Abstract
Background and Purpose
We tested the effect of bone marrow stromal cells (BMSCs) on neuronal remodeling of the corticospinal tract (CST) originating from the contralesional cortex in mice subjected to unilateral pyramidotomy (PT) followed by middle cerebral artery occlusion (MCAo).
Methods
Adult mice with transgenic yellow fluorescent protein (YFP) labeling in the CST were subjected to right hemispheric PT and right permanent or sham MCAo. One day later, the mice were treated intravenously with BMSCs or phosphate-buffered saline (PBS). A Foot-Fault test and a single pellet reaching test were performed before surgery, 3 days after MCAo and weekly thereafter. Pseudorabies virus (PRV)-614-monomeric red fluorescent protein (RFP) was injected into the left forelimb flexor muscles 28 days after surgery (4 days before sacrifice). The brain and cervical cord were processed for fluorescent microscopy to detect RFP and YFP labeling, respectively.
Results
Significant functional improvements were evident in PT-MCAo mice treated with BMSCs (n=9) compared with PBS treated mice (n=9, p<0.05), but not in mice suffering PT- sham MCAo treated with either PBS (n=9) or BMSCs (n=10). Furthermore, in PT-MCAo mice, both CST axonal density in the denervated side of the cervical gray matter and RFP-labeled pyramidal neurons in the left intact cortex were significantly increased compared with PT-sham MCAo mice (p<0.05). BMSCs significantly enhanced both CST density and RFP labeling in PT-MCAo mice (p<0.05) only. The behavioral outcome was highly correlated with CST density and RFP labeling.
Conclusions
BMSCs amplify stroke-induced contralesional neuronal remodeling, which contribute to motor recovery after stroke.
Keywords: functional recovery, middle cerebral artery occlusion, neuronal plasticity, pyramidotomy
Stroke is the leading cause of long-term disability in adults. Impaired hand function is one of the most frequent consequences of stroke. Early reduction of hand motor deficits is an excellent predictor of full or good recovery after stroke.1 Precise voluntary movement is specifically controlled by motor areas of the cerebral cortex, primarily through the corticospinal tract (CST). Therefore, rewiring CST innervation from the motor cortex to peripheral tissue may enhance post stroke performance of fine motor skill movements.
Bone marrow stromal cells (BMSCs) stimulate production of restorative factors by parenchymal cells and improve neurological function recovery after stroke.2 Transplantation of BMSCs significantly increases contralesional CST sprouting into the stroke-impaired side of the cervical spinal gray matter.3 The present study was carried out to further investigate whether such axonal innervation originating from the contralesional cortex contributes to functional recovery. We examined the correlations between neuronal remodeling using transgenic mice with yellow fluorescent protein (YFP) labeling in the CST4 and retrograde pseudorabies virus (PRV)-monomeric red fluorescent protein (mRFP)5 labeling in the cortical pyramidal neurons with forepaw performance in adult mice subjected to unilateral pyramidotomy (PT) followed by permanent or sham middle cerebral artery occlusion (MCAo).
Materials and Methods
Animals
Adult male CST-YFP mice (2 months-old, 25-30 g) were generated by in-house breeding colony using two transgenic mouse strains of B6.Cg-Tg(Thy1-EYFP)15Jrs/J and B6.129-Emx1tm1(cre)Krj/J purchased from Jackson Laboratories (Bar Harbor, ME), in which the CST axons are specifically and completely labeled by YFP.4 All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Pyramidotomy
To examine the contribution of the CST originating from the contralesional cortex to behavioral recovery after stroke, we transected the right hemispheric pyramidal tract rostral to the CST decussation at the medulla level with a ventral surgical procedure in advance of MCAo to eliminate the CST axons in the left stroke-impaired side of the spinal cord.6 Briefly, the animal was secured in a supine position. After a ventral midline incision of the neck skin, the ventral vertebral column and outer surface of the occipital bone were exposed by bluntly splitting the muscle layers under an operating microscope, and the ventrocaudal part of the bone was partially removed with rongeurs and blunt forceps. The middle portion of the right CST was transected with an iridectomy scissor. The esophagus, trachea and muscles were then repositioned. No animals died from the PT alone procedure. MCAo or sham surgery was performed using the same incision.
