Abstract
Background
Central obesity is associated with peripheral arterial disease (PAD), suggesting that ectopic fat depots may be associated with localized diseases of the aorta and lower extremity arteries. We hypothesized that individuals with greater amounts of peri-aortic fat are more likely to have clinical peripheral arterial disease (PAD) and a low ankle-brachial index (ABI).
Methods and Results
We quantified peri-aortic fat surrounding the thoracic aorta using a novel volumetric quantitative approach in 1205 individuals from the Framingham Heart Study Offspring cohort (mean age 65.9 years, 54% women); visceral abdominal fat (VAT) was also measured. Clinical PAD was defined as a history of intermittent claudication and ABI was dichotomized as low ABI≤0.9 or lower extremity revascularization vs normal ABI >0.9 to < 1.4. Regression models were created to examine the association between peri-aortic fat and intermittent claudication or low ABI (n=66 participants). In multivariable logistic regression, per 1 standard deviation increase in peri-aortic fat, the odds ratio (OR) for the combined end-point was 1.52 (p-value=0.004); these results were strengthened with additional adjustment for BMI (OR 1.69, p=0.002) or visceral abdominal fat (OR 1.67, p=0.009) whereas no association was observed for VAT (p=0.16). Similarly, per standard deviation increase in BMI or waist circumference, no association was observed after accounting for VAT (p=0.35 [BMI]; p=0.49 [waist circumference]).
Conclusions
Peri-aortic fat is associated with low ABI and intermittent claudication.
Keywords: obesity, atherosclerosis, peripheral arterial disease
Introduction
Peripheral arterial disease (PAD) affects more than 12% of adults in the United States and is strongly associated with multiple cardiovascular disease (CVD) risk factors.1;2 PAD is associated with an increased risk of cardiovascular disease and all-cause mortality,3;4 highlighting the need to better understand the pathogenesis of PAD.
While traditional CVD risk factors including smoking and diabetes are strong risk factors for PAD,5 only central obesity, but not generalized obesity, has been shown to be associated with PAD.6;7 In this context, body fat distribution is an important factor in determining overall cardiometabolic risk.8;9 Ectopic fat depots, defined as fat depots in non-classical locations,10 are typically thought to exert systemic effects on cardiometabolic risk. However, locally-acting ectopic fat depots may contribute to obesity-mediated vascular disease.10;11 In particular, peri-vascular fat, or fat that surrounds blood vessels, is a physiologic modulator of vascular tone and adipocyte hypertrophy that can lead to hypoxia, inflammation, and oxidative stress.12 Further, recent experimental work suggests that perivascular fat may provide a mechanistic link between metabolic signals and vessel wall inflammation13 and vascular smooth muscle cell proliferation.14
We have developed a reproducible protocol to quantify peri-aortic fat15 in order to examine whether peri-vascular fat may mediate diseases of the aorta. Because prior findings demonstrated an association of central but not generalized obesity with PAD, we hypothesized that individuals with greater amounts of peri-aortic fat will have a higher prevalence of low ABI values and clinical PAD.
Methods
Study Sample
The Framingham Offspring Study, in 1971, enrolled children and spouses of the original Framingham Heart Study cohort. Participants for the current analysis participated in the Multi-Detector Computed Tomography (MDCT) sub-study. From the Offspring Study, 1,422 participants underwent chest and abdominal MDCT from 2002–2005. Of the 1422 Offspring who underwent CT scanning, 1397 were analyzed for perivascular fat. Of these, 1295 had non-missing ABI ≤ 1.4. Of these, 1205 had non-missing covariates and were included in the analysis.
The institutional review board of Boston University Medical Center and Massachusetts General Hospital approved the study protocol. Written informed consent was provided by all participants.
