ligand, and TNF-alpha in the activation of dendritic cells and the expansion of viral specific CD8+ T cell memory responses in HIV-1-infected and HIV-1-uninfected individuals. J Immunol. 2003 Feb 15;170(4):1797–805. doi: 10.4049/jimmunol.170.4.1797. [DOI] [PubMed] [Google Scholar]
- 27.Ostrowski MA, Justement SJ, Ehler L, Mizell SB, Lui S, Mican J, et al. The role of CD4+ T cell help and CD40 ligand in the in vitro expansion of HIV-1-specific memory cytotoxic CD8+ T cell responses. J Immunol. 2000 Dec 1;165(11):6133–41. doi: 10.4049/jimmunol.165.11.6133. [DOI] [PubMed] [Google Scholar]
- 28.Fang ZY, Kuli-Zade I, Spearman P. Efficient human immunodeficiency virus ( HIV)-1 Gag-Env pseudovirion formation elicited from mammalian cells by a canarypox HIV vaccine candidate. J Infect Dis. 1999 Oct;180(4):1122–32. doi: 10.1086/315028. [DOI] [PubMed] [Google Scholar]
- 29.Cadoz M, Strady A, Meignier B, Taylor J, Tartaglia J, Paoletti E, et al. Immunisation with canarypox virus expressing rabies glycoprotein. Lancet. 1992 Jun 13;339(8807):1429–32. doi: 10.1016/0140-6736(92)92027-d. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowski MA, Justement SJ, Ehler L, Mizell SB, Lui S, Mican J, et al. The role of CD4(+) T cell help and CD40 ligand in the In vitro expansion of HIV-1-specific memory cytotoxic CD8(+) T cell responses. J Immunol. 2000;165(11):6133–41. doi: 10.4049/jimmunol.165.11.6133. [DOI] [PubMed] [Google Scholar]
- 31.Stone GW, Barzee S, Snarsky V, Kee K, Spina CA, Yu XF, et al. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. Journal of virology. 2006 Feb;80(4):1762–72. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mata M, Travers PJ, Liu Q, Frankel FR, Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J Immunol. 1998 Sep 15;161(6):2985–93. [PubMed] [Google Scholar]
- 33.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007 Oct 1;204(10):2473–85. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007 Jun 11;204(6):1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003 Dec;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 37.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994 Oct 1;180(4):1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998 Sep 1;161(5):2094–8. [PubMed] [Google Scholar]
- 39.Ignatius R, Marovich M, Mehlhop E, Villamide L, Mahnke K, Cox WI, et al. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor alpha secretion. J Virol. 2000 Dec;74(23):11329–38. doi: 10.1128/jvi.74.23.11329-11338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen J. AIDS research. Promising AIDS vaccine’s failure leaves field reeling. Science (New York, NY) 2007 Oct 5;318(5847):28–9. doi: 10.1126/science.318.5847.28. [DOI] [PubMed] [Google Scholar]
- 41.Douek DC, Kwong PD, Nabel GJ. The rational design of an AIDS vaccine. Cell. 2006 Feb 24;124(4):677–81. doi: 10.1016/j.cell.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Hanke T, McMichael AJ, Dorrell L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol. 2007 Jan;88(Pt 1):1–12. doi: 10.1099/vir.0.82493-0. [DOI] [PubMed] [Google Scholar]
- 43.Schoenly KA, Weiner DB. Human immunodeficiency virus type 1 vaccine development: recent advances in the cytotoxic T-lymphocyte platform “spotty business”. Journal of virology. 2008 Apr;82(7):3166–80. doi: 10.1128/JVI.01634-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barouch DH, McKay PF, Sumida SM, Santra S, Jackson SS, Gorgone DA, et al. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. Journal of virology. 2003 Aug;77(16):8729–35. doi: 10.1128/JVI.77.16.8729-8735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005 Jul 1;175(1):112–23. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 46.Kwissa M, Amara RR, Robinson HL, Moss B, Alkan S, Jabbar A, et al. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J Exp Med. 2007 Oct 29;204(11):2733–46. doi: 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrison JM, Bertram EM, Boyle DB, Coupar BE, Ranasinghe C, Ramshaw IA. 4–1BBL coexpression enhances HIV-specific CD8 T cell memory in a poxvirus prime-boost vaccine. Vaccine. 2006 Nov 17;24(47–48):6867–74. doi: 10.1016/j.vaccine.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Paoletti E, Tartaglia J, Taylor J. Safe and effective poxvirus vectors--NYVAC and ALVAC. Dev Biol Stand. 1994;82:65–9. [PubMed] [Google Scholar]
