Abstract
Administration of L-dopa or apomorphine to neonatal and adult 6-hydroxydopamine (6-OHDA)-treated rats resulted in different behavioral responses depending on the age at which dopaminergic fibers were destroyed. When neonatal 6-OHDA-treated rats were tested as adults, they exhibited marked stereotypies, self-biting and self-mutilation behavior (SMB) when given these dopamine agonists. Self-biting as well as the incidence of SMB in neonatal 6-OHDA-treated rats showed dose-related changes between 10 and 100 mg/kg of L-dopa. This SMB and self-biting after L-dopa was observed as early as 22 to 24 days of age. Adult 6-OHDA-treated rats did not exhibit SMB or self-biting to L-dopa (100 mg/kg) or apomorphine (10 mg/kg), but did display paw treading and head nodding—behaviors not observed in neonatal 6-OHDA-treated rats. In addition, the locomotor response to apomorphine (1 mg/kg) was significantly greater in adult 6-OHDA-treated rats than in neonatal 6-OHDA-treated rats. Brain dopamine was reduced markedly in striatum, nucleus accumbens and olfactory tubercles in both 6-OHDA treatment groups with the reduction being slightly greater in rats treated with 6-OHDA neonatally. Serotonin content was elevated in striatum of rats treated neonatally with 6-OHDA, but not in adult 6-OHDA-treated rats. SMB and behaviors observed after L-dopa in rats treated neonatally with 6-OHDA were not apparent after L-dopa in rats with brain serotonin or norepinephrine reduced. Rats with brain dopaminergic fibers destroyed neonatally exhibited self-biting and SMB after L-dopa, suggesting that neonatal reduction of this amine is responsible for the SMB and self-biting in neonatal 6-OHDA-treated rats. 5-Hydroxytryptophan administration to neonatal 6-OHDA-treated rats did not induce SMB, indicating that release of serotonin by L-dopa is not responsible for this behavior. Because inhibition of dopamine-β-hydroxylase did not alter the SMB response to L-dopa observed in neonatal 6-OHDA-treated rats, norepinephrine synthesized from L-dopa does not appear to contribute to the response. High closes of a decarboxylase inhibitor sufficient to inhibit conversion of dopa to dopamine in brain did not reduce the incidence of SMB. Administration of haloperidol (1 mg/kg) reduced the incidence of SMB, but did not antagonize the self-biting or the taffy pulling exhibited by L-dopa. In contrast, cisflupentixol completely blocked the SMB and self-biting induced by L-dopa. The latter findings suggest that these behaviors in neonatal 6-OHDA-treated rats are more associated with D-1 than D-2 receptor function. The age-dependent effects of dopamine agonists observed in these studies provide an explanation for the different symptomatology observed in Lesch-Nyhan patients and Parkinson’s disease—neurological disorders with reduced brain dopamine.
Lloyd et al. (1981) reported that several measures of dopaminergic function in striatum and other brain areas are reduced in patients with Lesch-Nyhan syndrome, a disease associated with an inborn deficiency of the enzyme hypoxanthine-guanine phosphoribosyl transferase (Seegmiller et al., 1967). Parkinson’s disease is another syndrome in which brain dopamine is reduced (Hornykiewicz, 1973). However, Parkinson’s patients do not demonstrate the choreoathetoid movements or the SMB observed in children with Lesch-Nyhan disease (Lesch and Nyhan, 1964; Kelley and Wyngaarden, 1983; Wilson et al., 1983). Apparent differences in these syndromes are the age of onset of the symptoms and the disordered purine metabolism in Lesch-Nyhan patients (Seegmiller et al., 1967).
The availability of 6-OHDA to destroy brain catecholamine-containing neurons in adult (Breese and Traylor, 1970) and developing (Breese and Traylor, 1972; Breese et al., 1978a; Smith et al., 1973) rats provides a pharmacological means to explore the possibility that motor function and susceptibility for SMB may differ depending upon the age at which these neurons are destroyed. Behaviorally, both adult and neonatal 6-OHDA-treated rats have avoidance and ingestive deficits (Breese et al., 1973a,b; Smith et al., 1973), but quantitative comparisons as to the magnitude of these deficits have not been made. In addition, both 6-OHDA treatments enhance the behavioral effects of dopamine agonists (Ungerstedt, 1971a,b; Uretsky and Schoenfeld, 1971; Hollister et al., 1974, 1979; Creese and Iversen, 1973). It is recognized that neonatal rats are more sensitive to the neurotoxic action of 6-OHDA on catecholamine-containing fibers than are adult rats (Breese and Traylor, 1972; Cooper et al., 1975). Furthermore, Stachowick et al. (1984) reported sprouting of serotonin-containing neurons in neonatal 6-OHDA-treated rats, a change not observed in adult 6-OHDA-treated rats. Recently, Breese et al. (1984) reported that L-dopa induced SMB in neonatal 6-OHDA-treated rats, but not in adult 6-OHDA-treated rats, evidence that age at which destruction of these fibers occurs influences a behavioral response. From such data, we hypothesized that the neonatal 6-OHDA-treated rat is a model of the susceptibility for SMB observed in Lesch-Nyhan patients (Breese et al., 1984).
The present experiments extend our investigation of the differences between neonatal and adult 6-OHDA-treated rats. Data will indicate that several behavioral responses to dopamine agonist challenge, other than the incidence of SMB, are different between rats treated as neonates or as adults with 6-OHDA. In addition, the present investigation examined possible mechanisms that might account for the neurobiological basis of differences in the two 6-OHDA treatment groups. The results are consistent with the view that adaptive mechanisms in the neonate after destruction of dopaminergic neurons differ from those in rats which have their fibers destroyed when adult. We postulate that this adaptation accounts for the different symptoms observed in Parkinson and Lesch-Nyhan syndromes.
Methods
General
Pregnant Sprague-Dawley female rats as well as adult male and female rats (200 g) were obtained from Charles River Laboratories (Wilmington, MA). Approximately 1 week before delivery, pregnant females were individually housed in clear plastic cages with wood chip bedding. Adult rats were housed four per cage. All rats were in rooms with environmentally controlled conditions (7:00 A.M. light, 7:00 P.M. dark cycle, temperature maintained at 23–25°C, with continuous access to Wayne Lab Blox laboratory chow and water). 6-OHDA HBr (prepared to contain 100 μg free base; Regis Chemical Co., Chicago, IL) was administered intracisternally (10 μl) under ether anesthesia to 5-day-old rats alone and in combination with desipramine (20 mg/kg i.p.) as previously reported (Breese and Traylor, 1972; Smith et al., 1973). Litters were limited to 10 pups each with an attempt to have equal numbers of males and females. Adult rats received the first 6-OHDA treatment (200 μg free base) 30 min after pargyline (50 mg/kg) and a second treatment 1 week later (200 μg 6-OHDA; Breese and Traylor, 1970). Serotonin reduction was accomplished by administering 50 μg (10 μl) of 5,7-DHT (Regis Chemical Co.) 60 min after desipramine (20 mg/kg i.p.) to 3-day-old rat pups (Breese and Cooper, 1975; Breese et al., 1978b). Apomorphine HCl (Merck Sharp & Dohme Research Laboratories, Rahway, NJ), dissolved in 0.05% ascorbic acid solution, was administered s.c.; L-dopa (Hoffmann-LaRoche, Nutley, NJ) was suspended in 0.5% methylcellulose and administered i.p. 60 min after 50 or 500 to 800 mg/kg of RO-4-4602 [N-(d/-seryl)-N-(2,3,4-trihydrox-ybenzyl)-hydrazine; Hoffmann-La Roche]. Haloperidol (McNeil Laboratories, Inc., Ft. Washington, PA) was dissolved in 0.3% tartartic acid and cisflupentixol HCl (Lundbeck, Copenhagen, Denmark) in saline and both were given i.p.
Evaluation of behavior
Most neonatal 6-OHDA-treated rats were used for behavioral testing when they reached 200 ± 12.7 g (85.5 ± 3.7 days old). Two litters of neonatal 6-OHDA-treated rats were given L-dopa (100 mg/kg) and a decarboxylase inhibitor when 22 or 24 days old. The rats with dopamine preferentially reduced were challenged with L-dopa when their weight reached 175 ± 15.8 g (53 ± 2.9 days old). Animals treated with 6-OHDA as adults (200 g) were allowed to recover from acute aphagia and adipsia before behavioral testing (1–3 weeks; Breese et al., 1973b). At the designated times, the experimental groups and appropriate control animals were given apomorphine (1 mg/kg) and the locomotor response determined using photocell activity monitors as previously described (Hollister et al., 1974). Rats were habituated to the donut-shaped test chambers for 1 hr before injection of the apomorphine and locomotor activity counts accumulated every 10 min after drug injection. At least 1 week after locomotor tests, behavioral observations were made in rats from the different treatment groups (saline, neonate; 6-OHDA, neonate; saline, adult; 6-OHDA, adult). Animals were given saline or their first dose of L-dopa (100 mg/ kg i.p.) and placed in clear plastic cages (46 × 24 × 20 cm high) with wood chip bedding on the floor. The behavioral responses for each animal were observed for 1 min once every 10 min for 2.5 hr or until SMB occurred. Each 1-min observation period was divided into four 15-sec intervals. At the end of each 15-sec interval, a trained observer recorded the codes for all behaviors that occurred during that interval. The behaviors observed included sniffing, rearing, head nodding, locomotion, “taffy pulling” (coordinated movement of front paws toward the mouth and then away from the body), paw treading, self-biting, licking, jumping, digging, eating wood chips and SMB. A high level of interobserver reliability was established before beginning the behavioral observations. At the beginning of each 1-min observation period, animals were inspected for evidence of self-mutilation. SMB was defined as self-biting that caused a break in the skin. Once SMB was observed, the rat was immediately anesthetized with pentobarbital sodium (40 mg/kg i.p.). Animals were not again used until the skin lesion was no longer apparent.