Multi-detector Computed Tomography (MDCT) Scan Protocol
MDCT of the abdomen and chest was performed with 8-slice MDCT (LightSpeed Ultra, General Electric, Milwaukee, Wisconsin). In the chest cavity, a series of 2.5 mm slices were acquired from the level of the carina to the diaphragm during an inspiratory breath-hold using prospective ECG triggering (120 kVp, 320 mA). In the abdomen, 2.5 mm slices (120 kVp, 320 mA) were obtained from the upper edge of the S1 vertebrae and 125 mm superiorly.
Measurement of Peri-aortic Fat Volume
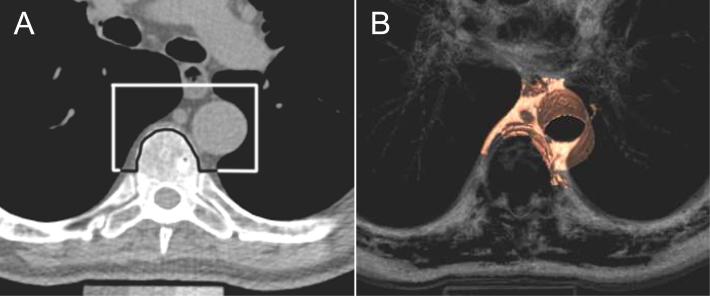
Using a dedicated workstation (Aquarius 3D, TeraRecon, San Mateo, California), image analyses were performed.15 Using a semi-automated method, adipose tissue quantification was performed which required manually defining the tissue borders. To calculate adipose tissue volumes, CT attenuation thresholds (window width −195 to −45 HU; window center −120 HU) were used. The anatomic borders to define thoracic peri-aortic fat were: 1) anteriorly, the area immediately surrounding the thoracic aorta (defined by a line drawn horizontally through the esophagus, which connected to the left costo-vertebral joint); 2) posteriorly, defined by the right lateral border of the vertebral body and the anterior edge of the vertebral body. This resulted in a 6.75 cm column of fat (27 slices) surrounding the thoracic aorta. The figure shows the region that was quantified. We also defined a measure consisting of abdominal peri-aortic fat, which consisted of tracing 5 mm rings that were calibrated to the vessel diameter. However, technical limitations, including the inherent relationship with the vessel diameter and the inability to visualize the retroperitoneal lining, limit the interpretation of these data and hence they are not included in the current analysis. Reproducibility was excellent for intra- and interreader measurements of the thoracic peri-aortic fat (intra-class correlation coefficient 0.99 and 0.98 respectively).15 We quantified visceral abdominal fat (VAT) as previously described.17 Briefly, the reader manually traced the abdominal muscular wall separating the subcutaneous from the visceral abdominal fat depot; semi-automatic quantification of fat volumes was facilitated with a window-width of −195 to −45 Hounsfield units.
Figure 1.
A demonstrates the upper boundaries of peri-aortic fat in an axial CT image, while figure B shows the corresponding 3D reconstruction. Peri-aortic fat, as measured by computed tomography, was defined as any pixel of attenuation between −195 and −45 HU within the region of interest
ABI and Peripheral Arterial Disease Assessment
At exam 8 (2005 to 2008), ankle and brachial blood pressures were routinely measured on all participants. Participants rested for a minimum of five minutes in the supine position on the examining table prior to blood pressure measurement. Blood pressure cuffs were applied to bare ankles with the midpoint of the bladder over the posterior tibial artery approximately three centimeters above the medial malleolus. Systolic blood pressure was obtained using a 9.6 megahertz Doppler pen probe and an ultrasonic Doppler flow detector (Parks Medical Electronics, Inc.). For each limb, the cuff was inflated rapidly to the maximal inflation level and deflated at a rate of 2 mmHg per second until the systolic blood pressure became audible. Measurements were obtained in the following order: right arm, right ankle, left ankle, left arm. All limb blood pressures were repeated in reverse order. Measurement was obtained from the dorsalis pedis artery only if the posterior tibial pulse could not be located by palpation or with the Doppler pen probe.
The ABI was calculated for each leg as the ratio of the average systolic blood pressure in the ankle divided by the average systolic blood pressure in the arm. The higher arm mean was used to calculate the ankle-brachial index for each leg. The lower of the ABIs from the two legs was used for analysis.