MCAo Model
A method of intraluminal vascular occlusion,7 modified in our laboratory was performed by advancing a 6-0 surgical nylon suture with an expanded (heated) tip from the right external carotid artery into the lumen of the internal carotid artery, to block the origin of the MCA. Ligation of the right external carotid artery was used as sham control. Within the first 5 days after surgery, four mice died out of the 22 subjected to PT followed by MCAo (3 in PBS treated group and 1 in BMSC treated group). This is a similar mortality rate as found in our previous studies (approximately 20%).
BMSC administration
Mouse BMSCs harvested from hind legs (C57BL/6J mice, 2-month old) were provided by Theradigm Inc (Baltimore, MD).8 One day after surgery, 1×106 mouse BMSCs in 0.4 ml phosphate-buffered saline (PBS) or PBS alone were injected into a tail vein. The animals were randomly divided into 4 groups, mice subjected to: (1) PT-sham MCAo and treated with PBS (n=9), (2) PT-sham MCAo transplanted with BMSCs (n=10), (3) PT-MCAo with PBS injection (n=9), and (4) PT-MCAo and treated with BMSCs (n=9).
Behavioral Tests
A Foot-Fault test and a single pellet reaching test were performed before surgery, 3 days after MCAo and weekly thereafter. The Foot-Fault test measures the accuracy of forepaw placement on a non-equidistant grid as the percentage of foot-faults of the left forepaw to total steps.9 The single pellet reaching test measures the ability of skilled forepaw use.10 The mice were placed in a Plexiglas box (195 mm long, 80 mm wide, and 200 mm high, a 10-mm wide vertical slot ran up the front, with a shelf mounted 11 mm from the floor on the front of the box). Animals were trained to use their left forepaw to extract 14 mg food pellets (Bio-Serv Inc, Frenchtown, NJ) placed in an indentation 10 mm away from the front wall and to the right of the slot midline for 5 days before surgery. The number of the left forepaw extensions through the slot and the number of pellets extracted were counted for each animal during a 10-min testing period. Performance was defined by the success rate=(number of pellets extracted/number of left forepaw attempts)*100.
Retrograde PRV Tracing
To verify neuronal innervation from the motor cortex to the stroke-impaired left forepaw, a trans-synaptic retrograde tracer, PRV-614-mRFP (Gift from Dr. Lynn Enquist, Princeton University, Princeton, NJ) was injected into the left forelimb radioulnar flexor muscles through a skin incision 4 days before sacrifice.11 The animals were then transferred to a Biosafety Level-2 room to survive for an additional 94-96 hrs.
Tissue Preparation
At 32 days after PT-MCAo or sham MCAo, animals were transcardially perfused with saline, followed by 4% paraformaldehyde. The brain and the cervical spinal cord were removed, and immersed in 4% paraformaldehyde overnight. A series of 100 μm-thick coronal sections were cut from seven 500 μm-thick forebrain blocks using a vibratome to examine RFP-positive pyramidal neurons. The remaining 400 μm brain blocks were embedded in paraffin for ischemic lesion volume measurements on a series of adjacent sections stained with Hematoxylin and Eosin. Three 100 μm-thick coronal sections were cut from the medulla caudal to the PT site to determine the completeness of PT surgery. The cervical spinal cord segments of C4-7 were cut into consecutive 100 μm-thick vibratome sections to measure the YFP-positive CST axons.
Data Analysis and Statistics
All analyses were performed blindly. Lesion volume was measured by NIH imaging software (Image J) and presented as a volume percentage of the lesion area compared with the contralesional hemisphere. A Bio-Rad MRC 1024 (argon and krypton) laser-scanning confocal imaging system mounted onto a Zeiss microscope (Bio-Rad, Cambridge, MA) was used to examine RFP-labeling in the cortical pyramidal neurons and YFP-labeling in the CST axons on the cervical cord. The number of RFP-positive cells was counted on coronal sections in 0.5 mm granularity to the bregma. The bilateral CST axonal densities in the gray matter of cervical cord were measured on 30 consecutive transverse sections with Image J. To correct for variation on fluorescent measurements, the percentage of CST density in the stroke-impaired side to the contralateral side on the same sections was used to assess axonal remodeling in the spinal cord.
