Abstract
A history of limited, intermittent intake of palatable food (sucrose drink) attenuates hypothalamic-pituitary-adrenal (HPA) axis stress responses and induces markers of neuronal plasticity in stress- and reward-regulatory brain regions. Synaptic plasticity could provide a mechanism for long-term changes in neuronal function, implying that sucrose stress-dampening may endure over long periods of time. The present study tests the persistence of HPA axis dampening and plasticity after cessation of palatable drinking. Adult, male Long-Evans rats (n = 10–13) with free access to water and chow were given additional twice-daily access to 4 ml sucrose (30%) or water for 14 days. Rats were subsequently tested for HPA responsiveness to an acute (20-minute) restraint stress at 1, 6 and 21 days after the cessation of sucrose. Brains were collected for immunohistochemical analysis of FosB/deltaFosB, a marker of long-term neuronal plasticity, in the basolateral amygdala and nucleus accumbens. Prior sucrose consumption significantly decreased the plasma corticosterone response to restraint at one day after the last palatable drink presentation, and also increased FosB/deltaFosB-positive cells in the basolateral amygdala and in the nucleus accumbens core. This HPA-dampening persisted through 21 days after the termination of the palatable drink, as did the increased FosB/deltaFosB immunoreactivity in both the BLA and the NuAc core. These data suggest that chronic palatable food intake causes lasting changes in stress/reward-modulatory circuitry and that the suppressed hormonal response to stress that can persist well beyond periods of palatable drink exposure.
Keywords: Sucrose, restraint stress, corticosterone, FosB/DeltaFosB, basolateral amygdala, nucleus accumbens, reward
1. INTRODUCTION
Activation of the HPA axis is a primary response to stress, and functions to maintain an organism's health and well-being in the face of a physiological or psychological challenge. Glucocorticoids (e.g., corticosterone in rats) are the major output of HPA axis cascade, and serve numerous physiologic functions that promote survival and adaptation. Higher brain regions, including limbic regions, can influence HPA axis regulation with respect to prior experience or innate predispositions [1,2,3,4]. Palatable food intake can buffer the physiological and behavioral responses to stress, an effect commonly referred to as stress relief by “comfort” foods [5,6,7,8,9,10]. However, the neural mechanism by which this regulation occurs and the persistence of stress buffering over time remain to be determined.
In our model of `snack' exposure, chronic, limited access to palatable food reliably decreases the plasma corticosterone response to a stress challenge. Moreover, this decrease persists at least 7 days after the end of the snacking paradigm [11]. Lesion studies demonstrate that the basolateral amygdala (BLA) is necessary for the palatable snack- induced stress-buffering effect [10,11]. In addition, two weeks of sucrose exposure increases expression of BLA genes associated with the long-term potentiation and calcium signaling pathways [11]. Finally, phosphorylated-CaMKII, phosphorylated-CREB, and synaptophysin proteins are all increased in the BLA at 16 hours after completing the sucrose snacking paradigm [11]. Together these data suggest that plastic changes in stress and reward brain regions, like the BLA, occur as a result of palatable snacking. Importantly, neural plasticity could produce long-term changes in neuronal function [12,13,14], suggesting that stress-dampening by sucrose may endure long after the last sucrose exposure. Previous work has linked expression of FosB/deltaFosB to long-term exposure to rewarding stimuli. For example, natural rewards such as sexual behavior, a high-fat diet, and ad libitum sucrose drinking each increase FosB/deltaFosB immunoreactivity in the nucleus accumbens (NuAc) [15,16]. In addition, in rats, chronic cocaine increases FosB/deltaFosB immunoreactivity in the BLA for several weeks after the last dose [12]. DeltaFosB is the truncated form of FosB that, after repeated stimuli, is believed to help convert short-term reactions into the long-term adaptations underlying neural and behavioral plasticity [13]. The present studies examine whether limited sucrose intake (in which sucrose accounts for approximately 10% of daily calories) is sufficient to buffer HPA responses in a long term fashion, and tests the hypothesis that stress-buffering actions are associated with long-term induction FosB/deltaFosB in critical brain reward circuitry, including the NuAc and/or BLA.