As part of routine FHS research exams, a physician-administered medical history interview was conducted that included queries about lower extremity revascularization. Medical records were obtained to verify self-report of all revascularization procedures. The physician also queried the participant about symptoms of intermittent claudication using a standardized questionnaire.2 Intermittent claudication was defined as exertional discomfort in the calf that appeared sooner with uphill or more rapid paced walking and was relieved with rest. An endpoint review panel of three senior investigators made the final determination of the presence of intermittent claudication. The mean time between CT scan acquisition and ABI measurement was 2.5 years.
Risk Factor Assessment
Cardiometabolic risk factors were quantified during the 8th Framingham Offspring Study Examination (2005–2008). Body mass index (BMI) was defined as the weight (kilograms) divided by height (in meters squared). Waist circumference was ascertained at the umbilicus. Fasting morning samples were collected for blood glucose and lipids. Diabetes was defined as fasting plasma glucose of at least 126 mg/dL or hypoglycaemic treatment. Current smokers were defined as individuals who smoked at least one cigarette/day in the year prior to their 8th examination. To define hypertension, systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or anti-hypertensive therapy was used.
Circulating fasting plasma levels of resistin and adiponectin were quantified by ELISA (R&D Systems, Minneapolis, MN). Intra-assay coefficients of variation were 9.0% for resistin and 5.8% for adiponectin.
Statistical Analysis
Thoracic peri-aortic fat and VAT volumes were normally distributed. ABI was dichotomized at ≤ 0.9; individuals with a history of lower extremity surgery were considered in the low ABI category. Participants with an ABI > 1.4 were excluded, none of whom had any intermittent claudication symptoms or a prior revascularization procedure. Low ABI and Intermittent claudication were combined as the primary analysis (n=66 cases). Analyses were modeled with peri-aortic fat and VAT as exposures, and low ABI and IC combined as outcomes. Body mass index (BMI) and waist circumference (WC) were also modeled as separate exposures; all adipose tissue data is presented per 1 standard deviation increase. The multivariable logistic regression model included the covariates of age, sex, smoking, diabetes, hypertension, total/HDL cholesterol, lipid treatment, and log triglycerides. Additional models with peri-aortic fat included BMI or VAT as covariates. Analyses were performed with SAS version 9.1.3. P-values <0.05 were considered statistically significant.
Results
Study Sample Characteristics
The mean age of the study sample was 65.8 years, and 53.7 % were women (Table 1). Overall, 45 individuals had ABI ≤0.9, 35 individuals had a history of intermittent claudication, and 66 individuals had either low ABI or intermittent claudication. Thoracic peri-aortic fat was strongly associated with VAT (r=0.74, p<0.001).
Table 1.
Study sample characteristics among participants. Data are presented as mean (standard deviation) or the percentage (n) having the characteristic.
| Overall (n=1205) | |
|---|---|
| Age, years | 65.9 (8.9) |
| Women, % | 53.7 (647) |
| Body Mass Index, kg/m2 | 28.4 (5.3) |
| Waist Circumference, cm | 99.1 (13.5) |
| Triglycerides, mg/dl* | 102 (73, 144) |
| Total/HDL cholesterol, mg/dl | 3.52 (1.05) |
| Lipid Treatment % | 42.9 (518) |
| Hypertension, % | 57.1 (688) |
| Diabetes mellitus, % | 8.1 (97) |
| Current Smoker, % | 8.8 (106) |
| Former Smoker, % | 53.7 (647) |
| Adiponectin**, μg/mL | 9.9 (6.0) |
| Resistin**, ng/mL | 14.6 (8.2) |
| Thoracic Peri-aortic Fat, cm3 | 16.3 (9.1) |
| Visceral Adipose Tissue, cm3 | 2089.9 (1099.9) |
| Ankle Brachial Index ≤0.9 (%, n) | 3.8 (45) |
| Intermittent Claudication (%, n) | 2.9 (35) |
| Lower Extremity Revascularization*** (%, n) | 0.6 (7) |
Median with 25th–75th percentiles. HDL indicates high density lipoprotein.
in sub-sample of 975 individuals
these 7 participants with lower extremity revascularization were part of the 66 participants with IC or low ABI; 3 also had prevalent IC, 1 also had ABI≤0.9, 1 had both IC and abnormal ABI, and 2 had neither.
