Abstract
The present study investigated if oral exposure to milk or amniotic fluid (AF) alters responsiveness to sensory stimulation in the neonatal rat, and whether these effects are mediated by the opioid system. Facial wiping evoked by intraoral lemon infusion was used as a measure of sensory responsiveness. Pups were tested in a supine posture, because they showed more paw-face strokes during facial wiping than pups tested prone (Experiment 1). Moreover, pups orally exposed to milk (Experiment 2) or AF (Experiment 3) showed a diminished wiping response to lemon compared to controls exposed to water. Blockade of opioid receptors with the non-selective antagonist naltrexone (Experiment 4) or the kappa antagonist nor-binaltorphimine (Experiment 5) reinstated higher levels of facial wiping after AF exposure. These findings confirm developmental continuity between fetal and neonatal behavioral responses to AF and the ability of AF to induce activity at kappa receptors of the endogenous opioid system.
Keywords: neonatal rat, kappa opioid, facial wiping, amniotic fluid, milk
The study of the prenatal period provides an opportunity to trace behavior to its developmental origin. Moreover, knowing how different mammalian species develop before birth permits better understanding of the emergence of postnatal behavior. As part of prenatal development, the fetus actively interacts with its intrauterine environment (Smotherman & Robinson, 1986), a dominant feature of which is amniotic fluid (AF). Fetuses are continually surrounded by AF during prenatal development, and contribute to the regulation of AF through fetal swallowing, breathing and micturition (Brace, 1997; Ross & Nijland, 1997). Although AF is a crucial characteristic of the fetal environment, it has been suggested that it is important not only for the prenatal setting but also for preparing the fetus for the transition of birth, the process of parturition, and the expression of postnatal behavior (Schaal, 2005). In this sense, previous work has concentrated on establishing behavioral continuity of AF, with a particular emphasis on the odor properties of AF that support adaptive learning responses in the fetus and neonate (see Schaal, 2005 for a review).
Several previous studies have provided evidence that human infants are attracted to the odor of AF only a few hours or days after birth. For example, when exposed to the odor of AF, neonates show a head-orientation response toward AF (Schaal, Marlier, Soussignan, 1995). A preference for AF over formula milk has been demonstrated in infants 2–4 days after birth (Marlier, Schaal & Soussignan, 1998a), or AF over a control stimulus of distilled water (Marlier, Schaal & Soussignan, 1998b). Schaal, Marlier, and Soussignan (1995) observed that neonates that were exposed to the odor of AF not only showed a preference by moving their heads toward the stimulus but also showed longer bouts of mouthing activity. Similarly, this preference toward AF also has been reported in newborn lambs that showed an attraction to AF relative to a control stimulus of distilled water (Schaal, Orgeur, & Arnould, 1995).
In addition to preferring AF over a neutral fluid such as water, infants also show a preference for their own AF in comparison to unfamiliar AF collected from different pregnancies (Marlier et al., 1998b; Schaal, Marlier & Soussignan, 1998). A comparable behavioral response has been demonstrated in rat pups, which prefer AF collected from their own litter over AF from an unrelated rat. Preferences for the odor of AF from self or siblings from the same pregnancy has been cited as evidence of the role of AF in the development of kin recognition (Hepper, 1987).
Previous findings suggest that in addition to promoting preference responses, AF also is important in the expression of other behavior. For example, it has been demonstrated experimentally that AF helps guide neonatal rats to the first nipple attachment (Teicher & Blass, 1977). Similarly, human neonates prefer to suck or grasp a nipple washed with AF versus an untreated one (Varendi, Porter & Winberg, 1996). Soothing is another property attributed to AF. This characteristic has been identified by observing that neonates exposed to a cloth moistened with AF show significantly less crying than controls or babies exposed to their mother’s breast odor (Varendi, Christensson, Porter, & Winberg, 1998). AF also can provide a medium for early olfactory experience. Postnatal olfactory preference for a specific odor can be induced by prenatal exposure via injection into the AF or through maternal exposure to the odor compound in her diet, suggesting that AF and its chemical properties may play an important role in the prenatal acquisition and perinatal transmission of olfactory learning (see Schaal & Orgeur, 1992 for a review). As an example, Hepper (1988) demonstrated in rats that the progeny of mothers that were fed garlic during gestation showed a preference for garlic after birth, unlike the offspring of mothers that did not eat garlic during pregnancy.