From the neonatal 6-OHDA treated animals tested with L-dopa, 53 rats were randomly chosen and a second dose of 100 mg/kg of L-dopa with RO-4-4602 or saline was administered. Behaviors and SMB were again recorded in these and corresponding control rats. Of those rats positive for SMB after the second 100 mg/kg dose of L-dopa, 30 mg/kg of L-dopa (plus RO-4-4602) was given and behavioral responses again monitored. Those rats positive for SMB at the 30 mg/kg L-dopa dose were subsequently given 10 mg/kg of L-dopa and behaviors recorded. At least a week was allowed between the various doses of L-dopa. When a response was negative for a given dose of L-dopa, it was assumed to be negative for all lower doses of L-dopa administered. The highest dose of L-dopa (with RO-4-4602) administered to adult 6-OHDA-treated rats was 300 mg/kg. The 300 mg/kg dose of L-dopa was also administered to all neonatal 6-OHDA-treated rats that did not elicit SMB at the 100 mg/kg dose. This procedure allowed an assessment of the dose-response relationships of L-dopa on SMB in the adult and neonatal 6-OHDA-treated groups. In addition, various doses of apomorphine (1–10 mg/kg) were administered to control and 6-OHDA treatment groups so that the incidence of behaviors and SMB could be evaluated.
Monoamine determinations
Animals were killed by decapitation, the brains removed and placed on an ice-cooled glass plate. Different brain areas (striatum, olfactory tubercle, nucleus accumbens, hypothalamus, rest of brain minus brain stem) were dissected, weighed and frozen on dry ice. Freezing of the brain stem on a cryostat pedestal and the cutting of three 300-μM sections allowed the substantia nigra to be dissected. Samples were stored at −70°C until monoamines and metabolites could be determined Dopamine, dihydroxyphenylacetic acid, homovanillic acid, serotonin and 5-HIAA were determined simultaneously in samples from various brain regions utilizing the reverse phase high-performance liquid chromatography procedure described by Kilts et al. (1981). The rest of brain, minus the areas dissected free, was homogenized in perchloric acid (0.4 N) and aliquots were analyzed for catecholamines as previously described (Breese and Traylor, 1970).
Statistical analysis
Locomotor response data were tested for significance with an analysis of variance. Accumulated counts were compared utilizing Dunnett’s t test. Data from behavioral observations were computed as described below. For each 1-min observation period, the proportion of intervals in which each behavior occurred was computed for each animal (Lewis et a/., 1984). An arc sin transformation was performed on the proportions to stabilize variances (Winer, 1971). The transformed scores were then analyzed with a two-factor ANOVA having one between subjects factor (treatment groups) and one within subjects factor (time, in 10-min intervals, from the L-dopa injection). A separate two-factor ANOVA was conducted for each behavior observed. Tests for sex differences within each treatment group were conducted. Because no consistent differences based on sex were found, the data for males and females were combined. When significant groups by trials interactions were observed, a one-factor ANOVA was conducted to test for differences between groups at each time point. For each one-factor ANOVA that yielded a significnt F ratio, the Tukey test was used to compare group means. Chi square was used to analyze the frequency of SMB data. Group differences for SMB induced by L-dopa were compared by plotting dose-response data of the percent responding according to the method of Litchfield and Wilcoxin (1949).
Results
Incidence of SMB in neonatal and adult 6-OHDA-treated rats to L-dopa administration: Dose-response relationships
In accord with earlier results (Breese et al., 1984), administration of 100 mg/kg of L-dopa to controls and rats treated as adults with 6-OHDA produced no self-biting or SMB (table 1; see fig. 2). However, this initial dose of L-dopa produced self-biting in 82% and SMB in 65% of the neonatal 6-OHDA-treated rats tested (table 1). Thus, the age at which 6-OHDA is administered plays a critical role in the susceptibility of rats to demonstrate SMB (Breese et al., 1984).
TABLE 1. Incidence of SMB in neonatal and adult 6-OHDA-treated rats after L-dopa.
Rats received 100 mg/kg of L-dopa i.p. 1 hr after treatment with 50 mg/kg of RO-4-4602, a peripheral decarboxylase inhibitor. A rat was considered positive for SMB if the skin was broken anytime during the 2.5-hr observation period. The mean ± S.E.M. time for the neonatal 6-OHDA-treated rats (N = 39) to show SMB was 83 ± 5 min after treatment. Fifty-three of the neonatal 6-OHDA-treated group were taken from this pool along with corresponding controls (neonatal-saline and adult-saline) and adult 6-OHDA-treated rats to examine dose-response relationships (fig. 1). Groups correspond to approximately equal numbers of male and female rats. No self-biting was observed in the adult 6-OHDA- or saline-treatment groups after L-dopa; this behavior was apparent in the majority of neonatal 6-OHDA-treated rats observed after this challenge (82%).
| Treatment | L-Dopa-Induced Incidence of SMB (No./Total) |
|---|---|
| Control | 0/20 |
| 6-OHDA (adult treated) | 0/18 |
| 6-OHDA (neonatal treated) | 39/60*** |
P < .001 when compared to control or 6-OHDA (adult treated).
Fig. 2.
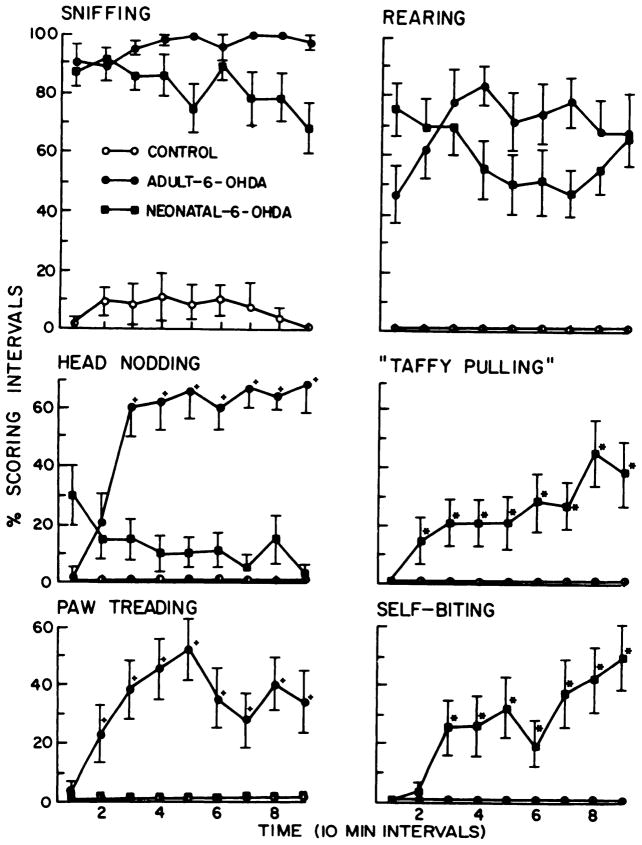
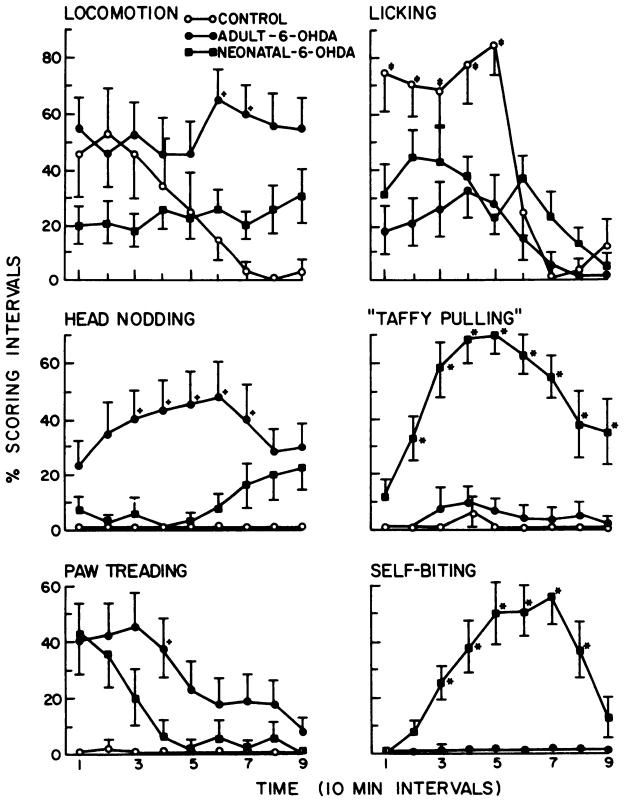
L-dopa-induced behaviors in control and neonatal and adult 6-OHDA-treated rats. The six behaviors illustrated are all significantly different from control (P < .05). The percent scoring interval is the proportion of the four 15-sec periods in which a behavior is observed at each observation point. The time points with a (+) or an (*) indicate significant differences (P < .05) when adult and neonatal 6-OHDA-treated rats are compared. Locomotor activity (not in graph) was significantly elevated in both groups over control (P < .05), but was not different between the 6-OHDA-treated groups (P > .1). The 6-OHDA-treated groups did not dig in wood chips or eat them to any significant degree (data not graphed; see fig. 6). *, p < .05: value in the neonatal 6-OHDA treatment is significant greater than that in the adult 6-OHDA treatment group. +, p < .05: value in the adult 6-OHDA treatment is significantly greater than that in the neonatal 6-OHDA treatment group.