Association between Peri-Aortic Fat and Combined Low ABI/Intermittent Claudication
In minimally adjusted models, per standard deviation increase in peri-aortic fat, the odds ratio (OR) for low ABI or IC was 1.79 (95% CI 1.40–2.30, p<0.001; Table 2). Further adjustment for clinical covariates modestly affected the OR (OR 1.52, p=0.004). Similarly, additional adjustment for BMI or VAT did not materially impact the results (OR 1.69 [BMI-adjusted]; OR 1.67 [VAT-adjusted]). In contrast, VAT was associated with low ABI or IC in minimally-adjusted models (OR 1.44, 95% CI 1.12–1.87, p=0.005), but these findings were attenuated after adjustment for standard covariates (OR 1.23, p=0.16).
Table 2.
Multivariable-adjusted regressions for Peri-aortic Fat and the combined end-point of Intermittent Claudication and low ABI (n=66 cases). Data presented as odds ratio per 1 standard deviation increase of thoracic abdominal fat, VAT, BMI, or WC.
| Thoracic Peri-Aortic Fat | VAT | BMI | WC | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age, Sex | 1.79 (1.40–2.30) | <0.001 | 1.44 (1.12–1.87) | 0.005 | 1.19 (0.92–1.52) | 0.18 | 1.37 (1.06–1.77) | 0.02 |
| Age, Sex, MV † | 1.52 (1.15–2.02) | 0.004 | 1.23 (0.92–1.65) | 0.16 | 1.03 (0.78–1.36) | 0.85 | 1.23 (0.93–1.65) | 0.15 |
| Age, Sex, MV† + BMI | 1.69 (1.22–2.34) | 0.002 | 1.40 (0.95–2.06) | 0.09 | - | - | 1.64 (1.03–2.62) | 0.04 |
| Age, Sex, MV† + VAT | 1.67 (1.14–2.45) | 0.009 | - | - | 0.83 (0.57–1.22) | 0.35 | 1.14 (0.78–1.67) | 0.49 |
Multivariable (MV) adjusted for smoking, diabetes, hypertension, total/HDL cholesterol, lipid treatment, log triglycerides VAT, visceral adipose tissue; BMI, body mass index; WC, waist circumference
We also examined the associations between BMI and WC with low ABI/IC (Table 2). BMI was not associated with the combined end-point in minimally-adjusted models (OR 1.19, p=0.18), whereas WC was modestly associated with low ABI/IC in age-sex adjusted models (OR 1.37, p=0.02), which was attenuated upon adjustment for VAT (OR 1.14, p=0.49).
Association between Peri-Aortic Fat and Low ABI or Intermittent Claudication
Results for Iow ABI or IC as separate outcomes were similar to the combined outcome models (Table 3).
Table 3.
Multivariable-adjusted regressions for Peri-aortic Fat and Low ABI and Intermittent Claudication individually. Data presented as odds ratio of Low ABI or IC per 1 standard deviation increase of peri-aortic fat.
| Low ABI n=45 | Intermittent Claudication n=35 | |||
|---|---|---|---|---|
| Model | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age, Sex | 1.89 (1.42–2.52) | <0.001 | 1.90 (1.39–2.59) | <0.001 |
| Age, Sex, MV † | 1.78 (1.27–2.48) | <0.001 | 1.54 (1.08–2.19) | 0.02 |
| Age, Sex, MV† + BMI | 2.07 (1.41–3.04) | <0.001 | 1.62 (1.09–2.41) | 0.02 |
| Age, Sex, MV† + VAT | 1.98 (1.25–3.13) | 0.004 | 1.69 (1.05–2.72) | 0.03 |
Multivariable (MV) adjusted for smoking, diabetes, hypertension, total/HDL cholesterol, lipid treatment, log triglycerides VAT, visceral adipose tissue; BMI, body mass index.