2. METHODS
All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee and were consistent with NIH guidelines. Single-housed, male Long-Evans rats (250 g) from Harlan Labs (Indianapolis, IN) were housed in a temperature- and humidity-controlled vivarium with a 12-hour/12-hour light cycle (lights on at 06:00 h). All rats received normal rat chow (LM-485 Mouse/Rat sterilizable diet; Harlan-Teklad, Madison, WI) and water ad libitum for the duration of the experiment. After a one-week period of acclimation, rats were randomly assigned to drink treatment groups of either 30% sucrose (Sigma Aldrich Co., St. Louis, MO) solution or water. Rats received a 14-d regimen of twice-daily (9:30 and 15:30 h), brief (maximum of 30 minutes), limited (up to 4 mL) access to their assigned drink solution in an additional sipper bottle on the homecage. Rats readily drank the sucrose in amounts near or at the maximum for the duration of the study, whereas the control rats drank little or none of their additional water (data not shown, see [10] for typical intake). Drink treatment terminated on Day 14, after which rats no longer received access to their respective experimental drink solution. To test persistence of the sucrose effects, cohorts of animals were killed 1, 6, and 21 days after the snacking paradigm ended (corresponding to days 15, 20 and 35 after commencement of the sucrose delivery (see Figure 1). Groups of animals killed at each time point included: 1) Water - No restraint stress (n=12), 2) Sucrose - No restraint stress (n=12), 3) Water - With restraint stress (n=12), and 4) Sucrose - With restraint stress (n=13) (a total of 147 rats in the study). The `no restraint stress' groups did not receive a stress challenge, and were injected with pentobarbital and perfused with 0.9% saline followed by 4% paraformaldehyde for collection of brains. The `with restraint stress' groups received a 20-min restraint stress challenge and blood samples were taken by tail clip at 0, 20, 40, and 60 min after the onset of stress. Briefly, rats were placed into well-ventilated restraint tubes and 0-min tail clip blood samples (200 μl) were quickly collected into chilled tubes containing EDTA. The 0-min sample was completed in less than 3 min from first handling each rat's cage, thereby ensuring plasma ACTH and corticosterone levels that were reflective of the basal, unstressed state [17]. Rats remained in the restrainers for 20 min, with a second tail blood collection occurring immediately prior to their removal from the restraint tubes (i.e., 20-min after the onset of restraint). At 40 and 60 min after the initiation of restraint, the rats were briefly returned to the restraint tubes (< 3 min) for collection of 40- and 60-min blood samples. It took less than 3 min to collect each post-stress blood sample. Blood samples were centrifuged (3000 g, 15 min, 4° C) and plasma was stored at −20° C until measurement of plasma corticosterone levels via radioimmunoassay (Corticosterone Double Antibody 125I RIA Kit, MPBiomedicals, Solon, OH). Sipper “snack” intake, food intake, and body weight were monitored throughout the experiment. In this paradigm, sucrose rats typically decrease their chow intake isocalorically, resulting in no effect on body weight or fat depot weights [10].
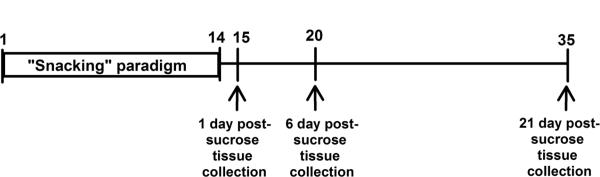
Figure 1. Experimental design.
Rats with ad libitum access to chow and water received 14 days of the snacking paradigm (limited, intermittent access to 30% sucrose solution or water (control)). Snack treatment ended on day 14 and on days 15, 20 and 35, separate cohorts of rats were sacrificed: non-stressed sucrose and non-stressed water rats were sacrificed for immunohistochemical analysis, and additional groups of sucrose and water rats received a 20-min restraint stress challenge for HPA assessment.