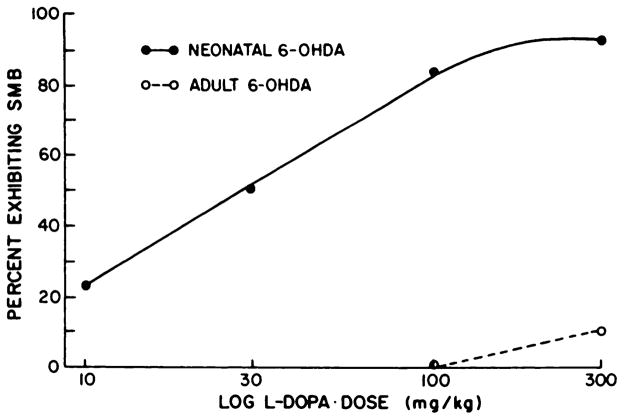
Several other doses of L-dopa also were administered to the two 6-OHDA treatment groups. Results in figure 1 illustrate that 300 mg/kg of L-dopa produced a 10% incidence of SMB in adult 6-OHDA-treated rats, indicating that SMB can occur in this treatment group if the dose is sufficiently high. In neonatal 6-OHDA-treated rats, the L-dopa treatment produced a dose-related increase in SMB from 10 to 300 mg/kg (fig. 1). At 10 mg/kg of L-dopa, SMB was evoked in 22% of the neonatal 6-OHDA-treated rats, twice the incidence of SMB elicited by 300 mg/kg of L-dopa in rats treated as adults with 6-OHDA.
Fig. 1.
Dose-response relationships of L-dopa to induce SMB in adult and neonatal 6-OHDA-treated rats. A second dose of L-dopa (100 mg/ kg) was administered to 53 of the neonatal 6-OHDA-treated rats and to 18 of the adult-treated 6-OHDA-treated rats in table 1. After this determination, 6 neonatal 6-OHDA-treated rats that were negative at 100 mg/ kg of L-dopa and 10 of the adult 6-OHDA-treated rats were given 300 mg/kg of 6-OHDA. The neonatal 6-OHDA-treated rats positive for SMB at the 100 mg/kg dose were given 30 mg/kg of L-dopa. Those SMB positive rats at 30 mg/kg of L-dopa were subsequently given 10 mg/kg of L-dopa. Percent exhibiting SMB was determined from the initial group tested (53) at the 100 mg/kg dose of L-dopa. Log L-dopa dose indicates that doses are plotted on a logarithmic scale.
Behavioral responses to L-dopa administration in various treatment groups
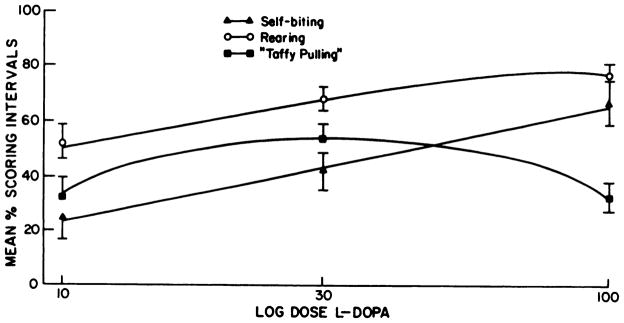
A summary of selected behavioral responses to 100 mg/kg of L-dopa is illustrated in figure 2. Generally, control rats given L-dopa demonstrated no change in behavior compared to animals that received saline (P > .1). These groups went to sleep after a few minutes of exploration. Both 6-OHDA treatment groups (neonatal- and adult-treated) demonstrated significant rearing and sniffing after L-dopa treatment. The dramatic difference between control and 6-OHDA treatment groups given L-dopa is indicative of the behavioral supersensitivity these rats have to this dopamine precursor (fig. 2). However, adult-treated rats had a different spectrum of behaviors when challenged with L-dopa than did rats treated as neonates with 6-OHDA. Paw treading and head nodding were characteristic behaviors of rats treated with 6-OHDA as adults; these behaviors were either not noted in neonatal 6-OHDA- or saline-treated rats after L-dopa or occurred only occasionally. Neonatal 6-OHDA-treated rats characteristically demonstrated self-biting and taffy pulling after 100 mg/kg of L-dopa; such behaviors were not observed in adult 6-OHDA-treated rats given this challenge. By plotting three behaviors observed in neonatal 6-OHDA-treated rats after various doses of L-dopa, it is apparent that self-biting and rearing in the neonatal 6-OHDA-treated rats are dose-related (fig. 3). The taffy pulling response was not found to be dose-related at doses examined (fig. 3).
Fig. 3.
Dose-response effects of L-dopa on selected behaviors in neonatal 6-OHDA-treated rats. Behaviors were assessed as described under “Methods” at 10,30 and 100 mg/kg of L-dopa. Animals are the same as those used to make the dose-response curve for SMB in neonatal 6-OHDA-treated rats (fig. 1). Log dose L-dopa refers to doses of L-dopa on a logarithmic plot.
Age at which SMB can be observed in rats treated with 6-OHDA as neonates
In two Utters of neonatal 6-OHDA-treated rats and a saline-treated control litter, 13 of the 6-OHDA-treated rats and the corresponding controls (5) were given 100 mg/kg of L-dopa with 50 mg/kg of RO-4-4602 (1 hr before) when 22 or 24 days old. In these neonatal 6-OHDA-treated rats, 11 of 13 rats showed self-biting and 3 of the 13 exhibited SMB. Control rats of like age (24 days) showed no SMB response or self-biting when given this L-dopa treatment (N = 5). These data indicate that the behaviors observed in older neonatal 6-OHDA-treated rats can be elicited quite early during development.
Monoamine content in various brain areas after neonatal and adult 6-OHDA treatment
Neonatal and adult 6-OHDA treatments reduced dopamine markedly in striatum, nucleus accumbens and olfactory tubercles (table 2). The substantia nigra showed a significant reduction of dopamine content, but this change was not as great as observed in areas containing dopaminergic terminals. Dopamine content was not altered in hypothalamus of neonatal 6-OHDA-treated rats, but content in adult 6-OHDA-treated rats was significantly reduced. Generally, dopamine metabolites in the 6-OHDA-treated rats were reduced in proportion to the reduction of dopamine (table 2). Neonatal 6-OHDA-treated rats not showing SMB to 100 mg/kg of L-dopa had greater concentrations of dopamine and its metabolites in the terminal regions examined than rats showing SMB, suggesting that the 6-OHDA treatment had been less effective in the former group. Adult 6-OHDA-treated rats had slightly, but significantly, less depletion in striatum than did neonatal 6-OHDA-treated rats showing a positive SMB response to L-dopa. Nevertheless, depletion of dopamine in adult 6-OHDA-treated rats was significantly greater in striatum, olfactory tubercle and nucleus accumbens than that observed in those neonatal 6-OHDA-treated rats which did not exhibit SMB after L-dopa challenge. Norepinephrine concentration was reduced in “rest of brain” to a similar degree in all groups (table 2).
TABLE 2. Effect of various 6-OHDA treatments on dopamine, serotonin and their metabolites in brain.
Neonatal 6-OHDA(+) refers to rats that were positive for L-dopa-induced SMB. Neonatal 6-OHDA(−) refers to rats that did not show SMB. Adult 6-OHDA refers to rats treated as adults. Controls are from neonatal-saline and adult-saline treatment groups and were combined because their values did not differ (P > .1). Brain areas were dissected as described under “Methods.” All groups have 10 rats (5 male and 5 female) in each group, except the neonatal 6-OHDA(−) negative for SMB. which contains 8 rats. No significant differences based upon sex were observed; therefore data were combined as presented above. Values are mean ± S.E.M. N.D., not detectable.
| Brain Area | Monoamine and Metabolites | Control Saline | Neonatal 6-OHDA (+) | Neonatal 6-OHDAH (−) | Adult 6-OHDA |
|---|---|---|---|---|---|
| ng/mg protein | |||||
| Striatum | Dopamine | 83.6 ± 3.2 | 0.8 ± 0.5* | 6.3 ±1.8* | 2.9 ± 0.5* |
| DOPACa | 15.2 ± 0.5 | 0.1 ± 0.04* | 4.4 ± 2.2* | 1.1 ±0.2* | |
| HVAb | 6.6 ± 0.4 | N.D.* | 1.3 ±0.9* | 0.7 ±0.1* | |
| Serotonin | 3.7 ± 0.2 | 4.6 ± 0.2* | 5.2 ± 0.4* | 3.2 ± 0.3 | |
| 5-HIAA | 4.0 ± 0.1 | 5.9 ± 0.2* | 5.5 ± 0.9* | 3.9 ± 0.4 | |
| Olfactory tubercle | Dopamine | 51.2 ± 2.2 | 1.5 ±0.7* | 14.0 ±4.2* | 3.6 ± 0.6* |
| DOPACa | 14.7 ± 0.9 | 0.4 ± 0.2* | 3.3 ±1.1* | 1.5 ±0.3* | |
| HVAb | 2.8 ± 0.7 | N.D.* | 0.4 ± 0.3* | 0.7 ±0.1* | |
| Serotonin | 9.8 ± 0.6 | 9.2 ± 0.5 | 10.3 ±0.6 | 10.4 ±1.2 | |
| 5-HIAA | 4.3 ± 0.5 | 5.4 ± 0.5 | 5.0 ± 0.4 | 6.0 ± 0.6 | |
| Nucleus accumbens | Dopamine | 69.2 ± 4.9 | 0.8 ± 0.3* | 15.0 ±5.2* | 1.8 ±0.5* |
| DOPACa | 28.9 ±1.5 | 0.8 ± 0.3* | 9.8 ± 5.3* | 1.1 ±0.2* | |
| HVAb | 5.7 ± 0.3 | N.D.* | 0.8 ± 0.6* | N.D.* | |
| Serotonin | 3.4 ± 0.3 | 4.7 ± 0.7 | 3.3 ± 0.4 | 4.5 ± 0.7 | |
| 5-HIAA | 4.7 ± 0.3 | 7.2 ± 0.6* | 5.7 ± 0.4* | 5.4 ± 0.6 | |
| Substantia nigra | Dopamine | 2.8 ± 0.2 | 1.0 ±0.1* | 1.3 ±0.4* | 0.1 ±0.1* |
| DOPACa | 0.8 ± 0.1 | 0.5 ± 0.03* | 0.6 ± 0.2 | N.D. | |
| Serotonin | 7.0 ± 0.3 | 6.8 ± 0.3 | 6.5 ± 0.2 | 6.6 ±1.0 | |
| 5-HIAA | 4.6 ± 0.3 | 4.5 ± 0.2 | 4.1 ±1.3 | 4.2 ± 0.4 | |
| Hypothalamus | Dopamine | 2.2 ± 0.3 | 2.0 ± 0.2 | 2.2 ± 0.4 | 1.4 ±0.1* |
| DOPACa | 0.8 ± 0.05 | 0.3 ± 0.06* | 0.6 ± 0.1 | 0.5 ± 0.03 | |
| Serotonin | 4.6 ± 0.5 | 4.1 ± 0.3 | 4.1 ± 0.5 | 3.3 ± 0.7 | |
| 5-HIAA | 4.3 ± 0.3 | 4.5 ± 0.3 | 5.0 ± 0.3 | 1.9 ±0.1 | |
| Rest of brain | Norepinephrine | 2.9 ± 0.2 | 0.2 ± 0.07* | 0.2 ± 0.05* | 0.2 ±0.1* |
Dihydroxyphenyl-acetic acid.