- 49.Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001 Dec 1;167(11):6330–7. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 50.Havari E, Lennon-Dumenil AM, Klein L, Neely D, Taylor JA, McInerney MF, et al. Expression of the B7.1 costimulatory molecule on pancreatic beta cells abrogates the requirement for CD4 T cells in the development of type 1 diabetes. J Immunol. 2004 Jul 15;173(2):787–96. doi: 10.4049/jimmunol.173.2.787. [DOI] [PubMed] [Google Scholar]
- 51.Bukczynski J, Wen T, Wang C, Christie N, Routy JP, Boulassel MR, et al. Enhancement of HIV-specific CD8 T cell responses by dual costimulation with CD80 and CD137L. J Immunol. 2005 Nov 15;175(10):6378–89. doi: 10.4049/jimmunol.175.10.6378. [DOI] [PubMed] [Google Scholar]
- 52.Kovarik J, Martinez X, Pihlgren M, Bozzotti P, Tao MH, Kipps TJ, et al. Limitations of in vivo IL-12 supplementation strategies to induce Th1 early life responses to model viral and bacterial vaccine antigens. Virology. 2000 Mar 1;268(1):122–31. doi: 10.1006/viro.1999.0159. [DOI] [PubMed] [Google Scholar]
- 53.Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. Journal of virology. 2002 Jan;76(1):243–50. doi: 10.1128/JVI.76.1.243-250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reali E, Canter D, Zeytin H, Schlom J, Greiner JW. Comparative studies of Avipox-GM-CSF versus recombinant GM-CSF protein as immune adjuvants with different vaccine platforms. Vaccine. 2005 Apr 22;23(22):2909–21. doi: 10.1016/j.vaccine.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 55.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006 Nov;28(3):209–19. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 56.MacLeod M, Kwakkenbos MJ, Crawford A, Brown S, Stockinger B, Schepers K, et al. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J Exp Med. 2006 Apr 17;203(4):897–906. doi: 10.1084/jem.20050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerloni M, Xiong S, Mukerjee S, Schoenberger SP, Croft M, Zanetti M. Functional cooperation between T helper cell determinants. Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):13269–74. doi: 10.1073/pnas.230429197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000 May 1;164(9):4465–70. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 59.Ryan EJ, Harenberg A, Burdin N. The Canarypox-virus vaccine vector ALVAC triggers the release of IFN-gamma by Natural Killer (NK) cells enhancing Th1 polarization. Vaccine. 2007 Apr 30;25(17):3380–90. doi: 10.1016/j.vaccine.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Q, Jones B, Hu N, Chang H, Ahmad S, Liu J, et al. Comparative analysis of tropism between canarypox (ALVAC) and vaccinia viruses reveals a more restricted and preferential tropism of ALVAC for human cells of the monocytic lineage. Vaccine. 2006 Sep 29;24(40–41):6376–91. doi: 10.1016/j.vaccine.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Lane HC, Depper JM, Greene WC, Whalen G, Waldmann TA, Fauci AS. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 62.Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nature medicine. 2000 Oct;6(10):1140–6. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- 63.Jin X, Ramanathan M, Jr, Barsoum S, Deschenes GR, Ba L, Binley J, et al. Safety and immunogenicity of ALVAC vCP1452 and recombinant gp160 in newly human immunodeficiency virus type 1-infected patients treated with prolonged highly active antiretroviral therapy. Journal of virology. 2002 Mar;76(5):2206–16. doi: 10.1128/jvi.76.5.2206-2216.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]