Brains were prepared for immunohistochemistry as described previously [18]. Total FosB protein (FosB/deltaFosB) was detected by free floating immunocytochemistry using standard protocols with a rabbit polyclonal primary antibody against a protein corresponding to amino acids 75–100 of the human FosB/deltaFosB (FosB (H75) 1:300 from Santa Cruz Biotechnology (Santa Cruz, CA)). Western blot analysis with this FosB/deltaFosB antibody immunoreacts with two bands, one at 35–37 kD and one at 45 kD corresponding with truncated deltaFosB (a truncated splice variant of the full length FosB protein) and the full length FosB protein respectively [19]. Primary antibody was detected using biotinylated goat anti-rabbit IgG (1:500 Vector Labs Burlingame, CA), incubated with avidin-horseradish peroxidase complex (1:500; ABC Elite Kit, Vector Laboratories), and reacted with 0.02% diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO) for 15 minutes, resulting in a brown reaction product. Sections were mounted onto slides, dehydrated in a graded ethanol series, cleared with xylene, and coverslipped with DPX. An observer blind to the treatment group assignments counted positively-stained neurons in the BLA and NuAc using Image J software (NIH).
The number of rats used in these studies was based on a priori power analyses using expected differences and variation based on our preliminary hormone and immunocytochemistry studies. Differences in the food intake, body weight and hormone data were determined using repeated measures two-way ANOVA. If group differences were present, then Fisher's least significant comparisons procedure was used to determine specific planned pairwise comparisons; no further adjustments were made to control for the experimentwise error rate. Differences in the numbers of FosB/deltaFosB-labeled cells between water and sucrose groups at each time point were determined using separate one-tailed t-tests based on the a priori hypothesis that sucrose snacking will increase synaptic plasticity and synaptic activity. Outliers were removed only if they differed from the mean by more than 1.96 times the standard deviation and they were outside the lower or upper quartiles by more than 1.5 times the interquartile range [20]. Data are shown as means ± SEM. Statistical significance was taken as p < 0.05.
3. RESULTS
3.1 Food intake and body weight
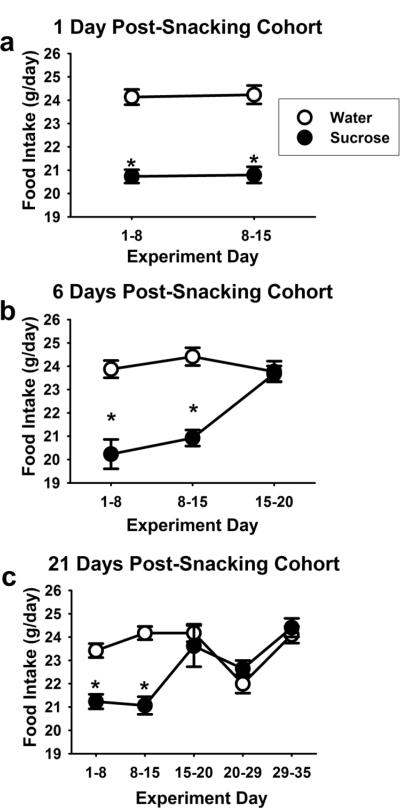
During the palatable snacking paradigm (Days 1–14), sucrose rats reduced their chow intake to compensate for the calories available in the sucrose drink (~10% of total daily intake) (Figure 2a–c). After the rats stopped receiving sucrose, chow intake quickly returned to baseline (Figure 2b,c). Moreover, sucrose drink did not affect body weight gain (data not shown).
Figure 2. Rats compensate for sucrose “snacks” by decreasing chow consumption.
During the 14 day snacking paradigm (experimental days 1–14), rats receiving sucrose decrease their chow intake ~10% as compared to the water control rats, and this returns to control levels once the sucrose snacking ceases. (a) 1-day post-sucrose cohorts, (b) 6-day post-sucrose cohorts, and (c) 21-day post-sucrose cohorts. An * indicates difference from water control (p<0.05).