All data are presented as mean±SD. One-way analysis of variance (ANOVA) was used to evaluate functional recovery, numbers of RFP-positive pyramidal neurons, and the index of axonal remodeling. To test the correlation between behavioral outcome and neuronal reorganization after MCAo, the Pearson's correlation coefficients between the left forepaw motor performance and the index of axonal density in the cervical cord and the total number of RFP-positive cortical neurons were calculated.
Results
BMSCs enhance functional recovery after MCAo
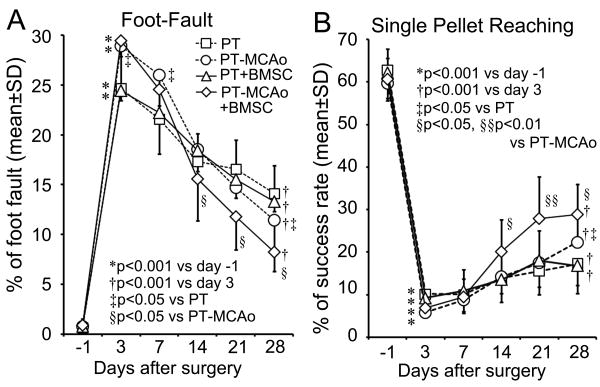
In all the animals, PT lesions were consistent and nearly complete in the right side of the pyramidal tract (less than 1% remaining compared to the left CST, Supplemental Figure S1). In mice with MCAo, the percent lesion volume was 20.4±3.8 and 19.7±4.2 for PBS and BMSC treated groups, respectively, without significant difference. After right side PT followed by sham MCAo or MCAo, all animals suffered severe functional deficits of the left forepaw as assessed by the Foot-Fault test and single pellet reaching test compared with the baseline before surgery (Figure 1A and B, p<0.001), while the mice subjected to PT-MCAo were more severely impaired than PT-sham MCAo mice in the Foot-Fault test measured 3 days after surgery (p<0.05). During the 28 day experimental period, significant progressive, however, incomplete behavioral recovery was observed with time in all animals (p<0.01 vs Day 3). In the PT-MCAo mice, BMSC administration significantly enhanced functional recovery 14 to 28 days after MCAo in both tests compared to PBS treated mice (p<0.05). However, such enhancement with BMSC treatment was not obtained in the animals subjected to PT-sham MCAo. In addition, the degree of functional recovery (%) in the pellet reaching task was worse than in the grid walking test 28 days after surgery (13.3±5.4 vs 44.0±7.1 in PT, 31.4±10.7 vs 62.9±6.9 in PT-MCAo, 14.6±7.0 vs 47.5±5.3 in PT+BMSC and 41.7±13.5 vs 74.2±4.8 in PT-MCAo+BMSC respectively, p<0.001).
Figure 1.
Line graphs show the temporal profile of left forepaw behavioral deficit and recovery after right PT followed by sham-MCAo or right MCAo with and without BMSC treatment, respectively, measured by the Foot-Fault test (A) and the single pellet reaching test (B). The left forepaw motor disability progressively decreased with time in all mice; while mice subjected to PT followed by MCAo were more seriously impaired in the Foot-Fault test, they exhibited more complete recovery. BMSC treatment significantly promoted the functional recovery in PT-MCAo mice.