While AF has been studied to explore perinatal continuity, other studies have explored the effect of AF to modulate behavioral responses of the fetus to chemosensory stimulation. When rat fetuses are exposed to novel chemosensory fluids, such as intraoral infusion of a lemon-odor solution, they show an increase in activity (Smotherman & Robinson, 1987, 1988). One of the components of the behavioral activation elicited by lemon infusion is the distinctive response of facial wiping (Smotherman & Robinson, 1987). Facial wiping involves moving one or both forepaws in a rostral direction by placing them along the side of the head, generally just behind the eyes, and sliding the paws forward over the face, nose and mouth (Robinson & Smotherman, 1991). This behavior is a species-typical response that has been described in many rodent species (Robinson and Smotherman, 1992b), which appears related to postnatal grooming and aversion responses (Berridge, 2000; Ganchrow, Steiner, & Canetto, 1986; Golani & Fentress, 1985; Johanson & Shapiro, 1986). Facial wiping also has been used as a measure of sensory responsiveness to study the behavioral effects of milk in neonatal and fetal rats (Smotherman & Robinson, 1992c). Perinatal rats that receive an experimental oral infusion of milk show diminished responsiveness to stimuli that ordinarily evoke facial wiping, including a perioral cutaneous stimulus or intraoral lemon infusion (Smotherman & Robinson, 1992a). These studies suggest that sensory responsiveness can be modulated by prior exposure to biologically relevant chemosensory cues, such as milk.
Fetuses are not ordinarily exposed to milk before birth, but they are regularly exposed to AF, another biologically relevant fluid. Korthank and Robinson (1998) described the effects of AF on the facial wiping response of rat fetuses exposed to lemon infusion. Fetuses were exposed to one of two stimuli—AF collected on gestational day 20 (E20) or isotonic saline—via intraoral infusion. One minute after exposure, fetuses received a second infusion of a novel test solution of lemon. Those fetuses exposed to AF before the lemon stimulus showed a diminished facial wiping response (fewer paw-face strokes) compared to subjects exposed to saline. This study also presented evidence that the behavioral effect exerted by AF is mediated by the endogenous opioid system in the fetus. Subjects that were pretreated with an injection of naloxone (a nonselective opioid antagonist) before exposure to AF showed a normal, robust facial wiping response to lemon. These findings suggest that blockade of opioid receptors by naloxone is effective in reinstating the facial wiping response to novel chemosensory stimulation in rat fetuses.
Because some studies have suggested that AF exerts effects on fetal behavioral responses that are opioid mediated, and other studies have demonstrated that AF possesses attractive properties after birth, it is reasonable to hypothesize that AF also is capable of exerting postnatal behavioral effects that are mediated by the endogenous opioid system. To test this possibility, the present study reports results from five experiments in which neonatal rats were tested 24-hr after birth (P1) following procedures similar to those described previously by Korthank and Robinson (1998). Using facial wiping as a behavioral index of sensory responsiveness, this study sought to provide evidence for neonatal responsiveness to AF and developmental continuity in the underlying neural substrates involved in the effects of AF exposure in neonates.
General methods
Subjects
Neonatal subjects were the offspring of Sprague-Dawley Norway rats (Rattus norvegicus) tested 24-hr after birth (Harlan Laboratories, Indianapolis, IN). Female rats were time-mated in our laboratory. The breeding period consisted of housing three adult female rats with an adult male during four days in standard breeding cages (38 × 48 × 20 cm) in a colony room with controlled temperature and humidity and a 12:12 hr light:dark photoperiod cycle (lights on at 0700 hr). Food and water were available ad libitum. Vaginal smears were collected daily during the 4-day breeding period to detect presence of sperm (designated as day of conception; E0 of gestation). A total of 146 P1 pups delivered vaginally at term (22 days of gestation, P0 = day of birth) from 28 adult female rats were included in the study. Maintenance of rats before and during the experiment was in accordance with guidelines established by the NIH (Institute for Laboratory Animal Resources, 1996) and approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Amniotic Fluid Collection
AF samples were collected from donor fetuses at gestational age E20. Previous work has reported the effectiveness of AF collected at this gestational age to induce a behavioral effect in the fetal response to chemosensory stimulation (Korthank & Robinson, 1998). These fetuses were obtained from different pregnancies not used for behavioral testing. The procedure of AF collection involved (a) rapid euthanasia of the pregnant rat by cervical dislocation, (b) externalization of the pregnant female’s uterus, (c) careful removal of fetuses and placenta, avoiding rupture of the amnion and chorion, and (d) extraction of AF into a sample tube that was immediately stored at −20° C until the day of testing. AF samples were thawed and presented to rat pups at incubator temperature (35°C).