Homovanillic acid.
P < .05 when compared to saline control.
Serotonin and 5-HIAA content (table 2) were not significantly affected in brain of rats treated as adults, confirming previous data (Breese and Traylor, 1970). In neonatal 6-OHDA-treated rats, serotonin concentration was found to be elevated in striatum with a tendency for an increase in nucleus accumbens (table 2), in agreement with Stachowiak et al. (1984). The major metbolite of serotonin, 5-HIAA, was significantly elevated in both striatum and nucleus accumbens in neonatal 6-OHDA-treated rats (table 2).
Effect of apomorphine on locomotion and behavior in 6-OHDA treatment groups
Hollister et al. (1979) provided evidence that the degree of decarboxylation of L-dopa in 6-OHDA-treated rats was in part dependent upon the integrity of serotonergic fibers. Becuse serotonin content was elevated in striatum of neonatal 6-OHDA-treated rats, it seemed essential to test the effects of a dopamine agonist, in these rats, whose action would not depend upon decarboxylation.
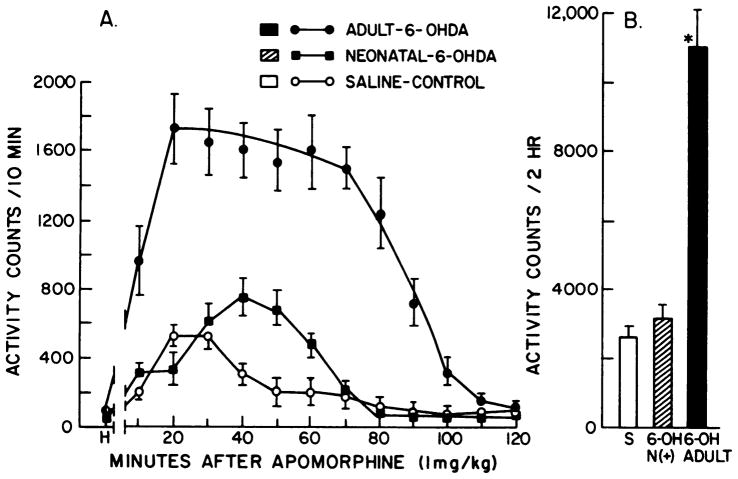
The first evaluation was on the locomotor stimulant effects of apomorphine. As shown in figure 4, administration of apomorphine (1 mg/kg) produced a marked elevation of locomotion in rats treated with 6-OHDA as adults, which was quantitatively similar to that observed in previous studies (Hollister et al., 1974). In contrast to the apomorphine-induced potentiated locomotor resonse in adult-treated rats, this dose of apomorphine in neonatal 6-OHDA-treated rats increased locomotion only slightly more than that observed in saline-treated rats (fig. 4). In a limited number of rats treated with 6-OHDA (n = 4), we compared the locomotor response to 10 mg/kg of L-dopa in each of the groups. The locomotor response to L-dopa remained significantly greater in the adult treated rats (18,800 ± 619 counts/2 hr for adult vs. 11,000 ± 563 counts/2 hr for neonates; P < .05). Because the locomotor response to apomorphine as well as to L-dopa was significantly less in neonatal 6-OHDA-treated rats compared to adult-treated rats, this seemed to minimize the possibility that a change in decarboxylation was responsible for the SMB. However, to obtain additional evidence, other behavioral responses after apomorphine administration were measured.
Fig. 4.
Effects of adult and neonatal 6-OHDA treatment on apomorphine-induced activity. Rats were allowed 1 hr habituation to the activity cages before receiving 1 mg/kg of apomorphine HCl s.c. Panel A shows consecutive 10-min period activity scores through 120 min. Panel B refers to the total activity counts collected for the 120 min session. Activity counts per 120 min for groups given only saline were as follows: control, 646 ± 70; adult 6-OHDA, 664 ± 170; neonatal 6-OHDA, 560 ± 165. Treatments are those described under “Methods.” H, counts accumulated during the last 10 min of habituation. S refers to saline controls that received apomorphine. 6-OH N(+) refers to neonatal 6-OHDA-treated rats positive for SMB and 6-OH adult refers to adult 6-OHDA-treated rats that received apomorphine. *p < .001 when compared to saline or to neonatal 6-OHDA-treated rats.
Whereas behavioral responses to 1 mg/kg of apomorphine in neonatal 6-OHDA-treated rats included increases in rearing, taffy pulling and self-biting, adult 6-OHDA-treated rats increased rearing (data not shown), head nodding and paw treading compared to control (fig. 5). Licking increased over saline treatment after apomorphine for all groups, but this response in 6-OHDA treatment groups was significantly less than for control (fig. 5). Sniffing (data not shown) was maximally elevated in all groups, but duration of this behavior was less for the control group. Adult 6-OHDA-treated rats did not demonstrate taffy pulling or self-biting behavior, but paw treading and head nodding were significantly greater in this group than in the neonatal 6-OHDA-treated rats. In addition, locomotion was significantly greater in adult 6-OHDA-treated rats given 1 mg/kg of apomorphine than in neonatal 6-OHDA-treated rats, a finding consistent with the recording obtained in the locomotor activity monitors (fig. 4).
Fig. 5.
Apomorphine-induced behaviors in control and neonatal and adult 6-OHDA-treated rats. The percent scoring interval is the proportion of the four 15-sec periods in which a behavior is observed at each observation point. At some point in each graph, behavior in controls given 1 mg/kg of apormorphine is significantly different from the other treatments. The time points with a (+), an (*), or a (±) indicate significant differences (P < .05) when adult and neonatal 6-OHDA-treated groups are compared (see below). Rearing activity was not significantly different between the 6-OHDA treatment groups (P > .1), but was significantly elevated (P < .05) in both groups compared to saline treatments (data not shown). Sniffing resembled the response pattern for licking [data not shown; i.e., 6-OHDA treatment responses were not significantly different, but were significantly increased (P < .05) compared to saline]. Duration for both was greater than for control. *, P < .05: value in the neonatal 6-OHDA-treated rats is significantly greater than the corresponding one in the adult 6-OHDA-treatment group. +, P < .05: value in the adult 6-OHDA-treated rats is significantly greater than the corresponding one in the neonatal 6-OHDA treatment group. ±, P < .05: value in saline-control group is significantly greater than the corresponding values for both 6-OHDA treatment groups.
The next experiment determined whether apomorphine would produce SMB in neonatal 6-OHDA-treated rats and whether this group would be more sensitive than adult 6-OHDA-treated rats or controls to induction of this behavior (table 3). Apomorphine (1 mg/kg) produced SMB in 2 of the 14 neonatal 6-OHDA-treated rats monitored in observation cages and self-biting in all of these rats (fig. 5). However, none of the rats (N = 20) given 1 mg/kg of apomorphine and placed in activity monitors showed signs of SMB when examined after removal from these chambers (fig. 4). Apomorphine (3 mg/kg) produced SMB in all neonatal 6-OHDA-treated rats tested (table 3). At 1, 3 or 10 mg/kg of apomorphine, controls and adult 6-OHDA-treated rats exhibited no self-biting or SMB (table 3).
TABLE 3. Incidence of SMB in neonatal and adult 6-OHDA-treated rats after apomorphine.
Rats from table 1 were used for this study at least 1 month after the dose-response investigations of L-dopa. The rats given 1, 3 or 10 mg/kg of apomorphine were visually monitored for 2.5 hr as previously described for L-dopa (see table 1) and the incidence of SMB noted. The neonatal 6-OHDA-treated rats had previously been demonstrated to be positive for SMB when given 100 mg/kg of L-dopa. The mean time ± S.E.M. for the neonatal 6-OHDA-treated rats to show SMB to 3 mg/kg of apomorphine was 53 ± 13 min. Behavioral responses to 1 mg/kg of apomorphine in the various groups are presented in figure 5.
| Treatment | Apomorphine Dose | Incidence of SMB (No./Total) |
|---|---|---|
| mg/kg | ||
| Saline | 1 | 0/12 |
| 3 | 0/6 | |
| 6-OHDA (neonatal treated) | 1 | 2/14 |
| 3 | 6/6** | |
| 6-OHDA (adult-treated) | 1 | 0/14 |
| 3 | 0/10 | |
| 10 | 0/10 |
p<.01 when compared to saline or adult 6-OHDA-treated rats
Thus, the observations concerning locomotion, behavioral responses and SMB induced by apomorphine provide additional data illustrating different responses to a dopamine agonist depending on whether the rats were treated with 6-OHDA as neonates or when adult. These data also provide evidence that the increase in serotonergic fibers observed in neonatal 6-OHDA-treated rats is not likely responsible for the results obtained with L-dopa.