3.2 HPA responses to stress
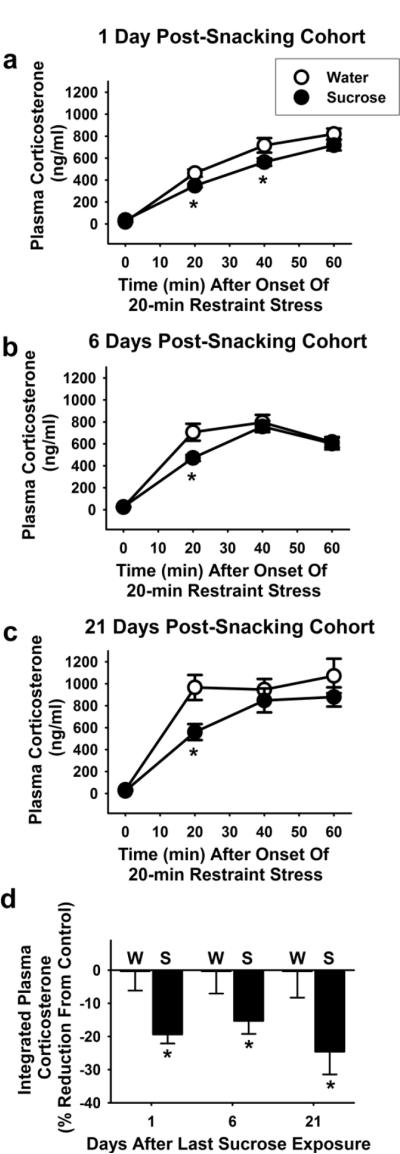
Rats tested one day (~16 hours) after the last sucrose snacking experience reduced stress-evoked plasma corticosterone response by 10–20% (Figure 3), similar to our previous results [10]. In addition, sucrose decreased stress-evoked plasma corticosterone at 6 and 21 days after the end of sucrose snacking (main effect of sucrose to decrease the plasma corticosterone response for the time-course data in Figure 3a–c, and the integrated corticosterone response in Figure 3d, p < 0.05).
Figure 3. Twice-daily sucrose snacks decrease the stress response at least 21days after the last access to sucrose snacks.
The timecourse of the plasma corticosterone response to a 20-min restraint stress challenge was reduced by sucrose snacks at (a) 1, (b) 6, and (c) 21 days after the last snack (significant main effect (p<0.05) of drink to decrease plasma corticosterone at each of the 1, 6, and 21 day timepoints). The integrated corticosterone response to restraint stress was also reduced by sucrose snacks at 1, 6, and 21 days after the last snack (d). An * indicates difference from water control (p<0.05).
3.3 FosB/deltaFosB in the BLA
Sucrose significantly increased the number of FosB/deltaFosB-positive cells in the BLA (Figures 4 and 5, p < 0.05) and the NuAC core (Figures 4 and 6) at 1 d after the snacking paradigm. This increase in FosB/deltaFosB-positive cells also occurred at 6 and 21 days after the last exposure to sucrose snacks in both the BLA and in the NuAc core (Figures 5 and 6, p < 0.05). There were no significant changes in the number of FosB/deltaFosB-positive cells in the NuAc shell (data not shown).
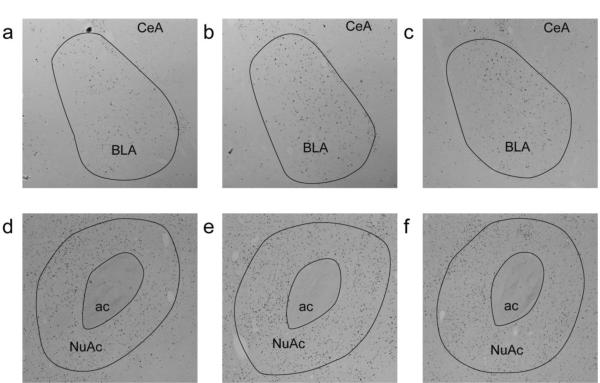
Figure 4. Representative images of FosB/deltaFosB immunolabeling in the BLA.
(a-c) (5× magnification) and NuAc core (d-f) (2.5× magnification) from (a and d) a rat receiving water one day after the snacking paradigm, (b and e) a rat receiving sucrose one day after the snacking paradigm, and (c and f) 21 days after the snacking paradigm. Abbreviations: Anterior commissure (ac), basolateral amygdala (BLA), central nucleus of the amygdala (CeA), NuAc (nucleus accumbens core).