BMSCs promote contralesional CST sprouting into the denervated side of the cervical cord after stroke
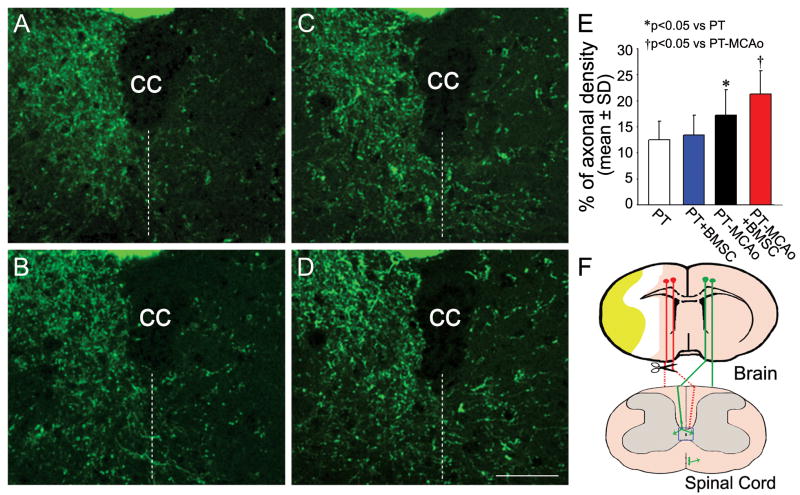
To examine the contralesional CST sprouting into the left stroke-impaired side of the spinal cord, the right pyramidal tract was transected rostral to the decussation (Figure 2F). YFP-labeling in the CST axons in the central area of the cervical gray matter was measured on transverse vibratome sections. After right PT, most CST axons were eliminated in the left side of the cervical cord in mice treated with either PBS (2A) or BMSCs (2B). In contrast, axonal sprouting from the right intact side of the cervical gray matter into the stroke-impaired left side was evident in mice subjected to PT-MCAo (2C), and was further enhanced by BMSC treatment (2D). The ratio of CST axonal density in the denervated side to the intact side on same sections in each animal was calculated as an index of contralesional axonal remodeling. Quantitative data showed that CST axonal sprouting was significantly increased in the PT-MCAo mice compared with sham MCAo mice (2E, p<0.05), while a significant stimulative effect of BMSCs was found in PT-MCAo mice (p<0.05), but not in PT-sham MCAo mice.
Figure 2.
Single layer confocal images show CST axons in the gray matter of the cervical cord in CST-YFP mice subjected to PT followed by Sham MCAo with PBS (A) or BMSC (B) treatment, and MCAo with PBS (C) or BMSC (D) treatment. Broken lines in A to D indicate the midline of the spinal cord. Note that in the cervical gray matter in PT-MCAo mice, the number of axons crossing the midline into the denervated left side from the right intact side was evident in both PBS and BMSC treated groups. Quantitative analysis of the percentage of CST axons in the denervated side to the contralateral side (E) demonstrated that BMSC treatment significantly increased axonal density in PT-MCAo mice but not in PT-sham MCAo mice. A scheme of the corticospinal projections and the lesion sites is shown in F. A rectangle field in the central area of the spinal gray matter indicates the position of the photomicrographs appearing in A to D. cc: central canal. Scale bar=50 μm.
BMSCs facilitate ipsilateral innervation in the contralesional cortex after stroke
A trans-synaptic fluorescent viral tracer, PRV-614-mRFP was injected into the left forepaw muscles to retrogradely trace the cortical neural innervating pathways. Four days after tracer injection, very few cortical pyramidal cells were labeled in the right cerebral cortex (Data not shown), further confirming the completeness of the PT. We counted the numbers of RFP-positive neurons in the left intact cortex on each one of five 100 μm-thick coronal sections of forebrain (Supplemental Figure S2 and Table 1). Compared with animals in the PT-sham MCAo group, significant increases of RFP-positive neurons were observed over both the left caudal forelimb area (-0.5∼0.5 mm rostral to the bregma) and the left rostral forelimb area (1.5∼2.0 mm rostral to the bregma) in the PT-MCAo mice (p<0.01), suggesting that the ischemic lesion induced additional pyramidal neurons in the contralesional cortex that connect with the stroke-impaired left forelimb. Moreover, the number of RFP-positive pyramidal neurons in the caudal forelimb motor area was further increased by BMSC treatment in PT-MCAo animals (p<0.05).
Table 1. Numbers of RFP-positive pyramidal neurons in the contralesional (left) cortex.
| Group | mm to bregma | -0.5 | 0 | 0.5 | 1.0 | 1.5 | 2.0 | Total |
|---|---|---|---|---|---|---|---|---|
| PT | 2.9±1.5 | 6.8±3.7 | 1.1±1.7 | 0±0 | 0.9±1.1 | 3.0±1.2 | 14.7±6.2 | |
| PT-MCAo | 7.1±4.2* | 16.8±3.3** | 7.8±4.8** | 0.1±0.3 | 4.0±1.9** | 6.3±1.3** | 43.2±9.8** | |
| PT + BMSC | 2.3±1.2 | 8.1±3.2 | 2.4±2.0 | 0.2±0.4 | 1.5±1.8 | 3.4±1.1 | 17.9±5.5 | |
| PT-MCAo + BMSC | 10.9±2.4† | 22.4±3.6†† | 8.6±2.5 | 0.3±0.7 | 4.1±1.8 | 8.2±3.7 | 54.6±9.8†† | |
Numbers are mean±SD.
p<0.01,
p<0.001 vs PT;
p<0.05,
p<0.01 vs PT-MCAo.