Secondary Analysis
In secondary analyses of a subset of participants (n=975), we additionally adjusted our primary model of TAT as a correlate for low ABI or IC for adiponectin and resistin; the results were not materially different (OR 1.73, p=0.01).
Discussion
Principal Findings
In our community-based sample of individuals from the Framingham Heart Study, we demonstrated that peri-aortic fat is associated with low ABI and intermittent claudication. We did not observe a similar association with BMI, waist circumference, or VAT. Our findings suggest a potential role for peri-aortic fat in the pathogenesis of peripheral arterial disease.
In the Context of Current Literature
The association between BMI and PAD has been inconsistent.18;19 Some studies demonstrate a linear association between BMI and ABI level20 while others show no association5 or an association with the highest BMI in individuals with the lowest ABI category21 or in individuals with a high ABI (>1.3).18 Central obesity, but not BMI, has previously been associated with PAD in a cohort of elderly men.6 Similarly, in a study of elderly individuals from the MrOS study, waist-to-hip ratio, but not BMI, was associated with low ABI.7 In the German cohort of the REACH registry, 50% of patients with PAD had abdominal obesity.22 Obesity has been previously associated with the severity of PAD.23 Obese patients report more calf pain as compared to the general population, and obese patients who undergo surgical treatment for obesity have a lower risk of developing calf pain.24 Taken together, the prior literature suggests that body composition, particularly individuals with increased central fat, may be at increased risk for PAD. The current work extends these prior findings by identifying an association between peri-aortic fat and peripheral arterial disease.
Potential Mechanisms
Experiments using perivascular adipose tissue from the rat aorta demonstrate that peri-aortic adipose tissue releases growth factors that stimulate smooth muscle cell proliferation that is enhanced in aged rats and rats fed a high fat diet.14 These findings suggest that perivascular adipose tissue may promote vascular disease through dysfunction of smooth muscle cells. Adipocytes secrete numerous other factors including proinflammatory cytokines and adipokines that may also promote development of vascular disease.13 Recent in vitro work demonstrates that under basal conditions, human perivascular adipocytes show evidence of a proinflammatory state and reduced adipocyte differentiation. Thus, perivascular adipocytes may contribute to adventitial inflammation and in turn the development of atherosclerosis. Greenstein et al isolated perivascular adipose tissue from small arteries taken from gluteal fat biopsy samples and demonstrated that the adipocytes secrete adiponectin, a physiologic modulator of vascular tone.12 Further, examining perivascular fat from obese subjects, the investigators noted loss of this vasodilatory effect due to adipocyte hypertrophy leading to inflammation and oxidative stress. In the obese Zucker rat, an animal model of obesity, hindlimb blood flow was reduced with concomitant stiffer vessels, and this was independent of muscle mass and physical activity,25 providing a potential mechanism for which obesity can lead to PAD. The pathophysiologic mechanisms by which local adipose tissue influences development of vascular disease remain to be determined and is an exciting area of active research.
Clinical and Research Implications
These findings highlight the potential toxic role of peri-aortic fat on the peripheral vasculature, and suggest a potential mechanism whereby obesity might lead to the development of PAD. Further research is necessary to uncover the specific mechanisms of disease. Whether reduction of peri-aortic fat can lead to reduced PAD or PAD progression requires further examination.