Figure 5. FosB/deltaFosB-positive cells in the BLA are persistently increased at least 21 days after the last access to sucrose snacks.
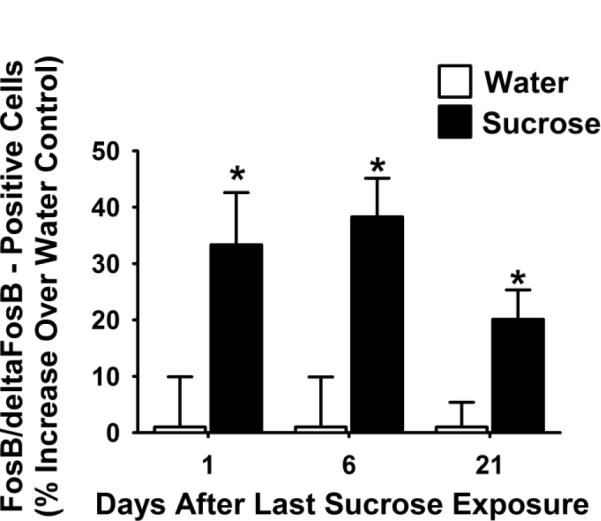
The number of cells positively labeled for FosB/deltaFosB in the BLA is significantly increased at 1, 6, and 21 days after the end of the sucrose snacking paradigm when compared to water controls. An * indicates difference from water control (p<0.05). Abbreviations: basolateral amygdala (BLA).
Figure 6. FosB/deltaFosB-positive cells in the NuAc core are persistently increased at least 21 days after the last access to sucrose snacks.
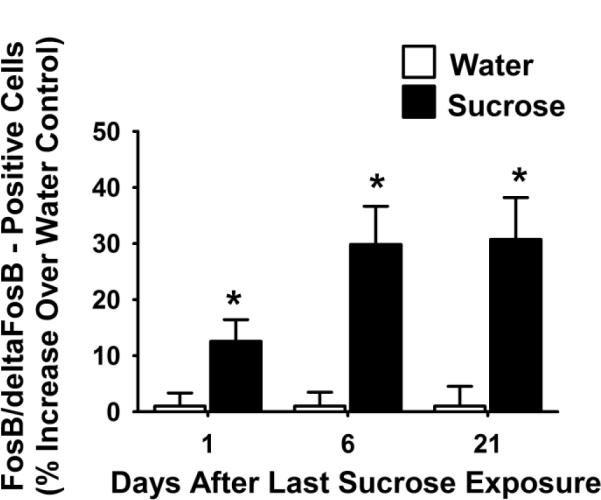
The number of cells positively labeled for FosB/deltaFosB in the NuAc core is significantly increased at 1, 6, and 21 days after the end of the sucrose snacking paradigm when compared to water controls. An * indicates difference from water control (p<0.05). Abbreviations: nucleus accumbens core (NuAc core).
4. DISCUSSION
The present study goals were to determine 1) whether sucrose snacking induces long-term stress buffering and 2) whether upregulation of FosB/deltaFosB, a marker of neuroadaptation and/or plasticity, in the BLA and NuAc persists across the same post-sucrose time frame. The results demonstrate that a history of sucrose snacking produces long-lasting increases in FosB/deltaFosB-positive cells in both the BLA and the NuAc core that coincide with decreases in the HPA response to stress. These results suggest that even small “snacks” of palatable food (that do not change body weight or adiposity) can have long-term consequences on stress regulation. Collectively, the data support the hypothesis that palatable food-induced plastic changes in the BLA and NuAC contribute to stress buffering.
4.1 Long-term modulation of the stress axis
The current studies demonstrate long-lasting effects of palatable snacking on the stress response. Specifically, rats have a decreased plasma corticosterone response to stress after a history of palatable snacking that persists at least 21 days after the termination of the access to palatable food. Previous studies demonstrate that the stress axis is plastic and can be persistently affected by a number of experiences. For example, early life stress exposure, such as maternal separation, can cause increases in the HPA response to stress and anxiety-like behavior that persist into the animal's adulthood [21,22]. Importantly, experiences during adulthood can also change stress modulation. In fact, the effects of maternal separation can be reversed in adulthood by environmental enrichment [23], and this reversal persists even after the animals are no longer housed in an enriched environment [24]. The current studies demonstrate that a relatively brief period of exposure to palatable food can also influence the HPA axis and produce long-term modulation of its responsivity.