Ng et al. (1970) showed that L-dopa reduced brain serotonin, suggesting that release of serotonin might be contributing to behavioral effects induced by L-dopa. To explore this possibility further, neonatal 6-OHDA-treated rats were given 100 mg/kg of L-5-HTP and the incidence of SMB determined. This treatment has previously been demonstrated to elevate serotonin in 6-OHDA-treated rats (Schlosberg and Harvey, 1979). None of the six neonatal 6-OHDA-treated rats demonstrated SMB after 5-HTP, providing further evidence that release of serotonin is not responsible for the SMB observed after L-dopa (data not shown).
Effects of preferential reduction of brain serotonin, norepinephrine or dopamine in neonatal rats on L-dopa-induced SMB
The possibility that disruption of any neural pathway in the neonate might cause a nonspecific change responsible for the SMB observed in neonates given L-dopa was investigated utilizing 5,7-DHT, the neurotoxin which destroys serotonin-containing fibers (Breese and Cooper, 1975). However, when 5,7-DHT-treated rats (N = 5; >80 days old) were challenged with L-dopa, none showed SMB or behaviors characteristic of those noted in neonatal 6-OHDA-treated rats (table 4). Measurement of monoamines documented that serotonin, but not catecholamines, was drastically reduced after neonatal 5,7-DHT treatment (table 5).
TABLE 4. Effect of preferential depletion of norepinephrine, dopamine and serotonin on L-dopa-induced SMB.
Rats (3 days old) were treated with desipramine (DMI) plus 5,7-DHT as described under “Methods” to reduce only serotonin (serotonin down). 6-OHDA was administered after DMI to reduce only dopamine (dopamine down) and given in two low doses (10,15 μg) at 1 and 3 days of age to reduce norepinephrine (norepinephrine down) (see “Methods” for details). When from 55 to 90 days of age, rats were given 100 mg/kg of L-dopa 1 hr after receiving 50 mg/kg of RO-4-4602. Incidence of SMB was assessed during the 2.5-hr observation period. The mean time ± S.E.M. for the dopamine down-treated rats to exhibit SMB was 95 ± 18 min. See table 5 for representative monoamine values for groups.
| Treatment± | L-dopa-induced SMB (No./Total) |
|---|---|
| Control | 0/18 |
| DMI + 5,7-DHT (serotonin down) | 0/5 |
| 6-OHDA (norepinephrine down) | 0/14 |
| DMI + 6-OHDA (dopamine down) | 7/12* |
P < .05 when compared to control.
TABLE 5. Effect of various neonatal treatments on concentrations of serotonin, norepinephrine and dopamine and their metabolites in brain.
Brain areas were dissected as described under “Methods.” AN groups are the mean nanogram per milligram of protein ± S.E.M. of at least six rats except for the neonatal 5,7-OHT treated rats (N = 5). N.D., not detectable. Dopamine down refers to rats treated to reduce dopamine preferentially; the (+) refers to those rats positive and the (−) refers to those rats negative for SMB. Neonatal 5,7-OHT refers to rats with brain serotonin reduced. Neonatal NE down refers to rats treated to reduce brain norepinephrine. See “Methods” for details.
| Brain Area | Mononines Metabolites | Control Saline | Dopamine Down(+) | Dopamine Down(−) | Neonatal5,7-OHT | Neonatal NE Down (6-OHDA) |
|---|---|---|---|---|---|---|
| ng/mg protein | ||||||
| Striatum | Dopamine | 95.7 ± 2.9 | 3.6 ± 0.9* | 15.6 ±7.7* | 69.8 ± 3.8 | 73.0 ± 8.8* |
| DOPAC a | 12.5 ±0.8 | 1.7 ±0.3* | 13.1 ±2.0 | 13.3 ±2.0 | 9.6 ± 0.9* | |
| HVA b | 8.7 ± 0.3 | 2.9 ± 0.6* | 12.6 ±5.4 | 6.3 ± 0.3 | 7.7 ± 0.7 | |
| Serotonin | 4.0 ± 0.1 | 5.1 ± 0.3* | 4.6 ± 0.3 | 0.9 ± 0.05* | 6.3 ± 0.6* | |
| 5-HIAA | 4.4 ± 0.2 | 5.6 ± 0.3* | 5.5 ± 0.5 | ND* | 4.9 ± 0.4 | |
| Olfactory tubercle | Dopamine | 46.4 ± 5.8 | 7.7 ±2.1* | 6.1 ±1.3* | 46.8 ± 3.4 | 32.0 ± 3.0* |
| DOPAC a | 15.7 ±1.0 | 3.9 ±1.5* | 3.1 ± 0.8* | 12.4 ±1.3 | 11.0 ±1.3* | |
| HVA b | 2.0 ± 0.4 | N.D.* | N.D.* | 2.0 ± 0.3 | 1.0 ±0.1* | |
| Serotonin | 10.6 ±1.0 | 14.3 ±1.7 | 10.8 ±0.8 | 0.9 ±0.1* | 11.0 ±0.6 | |
| 5-HIAA | 6.7 ± 0.3 | 9.2 ± 0.9* | 7.9 ± 0.6 | 0.3 ± 0.06* | 6.6 ± 0.4 | |
| Nucleus accumbens | Dopamine | 83.6 ± 4.2 | 1.9 ±0.8* | 3.1 ± 0.8* | 71.2 ±7.4 | 76.6 ± 9.5 |
| DOPAC a | 29.3 ± 2.0 | N.D.* | 0.5 ± 0.3* | 22.2 ± 7.9 | 27.7 ± 4.2 | |
| HVAb | 6.5 ± 0.6 | N.D.* | N.D.* | 5.1 ± 0.5 | 3.2 ± 0.7* | |
| Serotonin | 3.3 ± 0.7 | 3.4 ± 0.7 | 4.4 ± 0.8 | 0.3 ± 0.05* | 4.5 ±1.2 | |
| 5-HIAA | 5.7 ± 0.4 | 5.1 ± 0.5 | 6.0 ± 0.5 | N.D.* | 6.1 ± 0.9 | |
| Rest of Brain | Dopamine | 6.0 ± 0.4 | 0.8±0.1* | 1.1 ±0.1* | 5.7 ± 0.3 | 4.2 ± 0.2 |
| Norepinephrine | 3.4 ± 0.2 | 3.8 ± 0.3 | 3.8 ± 0.4 | 3.5 ± 0.2 | 0.9 ± 0.1 | |
Dihydroxyphenyt-acetic acid.
Homovanillic acid.
P < .05 when compared to saline.
In order to examine the relative importance of norepinephrine-and dopamine-containing fibers in the susceptibility for SMB observed in neonatal 6-OHDA-treated rats to dopamine agonists, neonatal rats were treated to reduce selectively dopamine or norepinephrine (Smith et al., 1973). Administration of 100 mg/kg of L-dopa to rats with selective reduction of norepinephrine-containing fibers produced no SMB (table 4). In contrast, L-dopa (100 mg/kg) produced SMB in 7 and self-biting in 11 of 12 neonatal 6-OHDA-treated rats with selective dopamine depletion. As before, no SMB was observed in saline-treated controls. Thus, the SMB observed in neonatal 6-OHDA-treated rats is apparently related to destruction of dopamine-containing fibers.
Among the rats with brain dopamine preferentially reduced, those positive for SMB after L-dopa had greater reductions of brain dopamine than those negative for SMB (table 5). Content of dopamine and its metabolites were markedly reduced by this neonatal 6-OHDA treatment in striatum, olfactory tubercle and nuclus accumbens. Hypothalamic dopamine (data not shown) as well as brain norepinephrine concentrations in rest of brain were not significantly reduced by this treatment. Similar to the change in neonatal 6-OHDA-treated rats (table 2), serotonin and 5-HIAA content were modestly, but significantly, increased in striatum of rats with brain dopamine reduced that were positive for SMB. There was a tendency for a change in serotonin content in the olfactory tubercles and 5-HIAA was significantly elevated in this brain area compared to saline (table 5). The “norepinephrine down” treatment group had the expected monoamine reduced more than any other transmitter (table 5).
Behavioral response to L-dopa in rats with brain dopamine selectively reduced neonatally
Behaviors induced by L-dopa in the rats treated neonatally to reduce dopamine selectively indicate that these animals do not display exactly the same behavioral profile as rats with both catecholamines reduced (fig. 6). Like neonatal 6-OHDA-treated rats with both amines reduced, animals with reduced dopamine demonstrated significant levels of taffy pulling, licking, self-biting, sniffing and rearing. However, licking was significantly greater in magnitude for those rats with brain dopamine reduced than for all other treatment groups (fig. 6). Furthermore, selective neonatal dopamine depletion was associated with significant amounts of eating wood chips and digging in the wood chips after L-dopa (fig. 6).
Fig. 6.