Correlation between functional recovery and neuronal remodeling
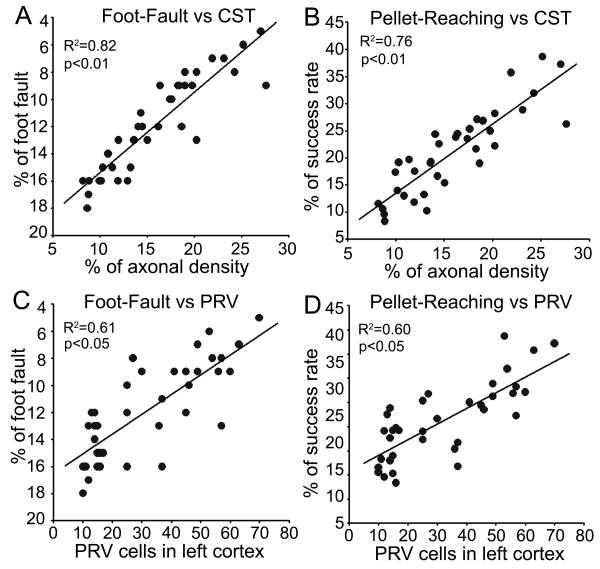
To test the hypothesis that contralesional neuronal remodeling functionally contributes to neurological outcome after stroke, we examined the relationship of behavioral performance with the neuronal status in mice with ablated right CST. Our data show that the forelimb skilled motor tasks assessed with the Foot-Fault test and the single pellet reaching test were highly correlated with the corticospinal innervation originating from the contralesional cortex indentified by transgenic YFP-labeling and retrograde viral tracing (Figure 3A-D, p<0.05).
Figure 3.
Data point graphs of correlations between neural remodeling and behavioral recovery. The behavioral outcome assessed by both Foot-Fault test and single pellet reaching test were highly correlated with the CST axonal density in the denervated side of the spinal cord and numbers of PRV-positive pyramidal neurons in the left contralesional cortex.
Discussion
In the present study, we examined the effect of BMSCs in adult mice subjected to unilateral PT followed by ipsilateral MCAo. In adult mice with the surgical elimination of the CST innervation originating from the right cortex, a second lesion of right side MCAo caused increased functional recovery of the impaired left forepaw, assessed by the Foot-Fault test and the single pellet reaching test, and increased the corticospinal innervation originating from the left contralesional cortex, detected by transgenic YFP-labeling in the CST and retrograde neural tracing with trans-synaptic PRV-RFP tracer from the impaired left forepaw. In addition, BMSC treatment significantly improved both behavioral outcome and increased neuronal innervation from the contralesional cortex to the denervated side of the spinal cord in the PT-MCAo but not in the PT-sham MCAo animals. A high correlation between behavioral outcome and ipsilateral innervation (left cortex to left forepaw) suggested the neurological recovery is in part mediated by contralesional neuronal remodeling after stroke.