4.2 FosB/DeltaFosB and neural plasticity
The current study demonstrates that after a history of palatable snacking, FosB/deltaFosB immunostaining in the BLA and the NuAc core is increased up to 21 days after the last snacking experience. DeltaFosB is a stable, long-lasting protein believed to be involved in persistent forms of neuronal plasticity in the brain [13]. This protein is induced in the NuAc by drugs of abuse and is extensively studied as a potential mechanism underlying addiction [14]. Moreover, sexual behavior, high-fat diet, and ad libitum sucrose drinking each increase FosB/deltaFosB immunoreactivity in the NuAc, indicating that FosB/deltaFosB can also accumulate in response to natural reward [15,16]. In the BLA, both chronic rewarding stimuli (e.g., cocaine) and chronic stressful stimuli (e.g., social defeat) can induce FosB/deltaFosB expression [12,25]. Importantly, the present work demonstrates that even limited sucrose intake, a relatively modest natural reward, is sufficient to induce FosB/deltaFosB expression in both the NuAc core and BLA.
As a technical consideration, it should be noted that no available antibodies can distinguish between FosB and the truncated FosB isoforms (deltaFosB), and therefore we cannot definitively determine which isoform is being detected in the current study. However, other studies demonstrate that sucrose drinking (ad libitum 10% sucrose for 10 days) increases FosB/deltaFosB immunostaining in the nucleus accumbens, with western blot analysis ascribing the difference to the deltaFosB (35–37 kD) isoform [16]. In addition, in our study the last exposure to rewarding stimuli was more than 16 hours prior to any measurement, well after the transiently-induced FosB induction returns to baseline [16,26], suggesting that these studies primarily assessed the more stable (and more long-lasting) deltaFosB protein.
The truncated FosB isoform deltaFosB is associated with behavioral and neural plasticity [13]. Increased deltaFosB in the NuAc regulates behaviors indicative of reward sensitivity in both natural and non-natural (drug) reward paradigms. For example, overexpression of deltaFosB in the NuAc causes rats to self-administer cocaine at lower doses, enhances lever presses for cocaine, and enhances instrumental responding for food reward [27,28]. In addition, chronic deltaFosB upregulation in the NuAc by one natural reward (i.e. sex) increases consummation of another (i.e. sucrose) [16]. Taken together, the literature indicates that increases in deltaFosB in the NuAc (a key reward brain region) can change sensitivity to future experiences with rewarding stimuli. The downstream effects of deltaFosB overexpression in the BLA are not currently known. However, it stands to reason that overexpression of deltaFosB in regions that are important in processing both stress and reward, such as the BLA, may change the sensitivity to reward or stress after rewarding experience.
The BLA is necessary for sucrose stress-dampening [11], and the current data suggest that the NuAc may also contribute to this effect. Anatomical and electrophysiological studies demonstrate synaptic connections between the BLA and the NuAc core [29,30]. These connections appear to be important for reward processing and responding, and are engaged during reward cue presentation for both natural reward (i.e., palatable food) and non-natural reward (i.e., drugs of abuse) [31,32,33]. These data, along with data from the current studies, suggest that the BLA and NuAc may jointly mediate the stress relief provided by palatable “comfort” foods.
Our data demonstrate that palatable snacking persistently decreases the HPA stress response, and this decreased stress response coincides with increased BLA and NuAc core FosB/deltaFosB, a neural plasticity marker. The current evidence supports the hypothesis that neural plasticity in the BLA and NuAc is responsible for buffering the stress response to future challenges after a history of palatable food. Future studies will more directly explore the causal relationship between neural modulation in the BLA and NuAc and the diminished stress response after palatable snacking.