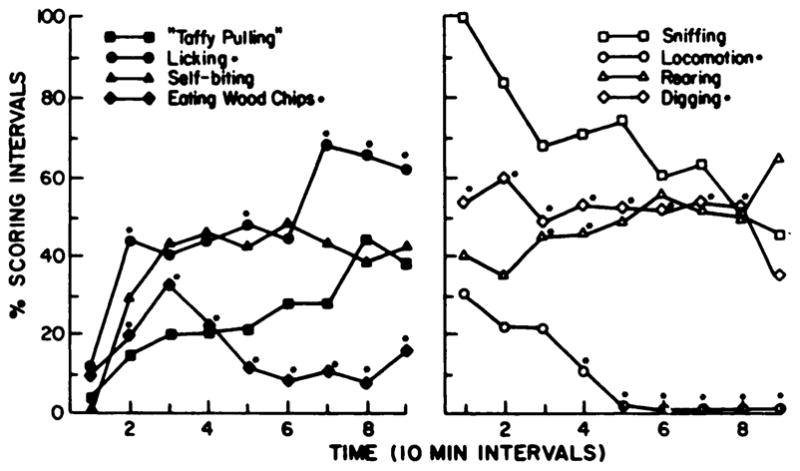
Effects of neonatal 6-OHDA-treatment causing preferential reduction of brain dopamine on behavioral responses to L-dopa. Behaviors illustrated are in rats treated with desipramine (20 mg/kg) 1 hr before 6-OHDA (100 μg intracistemally) to reduce only brain dopamine and not norepinephrine at 5 days of age (see table 5). Behaviors were scored as described under “Methods.” Over time, behaviors induced by L-dopa (100 mg/kg) in rats with brain dopamine preferentially reduced were compared statistically to behavioral responses observed in 6-OHDA-treated neonates, controls and adult 6-OHDA-treated rats. Behaviors illustrated are significantly different from control. Notations on the graph refer to differences with behaviors observed in neonatal 6-OHDA-treated rats with both catecholamines reduced (see fig. 2 for comparison with some of the behaviors). *p < .05 when compared to neonatal 6-OHDA-treated rats.
The rats with dopamine preferentially reduced neonatally with 6-OHDA were also administered apomorphine (1 mg/kg i.p.). The locomotor activity response in these rats was compared to the response in saline-treated controls. Activity counts in saline-treated controls were 2744 ± 349 per 120 min (N = 7), whereas the response in the “dopamine down” treatment group was 4798 ± 374 counts (N = 11), which is significantly higher than the response in controls (P < .001). This response, then, is significantly greater than the apomorphine-induced activity observed in neonatal 6-OHDA-treated rats (fig. 4), but is consideraby less than the increased activity counts induced by apomorphine in rats with dopamine reduced when adult (Hollister et a/., 1979). Thus, while susceptibility for SMB persists when damage is limited to dopaminergic neurons, the simultaneous reduction of norepinephrine may have a modulatory effect on the degree to which a specific behavioral response is observed as well as the magnitude of the response.
Central dopa decarboxylase inhibition, dopamine-β-hydroxylase inhibition and neuroleptics on SMB induced by L-dopa in neonatal 6-OHDA-treated rats
Because dopamine formed from L-dopa can be converted to norepinephrine, a dopamines-hydroxylase inhibitor was administered to block this conversion, to determine if this treatment would antagonize or alter the SMB response induced by 30 mg/kg of L-dopa in neonatal 6-OHDA-treated rats. This treatment had no effect on the SMB induced in these rats (table 6). In our next experiment, large doses of the decarboxylase inhibitor, RO-4-4602, were administered to block conversion of dopa to dopamine in brain (Porter, 1971; Hollister et al., 1979), which in turn would be expected to antagonize the SMB induced by L-dopa. Unexpectedly, this treatment did not antagonize the SMB response to L-dopa. These latter results subsequently led to a trial of haloperidol against the SMB induced by L-dopa. This pharmacological treatment attenuated the SMB observed in the majority of the animals (table 6), but did not attenuate self-biting and taffy pulling responses to L-dopa in any of the rats (data not shown). In contrast to the action of haloperidol against L-dopa-induced SMB, another neuroleptic, cisflupentixol, effectively antagonized the SMB and self-biting induced by 100 mg/kg of L-dopa in all rats (table 6) and partially blocked the taffy pulling behavior (data not presented). These latter observations are particularly significant because the dose of L-dopa (100 mg/kg) was a log unit higher in the rats given cisflupentixol than those given haloperidol before L-dopa (30mg/kg; see table 6).
TABLE 6. Effect of central dope decarboxylase inhibition, a dopamine-β-hydroxytase inhibitor, and neuroleptics on SMB induced by L-dopa.
Rats were treated with RO-4-4602 (50 mg/kg or higher doses) 60 min before injection of L-dopa (30 mg/kg). The oopamine-β-hydroxylase inhibitor, U-14,624, haloperidol and cisflupentixol were administered 45 min before administration of L-dopa.
| Treatment | Dose | L-dope-induced Incidence of SMB (No./Total) |
|---|---|---|
| (mg/kg) | ||
| Saline | 18/18 | |
| RO-4-4602 | 500–800 | 7/7 |
| U-14,624 | 50 | 6/6 |
| Haloperidol | 1 | 2/7* |
| Flupentixola | 1 | 0/6* |
Rats pretreated with cisflupentixol received 100 mg/kg of L-dopa rather than the 30 mg/kg received by the other rats.
P < .05 when compared to saline.
Discussion
The present findings demonstrate that certain responses to L-dopa and apomorphine in neonatal 6-OHDA-treated rats are qualitatively different from responses observed in adult 6-OHDA-treated rats. In general, increased locomotion, paw treading and head nodding were behaviors characteristically associated with dopamine agonists administered to rats treated with 6-OHDA as adults, whereas self-biting and taffy pulling were common responses in neonatal 6-OHDA-treated rats administered a dopamine agonist. Both groups showed elevated rearing after either L-dopa or apomorphine indicative of their supersensitivity to these challenges. In rats treated neonatally, a high percentage exhibited SMB to dopamine agonist challenge, whereas only one adult 6-OHDA-treated rat exhibited this behavioral response at doses tested. The marked increase in apomorphine-induced locomotion in adult 6-OHDA-treated rats is consistent with earlier findings (Hollister et al., 1974, 1979) and probably indicates a maximal adaptation of systems essential for this “supersensitive” response to dopamine agonists. Behaviors observed after apomorphine in our adult 6-OHDA-treated rats appear to be like those described by Schoenfeld and Uretsky (1972), who describe no self-mutilation in their 6-OHDA-treated rats after administration of 3 mg/kg of apomorphine. Ungerstedt (1971a) described SMB after apomorphine treatment of rats with 6-OHDA placed in substantia nigra to reduce brain catecholamines, a finding consistent with our result that high doses of L-dopa could elicit SMB in adult 6-OHDA-treated rats. Creese and Iversen (1973) reported that neonatal 6-OHDA treatments enhanced stereotyped behaviors induced by apomorphine and described SMB at doses of 1.5 mg/kg of apomorphine after neonatal 6-OHDA treatment. Thus, there is considerable data demonstrating that 6-OHDA treatment of developing and adult rats induces supersensitivity to dopamine agonists. However, this literature has not stressed that the character of the behavioral responses to dopamine agonists in neonatal 6-OHDA-treated rats can differ from those in adult 6-OHDA-treated animals.
The observation that self-mutilation and self-biting could be exhibited in neonatal 6-OHDA-treated rats given L-dopa as early as 22 days of age was unexpected. Generally, gnawing is not elicited by dopamine agonists until after this age (Lai and Sourkes, 1973). Certainly, SMB is not observed at any age in control rats given 100 mg/kg of L-dopa. This particular observation suggests not only that supersensitivity to dopamine agonists is in place at this early age, but also that any other adaptive changes responsible for this behavior have occurred by the time the rat is 22 to 24 days old.
Because L-dopa-induced SMB was apparent in rats with only dopamine-containing fibers destroyed neonatally, the neural basis of this behavioral response to dopamine agonists in neonatal 6-OHDA-treated rats appears to be associated with destruction of dopamine-containing neurons during development. The inability of the dopamines-β-hydroxylase inhibitor to antagonize L-dopa-induced SMB suggests that synthesis of norepinephrine from L-dopa is not contributing to the response. Because norepinephrine is equally reduced in neonatal 6-OHDA-treated rats negative or positive for SMB and L-dopa does not produce SMB in rats with norepinephrine preferentially reduced, it does not appear that altered noradrenergic function is responsible for the SMB exhibited after L-dopa. Nevertheless, whereas reduction of dopaminergic fibers appears to be essential for SMB induced by dopamine agonists, there were differences between behavioral responses to L-dopa in neonates with dopamine reduced and those with both catecholamines reduced (fig. 6). Because degree of dopamine depletion in rats with brain dopamine preferentially reduced was significantly less than that in rats with both amines reduced, one cannot determine with certainty whether this small change in the pattern of dopamine depletion or the absence of noradrenergic fibers is contributing to the different spectrum of behaviors in these groups. A possible explanation would be that noradrenergic fibers modulate dopamine output which results in qualitatively different behaviors in the group with dopamine preferentially reduced.
Several possibilities could explain the different behavioral responses to dopamine agonists observed in adult and neonatal 6-OHDA-treated rats. The first considered was that there were differences in the depletion of dopamine due either to sprouting or differential effects of 6-OHDA administration at the two different ages. However, assessment of dopamine depletion in neonatal and adult 6-OHDA-treated rats does not support this possibility. This latter point is illustrated with changes in striatal dopamine induced by the various treatments (table 7). Although rats treated neonatally exhibited SMB when challenged with L-dopa, they had only slightly greater reductions of dopamine in striatum than adult rats (table 7). This minor difference in dopamine depletion did not seem sufficient to explain the major shift in the character of the response to dopamine agonists exhibited between neonatal and adult 6-OHDA-treated rats. Furthermore, neonatal 6-OHDA-treated rats with only dopamine content reduced had dopamine concentrations in striatum similar to those in adult 6-OHDA-treated rats. The former were positive for SMB and the latter were not. It also is important to note that the degree of potentiation of rearing was not significantly different after L-dopa in the two 6-OHDA treatment groups (fig. 2), suggesting equivalent potentiation of these responses. Because there were no major regional differences in dopamine reduction among the various 6-OHDA treatment groups in the brain areas analyzed, a regional change does not appear to provide an explanation. Thus, it appears that a mechanism(s) other than a difference in depletion of dopamine is responsible for the altered behavioral responses to dopamine agonists exhibited by neonatal and adult 6-OHDA-treated rats. Nevertheless, monoamine determinations in neonatal 6-OHDA-treated rats positive and negative for SMB induction by L-dopa clearly demonstrated that degree of dopamine depletion contributes to the susceptibility for SMB induced by dopamine agonists in the group with both catecholamines reduced as well as in the dopamine-down group (table 7).