Previous studies have provided insights into the cellular and molecular events underlying the functional recovery after stroke.12 A unilateral infarct is associated with an increase of growth-related factors, which lead to structural changes in axons, dendrites, and synapses in the peri-infarct area, homologous sites in the contralesional hemisphere, and remote regions that are generally connected to the site of injury.13 Furthermore, our previous studies demonstrated that the ischemic brain environment promotes a responsive secretion of an array of neurotrophins and bioactive factors by BMSCs, and the BMSCs transplanted into the ischemic brain further stimulate brain parenchymal cells, especially astrocytes to produce neurotrophic factors.14 Additionally, BMSCs also reduce the gliosis and scar formation in the ischemic brain that inhibit axonal outgrowth.15 The finding that BMSCs provide therapeutic benefits after stroke but not after surgical PT lesion alone, further demonstrate that BMSCs amplify the spontaneous repair responses after stroke. A recent study shows that BMSCs embolized in the lung secrete tumor necrosis factor-stimulated gene 6 protein which reduced myocardial infarct size, and improved cardiac function, suggesting such humoral effects may also benefit to stroke recovery.16 The contribution of the contralesional cerebral hemisphere to recovery after stroke is controversial in clinical studies. Although increased task-related activation within contralesional motor structures had been observed in patients with poor recovery,17 our finding in mice with ablated ipsilesional CST support the hypothesis that contralesional motor areas significantly contribute to motor performance in patients with subcortical stroke.18
Clinical studies demonstrated that the extent of functional disability and the potential for functional recovery is dependent on the CST integrity after ischemic stroke.19 As the CST is the primary transmission tract from the sensorimotor cortex controlling voluntary movements of the peripheral muscles, the CST plays an important role during tasks requiring skilled sensorimotor integration, such as skilled fore-paw usage.20 Although a previous study showed no detectable impairment of forepaw use resulting from loss of the uncrossed portion of the CST on one side or compensation provided by the uncrossed CST on the other side,21 we found that the increase of CST innervation originating from the opposite hemisphere (re-crossed) is associated with enhanced skilled forepaw performance following ischemic stroke in mice with loss of the crossed CST. Nevertheless, a previous study indicates that the reach-to-grasp function did not show recovery over a 8-week period after cervical dorsolateral funiculotomy to transect the rubrospinal axons.22 Since the rubrospinal axons possess similar branching patterns in the spinal cord with the CST,23 and the red nucleus is active during the reaching movement,24 the finding that animals retained some residual forepaw usage after PT, and that there was further impairment in the Foot-Fault test following a secondary MCAo, suggest that the skilled forepaw movement is partially under rubrospinal control. However, the more severe deficit and incomplete recovery in the pellet reaching task compared to Foot-Fault suggests that the digit control may be more dependent on the CST. Further studies on the cortico-ruburo-spinal system are warranted.
Conclusions
Taken together, the present findings demonstrate that corticospinal innervation originating from the contralesional motor cortex stimulated by ischemic stroke is amplified by BMSC treatment and is highly correlated with skilled forepaw performance, suggesting that such neuronal remodeling contributes to functional recovery after stroke.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by Henry Ford Health System internal grant (ZL), American Heart Association National Scientist Development Grant 0835397N (ZL), NIH P01NS023393 (MC) and R01NS066041 (YL).
Footnotes
Conflict of Interest: None
References
- 1.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 2.Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40:S143–145. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats following stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 5.Banfield BW, Kaufman JD, Randall JA, Pickard GE. Development of pseudorabies virus strains expressing red fluorescent proteins: New tools for multisynaptic labeling applications. J Virol. 2003;77:10106–10112. doi: 10.1128/JVI.77.18.10106-10112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starkey ML, Barritt AW, Yip PK, Davies M, Hamers FP, McMahon SB, Bradbury EJ. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp Neurol. 2005;195:524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of vegf and bdnf promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 10.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16:638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 14.Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 16.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hmscs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein tsg-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 19.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 20.Kanagal SG, Muir GD. Effects of combined dorsolateral and dorsal funicular lesions on sensorimotor behaviour in rats. Exp Neurol. 2008;214:229–239. doi: 10.1016/j.expneurol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Whishaw IQ, Metz GA. Absence of impairments or recovery mediated by the uncrossed pyramidal tract in the rat versus enduring deficits produced by the crossed pyramidal tract. Behav Brain Res. 2002;134:323–336. doi: 10.1016/s0166-4328(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 22.Stackhouse SK, Murray M, Shumsky JS. Effect of cervical dorsolateral funiculotomy on reach-to-grasp function in the rat. J Neurotrauma. 2008;25:1039–1047. doi: 10.1089/neu.2007.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinoda Y, Futami T, Mitoma H, Yokota J. Morphology of single neurones in the cerebello-rubrospinal system. Behav Brain Res. 1988;28:59–64. doi: 10.1016/0166-4328(88)90076-9. [DOI] [PubMed] [Google Scholar]
- 24.Jarratt H, Hyland B. Neuronal activity in rat red nucleus during forelimb reach-to-grasp movements. Neuroscience. 1999;88:629–642. doi: 10.1016/s0306-4522(98)00227-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.