4.3 Perspectives
These studies suggest that modest amounts of palatable food, which are insufficient to cause weight gain, are sufficient to cause long-lasting changes in the brain and the stress response. This work suggests that even limited quantities of palatable food have effects on the brain of a similar nature to those of drugs of abuse [12]. The stress-relieving and “addictive” qualities of palatable food may be partially explained by their ability to alter long-term activity of neurocircuitry in reward- and stress-regulatory brain regions, such as the NuAc and BLA.
The idea that palatable snacking has long-term effects on the stress response has new implications for the idea of “comfort food”. The drive to “self-medicate” with palatable food may occur because of an acute decrease in perceived stress and/or improved mood that is reinforced by long-term physiological reductions in the response to stress. Importantly, the work suggests that individuals may not need to consume large amounts of palatable foods on an ongoing basis in order to achieve physiological stress relief.
ACKNOWLEDGEMENTS
The authors would like to thank Kenny Jones, Ben Packard, and Kristen Halcomb for their technical assistance. This work was supported by DK059803 (AMC), MH069725 (JPH), MH049698 (JPH), DK067820 (YMU), and DK078906 (YMU).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2000;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- [2].Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [3].Hajizadeh Moghaddam A, Roohbakhsh A, Rostami P, Heidary-Davishani A, Zarrindast MR. GABA and histamine interaction in the basolateral amygdala of rats in the plus-maze test of anxiety-like behaviors. Pharmacology. 2008;82:59–66. doi: 10.1159/000131110. [DOI] [PubMed] [Google Scholar]
- [4].Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60:1039–42. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- [6].Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272:R840–8. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- [7].Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70:333–42. doi: 10.1016/s0031-9384(00)00265-1. [DOI] [PubMed] [Google Scholar]
- [8].Dube L, LeBel JL, Lu J. Affect asymmetry and comfort food consumption. Physiol Behav. 2005;86:559–567. doi: 10.1016/j.physbeh.2005.08.023. [DOI] [PubMed] [Google Scholar]
- [9].Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- [10].Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, et al. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci (USA) 107:20529–34. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harris GC, Hummel M, Wimmer M, Mague SD, Aston-Jones G. Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience. 2007;147:583–91. doi: 10.1016/j.neuroscience.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nestler EJ, Kelz MB, Chen J. DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res. 1999;835:10–17. doi: 10.1016/s0006-8993(98)01191-3. [DOI] [PubMed] [Google Scholar]
- [14].Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–6. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Teegarden SL, Bale TL. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol Behav. 2008;93:713–23. doi: 10.1016/j.physbeh.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, et al. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–7. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:5823–8. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- [18].Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, et al. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marttila K, Raattamaa H, Ahtee L. Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice. Neuropharmacology. 2006;51:44–51. doi: 10.1016/j.neuropharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- [20].McClave J. a. D., F. Statistics. McMillian College Publishing Company, Inc.; Edgewood Cliffs, NJ: 1994. [Google Scholar]
- [21].Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- [22].Penke Z, Felszeghy K, Fernette B, Sage D, Nyakas C, Burlet A. Postnatal maternal deprivation produces long-lasting modifications of the stress response, feeding and stress-related behaviour in the rat. Eur J Neurosci. 2001;14:747–55. doi: 10.1046/j.0953-816x.2001.01691.x. [DOI] [PubMed] [Google Scholar]
- [23].Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–3. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol Biochem Behav. 2002;73:193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- [25].McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–54. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- [26].Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, et al. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–69. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kelz MB, Chen J, Carlezon WA, Jr., Whisler K, Gilden L, Beckmann AM, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–6. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- [28].Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mulder AB, Hodenpiji MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci. 1998;18(13):5095–102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mitrano DA, Pare JF, Smith Y. Ultrastructural relationships between cortical, thalamic, and amygdala glutamatergic inputs and group I metabotropic glutamate receptors in the rat accumbens. J Comp Neurol. 2010;518(8):1315–29. doi: 10.1002/cne.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jones JL, Day JJ, Wheeler RA, Carelli RM. The basolateral amygdala differentially regulates conditioned neural responses within the nucleus accumbens core and shell. Neuroscience. 2010;169(3):1186–98. doi: 10.1016/j.neuroscience.2010.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Theberge FR, Milton AL, Belin D, Lee JL, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory consolidation. Learning Memory. 2010;17(9):444–53. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]