TABLE 7.
Comparison of striatal dopamine concentration with SMB response in neonatal 6-OHDA-treated rats
| Treatment | SMB Response to L-dopa | Striatal Dopaminea (% Control) |
|---|---|---|
| Neonatal 6-OHDA | Positive | 1.0 ± 0.6 |
| Neonatal 6-OHDA | Negative | 7.4 ± 1.2 |
| Adult 6-OHDA | Negative | 3.0 ± 0.5 |
| Dopamine down | Positive | 3.5 ± 1.0 |
| Dopamine down | Negative | 16.4 ± 8.0 |
The specific increase of serotonin content in the striatum of rats treated neonatally with 6-OHDA confirm earlier reports (Stachowiak et al., 1984; Mailman et al., 1983). Recent data (Mailman et al., 1983) suggest that this increase in serotonin concentration after neonatal 6-OHDA-treatment is associated with increased serotonergic innervation. Therefore, based upon the observation that serotonergic fibers contribute in part to the conversion of L-dopa to dopamine in adult 6-OHDA-treated animals (Hollister et al., 1979), it was possible that different responses to L-dopa in neonatal and adult 6-OHDA-treated rats were related to differences in the degree of decarboxylation of L-dopa in serotonergic fibers. However, data collected in this study suggest that this mechanism does not explain the behavioral differences between neonatal and adult 6-OHDA-treated rats, because similar behavioral differences were exhibited by these groups when given apomorphine, a dopamine agonist not dependent upon decarboxylation. Furthermore, serotonin released from these fibers by L-dopa does not appear to be responsible for the SMB or other unique behaviors exhibited by L-dopa in neonatal 6-OHDA-treated rats, because administration of 5-HTP did not produce such changes in behavior or induce SMB in these rats. Nevertheless, the serotonin hyper-innervation in striatum is unique to the neonatal 6-OHDA-treated rat with or without norepinephrine depletion and provides evidence for one type of neural adaptation which differs from that occurring in adult 6-OHDA-treated animals. Altering serotonin content is known to influence responses of indirect acting dopamine agonists (Neill et al., 1972; Breese et al., 1974; Hollister et al., 1976). Segal et al. (1976) suggested that the enhanced locomotor response to d-amphetamine in rats with decreased serotonin function was dependent upon a reduction of stereotyped behavior. It is not known whether elevated content or release of serotonin can produce the reverse consequences on motor function changes induced by dopamine agonists (i.e., increase stereotyped behavior). Additional work will be necessary to define whether this neural change in serotonin-containing fibers contributes in some way to the differences in response to dopamine agonists noted between adult and neonatal 6-OHDA-treated rats.
A surprising finding was the inability of large doses of the decarboxylase inhibitor, RO-4-4602, to inhibit SMB induced by L-dopa, because doses used are reported to inhibit central decarboxylation of L-dopa (Porter, 1971). Because Hollister et al. (1979) antagonized the locomotor stimulant action of L-dopa in adult 6-OHDA-treated rats with doses of RO-4602 used in our study, it was possible that mechanisms other than those associated with dopamine might be involved in the SMB. The prolonged period (>80 min) before SMB develops after L-dopa administration could be consistent with this view (see legends to Tables 1 and 4). Additional doubt about the involvement of dopamine in the induction of SMB was raised by the observation that a high dose of haloperidol did not reduce self-biting and only attenuated SMB in neonatal 6-OHDA-treated rats. However, resistance to the action of haloperidol-like neuroleptics to antagonize dopamine agonists has been observed in adult 6-OHDA-treated rats (Fuxe et al., 1975; Ondrusek et al., 1981). Thus, additional work is necessary to determine whether responses to haloperidol differ in neonatal and adult 6-OHDA-treated rats. In spite of these latter findings, positive effects with apomorphine, a dopamine agonist, and the observation that cisflupentixol antagonizes the effects of L-dopa in neonatal 6-OHDA-treated rats would seem to implicate a dopamine receptor mechanism in behavioral responses to dopamine agonists observed in the neonatal 6-OHDA-treatment group. If one assumes that this is the case, the inability of the dopa-decarboxylase inhibitor to antagonize the effects of L-dopa is likely associated with the marked supersensitivity of these animals to dopamine.
From previous experiments, an increase in spiperone receptor binding has not been observed in neonatal and adult 6-OHDA-treated rats in spite of their demonstrated supersensitivity to dopamine agonists (Mailman et al., 1981, 1983), reducing the likelihood that a change in this receptor system explains the resistance 6-OHDA-treated rats have to haloperidol. Because cisflupentixol has potent effects to block dopamine-stimulation of adenylate cyclase, with less affinity for spiperone binding sites (Hyttel, 1978; Hyttel and Christensen, 1983), the effectiveness of cisflupentixol to block SMB and self-biting induced by L-dopa suggests that sites other than those associated with haloperidol are responsible for the behavioral responses to dopamine agonists in the neonatal 6-OHDA-treated rats. Because preliminary data indicates that Sch-23390, a D-l blocker (Iorio et al., 1983), antagonizes SMB induced by L-dopa in neonatal 6-OHDA-treated rats (G. R. Breese, unpublished data), the SMB probably depends upon supersensitive D-1 receptors.
The present results, exemplifying the remarkable plasticity of the neonate to neuronal damage and their varied responses compared to adults, appear to be relevant to understanding the clinical symptoms associated with Parkinsonism and the Lesch-Nyhan syndrome. Both of these clinical disorders are associated with reduced brain dopamine and motor dysfunction (Hornykiewicz, 1973; Lloyd et al., 1981; Wilson et al., 1983). However, in spite of this common biochemical deficiency, the character of their motor symptoms differ. Parkinson patients display tremor, bradykinesia and stiffness, whereas Lesch-Nyhan syndrome is characterized by choreoathetoid movements and hypertonicity (Wilson et al., 1983; Kelley and Wyn-gaarden, 1983). The latter symptoms bear some resemblance to those observed in Huntington’s chorea (Coyle et al., 1977). It may be relevant, too, that a juvenile form of Huntington’s chorea has many symptoms resembling those of Parkinsonism rather than those observed in the adult onset form of this genetic disorder (Coyle et al., 1977). Data in the present investigation provide support for the view that the age at which dopaminergic pathways are destroyed in these clinical conditions can have an important bearing on the type of motor dysfunction exhibited. The adult 6-OHDA-treated rat has been used for some time as a neurochemical model of Parkinson’s disease (Ungerstedt, 1971a; Uretskey and Schoenfield, 1971; Hollister et al., 1974). We suggest that the neonatal 6-OHDA-treated rat may serve as a model of central dysfunctions observed in childhood diseases with reduced brain dopamine. The Lesch-Nyhan syndrome would be one of those diseases (Lloyd et al., 1981; Kopin, 1981).
The Lesch-Nyhan syndrome is also characterized by a unique compulsive self-mutilation of digits and tissue about the mouth (Lesch and Nyhan, 1964). The present work provides further support for the hypothesis that the increased susceptibility for SMB observed in children with Lesch-Nyhan syndrome is associated with a neonatal destruction of dopaminergic pathways (Breese et al., 1984). This is supported by the remarkable sensitivity neonatal 6-OHDA-treated rats show for SMB when challenged with L-dopa or apomorphine (Creese and Iversen, 1973; Breese et al., 1984). Although the neonatal 6-OHDA-treated rats do not have the biochemical error in purine metabolism characteristic of Lesch-Nyhan disease, the availability of this treatment model should allow independent investigations as to whether purine metabolites contribute to symptoms, such as SMB, when administered to rats with reduced brain dopamine. The neonatal 6-OHDA-treated rat will also permit us to determine if hypoxanthine-guanine phosphoribosyl transferase is in dopamine-containing fibers or in other neural elements within the striatum. This latter information would allow an assessment of the basis for the destruction of dopamine-containing fibers in this condition (i.e., lack of enzyme in dopaminergic neurons). It should be emphasized that L-dopa- and apomorphine-induced SMB in neonatal 6-OHDA-treated rats should only be interpreted to reflect the enhanced susceptibility animals have for induction of SMB. Activation of dopamine receptors need not be the mechanism by which SMB is induced in the Lesch-Nyhan syndrome. Whereas endogenous release of dopamine from neurons remaining after the destructive process may well contribute to SMB in patients, it is also possible that other mechanisms can precipitate SMB once the increased susceptibility is in place. The availability of this neurochemical model of increased susceptibility for SMB may also permit screening of a variety of pharmacological agents that could minimize the SMB observed in Lesch-Nyhan patients. Future investigations in the neonatal 6-OHDA-treated rats will test the usefulness of this proposed model.
Acknowledgments
The authors are grateful to Marcine Garrison and Edna Edwards for their technical assistance and to Sue Ellis for typing the manuscript.
ABBREVIATIONS
- SMB
self-mutilation behavior
- 6-OHDA
6-hydroxydopamine
- 5,7-DHT
5,7-dihydroxytryptamine
- 5-HIAA
5-hydroxyindole-acetic acid
- ANOVA
analysis of variance
- 5-HTP
5-hydroxytryptophan
Footnotes
This work was supported by U.S. Public Health Services Grants HD-03110, MH-36294, HL-31424 and HD-07201.