Strengths and Limitations
Strengths of the present analysis include a detailed characterization of ectopic fat depots, allowing us to examine the associations between thoracic peri-aortic fat and VAT with low ABI and intermittent claudication. Important covariates were routinely collected, limiting any potential for recall bias. Some limitations warrant mention. First, the sample is white, limiting generalizability to other ethnicities. Second, our measure of abdominal peri-aortic fat is not reliable, limiting our ability to directly quantify this fat depot in the abdomen. We use thoracic peri-aortic fat as a proxy measure of peri-vascular fat through the entire arterial tree, as we are unable to quantify peri-femoral artery fat. Further research is necessary to better understand the distribution of peri-aortic fat through the vascular territory. Ectopic fat depots are hypothesized to have primarily have systemic effects (such as VAT)8, or local effects, such as pericardial fat or peri-aortic fat. The results from the present paper suggest that only peri-aortic fat and not BMI, WC, or VAT are associated with PAD, rendering a systemic effect of peri-aortic fat unlikely. However, this is a cross-sectional study therefore causation can not be inferred. Smoking was defined as cigarette smoking with the last 12 months; some degree of misclassification may occur among individuals who stopped smoking within this time interval.
Conclusions
Peri-aortic fat is associated with low ABI and intermittent claudication.
Acknowledgments
Sources of Funding This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (N01-HC-25195), the General Clinical Research Centers Program (Grant Number M01-RR-01066), and by a Career Development Award from the American Diabetes Association and NIDDKK24 DK080140 (JBM).
JBM currently has research grants from GlaxoSmithKline and Sanofi-Aventis, and has consulting agreements with Eli Lilly and Interleukin Genetics.
Footnotes
Disclosures None of the other authors report any relevant disclosures.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2007;55:583–589. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 2.Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 3.Saw J, Bhatt DL, Moliterno DJ, Brener SJ, Steinhubl SR, Lincoff AM, Tcheng JE, Harrington RA, Simoons M, Hu T, Sheikh MA, Kereiakes DJ, Topol EJ. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol. 2006;48:1567–1572. doi: 10.1016/j.jacc.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 4.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, D'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 6.Planas A, Clara A, Pou JM, Vidal-Barraquer F, Gasol A, de MA, Contreras C, Marrugat J. Relationship of obesity distribution and peripheral arterial occlusive disease in elderly men. Int J Obes Relat Metab Disord. 2001;25:1068–1070. doi: 10.1038/sj.ijo.0801638. [DOI] [PubMed] [Google Scholar]
- 7.Vogt MT, Cauley JA, Kuller LH, Hulley SB. Prevalence and correlates of lower extremity arterial disease in elderly women. Am J Epidemiol. 1993;137:559–568. doi: 10.1093/oxfordjournals.aje.a116709. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr., O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 9.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 10.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S58–S65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 11.Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 12.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–H1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- 15.Schlett CL, Massaro JM, Lehman SJ, Bamberg F, O'Donnell CJ, Fox CS, Hoffmann U. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int J Obes (Lond) 2009;33:226–232. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr., Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: Design, Recruitment, and Initial Examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 17.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 18.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 19.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 20.O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 21.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 22.Zeymer U, Parhofer KG, Pittrow D, Binz C, Schwertfeger M, Limbourg T, Rother J. Risk factor profile, management and prognosis of patients with peripheral arterial disease with or without coronary artery disease: results of the prospective German REACH registry cohort. Clin Res Cardiol. 2009;98:249–256. doi: 10.1007/s00392-009-0754-1. [DOI] [PubMed] [Google Scholar]
- 23.Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45:40–46. doi: 10.1016/j.jvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Karason K, Peltonen M, Lindroos AK, Sjostrom L, Lonn L, Torgerson JS. Effort-related calf pain in the obese and long-term changes after surgical obesity treatment. Obes Res. 2005;13:137–145. doi: 10.1038/oby.2005.18. [DOI] [PubMed] [Google Scholar]
- 25.Stepp DW, Pollock DM, Frisbee JC. Low-flow vascular remodeling in the metabolic syndrome X. Am J Physiol Heart Circ Physiol. 2004;286:H964–H970. doi: 10.1152/ajpheart.00836.2003. [DOI] [PubMed] [Google Scholar]