References
- Breese GR, COOPER BR. Behavioral and biochemical interactions of 5,7-dihydroxytryptamine with various drugs when administered intracisternally to adult and developing rats. Brain Res. 1975;98:517–527. doi: 10.1016/0006-8993(75)90370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Traylor TD. Effects of 6-hydroxydopamine on brain norepinephrine and dopamine: Evidence for selective degeneration of catecholamine neurons. J Pharmacol Exp Ther. 1970;174:413–420. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Traylor TD. Developmental characteristics of brain catecholamines and tyrosine hydroxylase in the rat: Effects of 6-hydroxydopamine. Br J Pharmacol. 1972;44:210–222. doi: 10.1111/j.1476-5381.1972.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Cooper BR, Mueller RA. Evidence for an involvement of 5-hydroxytryptamine in the actions of amphetamine. Br J Pharmacol. 1974;52:307–319. doi: 10.1111/j.1476-5381.1974.tb09714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Cooper BR, Smith RD. Biochemical and behavioral alterations following 6-hydroxydopamine administration into brain. In: Usdin E, Snyder S, editors. Frontiers in Catecholamine Research. Pergamon Press; New York: 1973a. pp. 701–706. [Google Scholar]
- Breese GR, McCown TJ, Baumeister AA, Emerick SG, Frye GD, Mueller RA. L-DOPA-induced self-biting in rats treated with 6-hydroxydopamine (6-OHDA) as neonates; Model of self-mutilation observed in the Lesch-Nyhan Syndrome. Fed Proc. 1984;43:928. [Google Scholar]
- Breese GR, Mueller RA, Mailman RB, Frye GD, Vogel RA. Study of drug mechanisms and disease symptoms: Alternatives to animal models for CNS disorders. Prog Neuro-Psychopharmacol. 1978a;2:313–325. [Google Scholar]
- Breese GR, Smith RD, Cooper BR, Grant LD. Alterations in consummatory behavior following intracisternal injection of 6-hydroxydopamine. Pharmacol Biochem Behav. 1973b;1:319–328. doi: 10.1016/0091-3057(73)90124-x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Vogel RA, Kuhn CM, Mailman RB, Mueller RA, Schanberg SM. Behavioral and prolactin responses to 5-hydroxytryptophan in rats treated during development with 5,7-dihydroxytryptamine. Brain Res. 1978b;155:263–275. doi: 10.1016/0006-8993(78)91022-3. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Smith RD, Konkol RJ, Breese GR. Alteration of growth and development after neonatal treatments with 6-hydroxydopamine. In: Jonsson G, Malormfors T, Sachs CN, editors. Chemical Tools in Catecholamine Research. Vol. 1. North Holland Publishing Co; New York: 1975. pp. 197–210. [Google Scholar]
- Coyle JT, Schwarcz R, Bennett JP, Campochiaro P. Clinical, neuropathological, and pharmacological aspects of Huntington’s disease: Correlates with a new animal model. Prog Neuro-psychopharmacol. 1977;1:13–30. doi: 10.1016/0364-7722(77)90025-x. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. Blockade of amphetamine-induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Hokfelt T, Jonsson G, Lidbrink P, Ljungdahl A, Lofstrom A, Ungerstedt U. The effect of dopamine receptor stimulating and blocking agents on the activity of supersensitive dopamine receptors and on the amine turnover in various dopamine nerve terminal systems in the rat brain. J Pharmacol. 1975;6:117–129. [Google Scholar]
- Hollister AS, Breese GR, Cooper BR. Comparison of tyrosine hydroxylase and dopamines-β-hydroxylase inhibition with the effects of various 6-hydroxydopamine treatments on d-amphetamine induced motor activiy. Psychopharmacologia. 1974;36:1–16. doi: 10.1007/BF00441377. [DOI] [PubMed] [Google Scholar]
- Hollister AS, Breese GR, Kuhn CM, Cooper BR, Schanberg SM. An inhibitory role for brain serotonin-containing systems in the locomotor effects of d-amphetamine. J Pharmacol Exp Ther. 1976;198:12–22. [PMC free article] [PubMed] [Google Scholar]
- Hollister AS, Breese GR, Mueller RA. Role of monoamine neural systems in L-dihydroxyphenylalanine-stimulated activity. J Pharmacol Exp Ther. 1979;208:37–43. [PubMed] [Google Scholar]
- Hornykiewicz O. Parkinson’s disease: From brain homogenate to treatment. Fed Proc. 1973;32:183–190. [PubMed] [Google Scholar]
- Hyttel J. A comparison of the effect of neuroleptic drugs on the binding of 3H-haloperidol and 3H-(Z)-flupentixol and on adenylate cyclase activity in rat striatal tissue in vitro. Prog Neuro-psychopharmacol. 1978;2:329–335. [Google Scholar]
- Hyttel J, Christensen AV. Biochemical and pharmacological differentiation of neuroleptic effect on dopamine D-1 and D-2 receptors. J Neurol Transm. 1983;18(suppl):157–164. [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba A. Sch 23390, a potent benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- Kelley WN, Wyngaarden JB. Clinical syndromes associated with hypoxanthine-guanine phophoiibosyl-transferase deficiency. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, editors. The Metabolic Basis of Inherited Disease. 5. McGraw-Hill; New York: 1983. pp. 1115–1143. [Google Scholar]
- Kilts CD, Breese GR, Mailman RB. Simultaneous quantification of dopamine, 5-hydrozytryptamine and four metabolically related compounds by means of reverse phase HPLC with electrochemical detection. J Chromatogr Biol Med Appl. 1981;225:347–357. doi: 10.1016/s0378-4347(00)80283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ. Neurotransmitters and the Lesch-Nyhan syndrome, N. Engl J Med. 1981;305:1148–1149. doi: 10.1056/NEJM198111053051910. [DOI] [PubMed] [Google Scholar]
- Lal S, Sourkes TL. Ontogeny of stereotyped behavior induced by apomorphine and amphetamine in the rat. Arch Int Pharmacodyn Ther. 1973;202:171–182. [PubMed] [Google Scholar]
- Lewis MH, Baumeister AA, McCorkle DL, Mailman RB. An improved analyses of drug induced stereotyped behavior using a computer supported observational method. Psychopharmacology. 1984 in press. [Google Scholar]
- Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am J Med. 1964;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxin F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–117. [PubMed] [Google Scholar]
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, Shibuya M, Kelley WN, Fox IH. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 1981;305:1106–1111. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- Mailman RB, Kilts CD, Beaumont K, Breese GR. “Supersensitivity” of dopamine systems: Comparisons between haloperidol withdrawal, intracisternal (I.C.) and unilateral (UNI) 6-hydroxydopamine (OHDA) treatments. Fed Proc. 1981;40:291. [Google Scholar]
- Mailman RB, Towle A, Schulz DW, Lewis MH, Breese GR, DeHaven DH, Krigman MR. Neonatal 6-OHDA treatment of rats: Changes in dopamine (DA) receptors, striatal neurochemistry and anatomy. Soc Neurosci Abstr. 1983;9:932. [Google Scholar]
- Ng KY, Chase TN, Colburn RW, Kopin IJ. L-DOPA induced release of cerebral monoamines. Science (Wash DC) 1970;170:76–77. doi: 10.1126/science.170.3953.76. [DOI] [PubMed] [Google Scholar]
- Neill DB, Grant LD, Grossman SP. Selective potentiation of locomotor effects of amphetamine by midbrain raphe lesions. Physiol Behav. 1972;9:655–657. doi: 10.1016/0031-9384(72)90026-1. [DOI] [PubMed] [Google Scholar]
- Ondrusek MG, Kilts CD, Frye GD, Mailman RB, Mueller RA, Breese GR. Behavioral and biochemical studies of the scopolamine- induced reversal of neuroleptic activity. Psychopharmacology. 1981;73:17–22. doi: 10.1007/BF00431093. [DOI] [PubMed] [Google Scholar]
- Porter C. C: Aromatic amino acid inhibitors. Fed Proc. 1971;30:871–876. [PubMed] [Google Scholar]
- Schlosberg AJ, Harvey JA. Effects of L-dopa and 1–5-hydroxytryptophan on locomotor activity of the rat after selective or combined destruction of central catecholamine and serotonin neurons. J Pharmacol Exp Ther. 1979;211:296–304. [PubMed] [Google Scholar]
- Schoenfeld R, Uretsky N. Altered response to apomorphine in 6- hydroxydopamine-treated rats. Eur J Pharmacol. 1972;19:115–118. doi: 10.1016/0014-2999(72)90085-4. [DOI] [PubMed] [Google Scholar]
- Seegmiller JE, Rosenbloom FM, Kelley WN. An enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science (Wash DC) 1967;155:1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Segal DS. Differential effects of PCPA on amphetamine-induced locomotion and stereotypy. Brain Res. 1976;116:267–276. doi: 10.1016/0006-8993(76)90904-5. [DOI] [PubMed] [Google Scholar]
- Smith RD, Cooper BR, Breese GR. Growth and behavioral changes in developing rats treated intracisternally with 6-hydroxydopamine: Evidence for involvement of brain dopamine. J Pharmacol Exp Ther. 1973;185:609–619. [PubMed] [Google Scholar]
- Stachowiak MK, Bruno JP, Snyder AM, Stricker EM, Zigmond MJ. Apparent sprouting of striatal serotonergic terminals after dopamine-depleting brain lesions in neonatal rats. Brain Res. 1984;291:164–167. doi: 10.1016/0006-8993(84)90665-6. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971a;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-OHDA induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971b;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Uretsky NJ, Schoenfeld RI. Effect of L-DOPA on the locomotor activity of rats pretreated with 6-hydroxydopamine. Nat New Biol. 1971;234:157–159. doi: 10.1038/newbio234157a0. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Young AB, Kelley WN. Hypoxanthine-guanine phosphoribosyl transferase deficiency: The molecular basis of the clinical syndromes. N Engl J Med. 1983;309:900–910. doi: 10.1056/NEJM198310133091507. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. McGraw Hill; New York: 1971. [Google Scholar]