Cannula implantation
Single or dual intraoral cannulae prepared with polyethylene tubing (PE-10; Clay -Adams) with a heat-formed flange at one end were implanted in pups following a method originally described by Hall and Rosenblatt (1977). To facilitate handling during the cannulation procedure, pups were briefly immobilized by immersion to neck depth in cold water for 1–2 min. Moderate hypothermia induced by this method is an accepted and effective form of general anesthesia in neonatal rats (Danneman & Mandrell, 1997; Phifer & Terry, 1986). When the pup was immobilized, a fine wire was passed through the lower jaw and tongue, the cannula slipped onto the wire, and the wire carefully pulled back through the jaw. This method allowed the flange end to rest on the tongue in a mid-anterior position (Kehoe & Blass, 1985). An alternative method of cannula implantation involves the cannula placed in the cheek of the pup rather than an anterior position on the tongue. Cheek cannulation was reported to result in reduced corticosterone responses in older rat pups compared to anterior or posterior tongue cannulae (Spear, Specht, Kirstein, & Kuhn, 1989). However, Smotherman and Robinson (1988) reported that the anterior tongue cannula results in no behavioral changes when compared to untreated or saline-infused control subjects. Moreover, the anterior tongue cannula is a standard method used in previous studies of facial wiping behavior in perinatal rats (Brumley & Robinson, 2004; Korthank & Robinson, 1998; Robinson & Smotherman, 1991). Placement of the cannula in an anterior position is necessary to prevent interference of the cannula with the expression of facial wiping behavior: pups cannot perform facial wiping with a cheek cannula because the tubing physically obstructs movement of the paw across the side of the face. After cannulation, pups were placed in a thermoneutral incubator for 1-hr at a temperature of 35°C to recover from anesthesia and acclimate before testing.
Presentation of Stimuli
Delivery of chemosensory stimuli involved attaching the external end of the intraoral cannula to polyethylene tubing (PE-50) attached to a syringe that contained the exposure or test fluid. This procedure permitted precise infusion (± 1 µl) of the exposure and test stimuli in a 2-s pulse. The fluids used for chemosensory exposure were AF, milk, or distilled water (DW); the fluids used for behavioral testing were pure lemon extract (McCormick brand) or DW. The lemon extract contained pure lemon oil dissolved in alcohol (83%), which provides a complex chemosensory cue that stimulates olfactory, gustatory and trigeminal systems in the mouth (Smotherman & Robinson, 1990). The milk used in Experiment 2 was a commercially available bovine light cream (half-and-half), which has been previously used in studies with fetal and neonatal rats (Hall & Rosenblatt, 1977; Robinson & Smotherman, 1994). All infusions of exposure and test fluids were delivered in a volume of 20 µl. Previous studies have demonstrated that an infusion of 20 µl of undiluted lemon extract is highly effective in eliciting the wiping response in E20 rat fetuses (Brumley & Robinson, 2004).
Behavioral observations
Behavioral observations during test sessions consisted of 2- or 3-min sessions. Behavioral scoring involved quantifying facial wiping strokes, which were identified every time the paw made contact with the face. The occurrence of the wiping cycle involves two phases; (a) when movements are made in a rostral direction (from ear to nose) maintaining contact with the side of the face, and (b) when the forelimbs are moved from nose to ear without contacting the face in order to prepare the limb for placement on the face again (Robinson & Smotherman, 1991). Unilateral wipes (single-paw) were distinguished from bilateral (dual-paw) facial wipes. Unilateral wipes were scored when one paw made contact with the face without overlapping with contact by the opposite paw. Scoring of bilateral wipes was made only when contact of both paws with the face coincided during the wiping cycle.
In addition to facial wiping, some experiments also report general motor activity, which was quantified by scoring movements of forelimbs, hindlimbs, head, and mouth. The activity of forelimbs and hindlimbs involved scoring each limb movement as an independent event (left vs. right, fore vs. hind). Scoring of general activity of forelimbs included movements that also were scored as facial wiping responses. In order to score head activity, only movements consisting of ventral, dorsal, lateral or rotary motion were quantified. Mouthing consisted of opening and closing of the mouth, sometimes involving extension of the tongue. The sum of forelimb, hindlimb, head and mouth movements provided a measure of overall activity before and after each infusion.
All experimental sessions were recorded to DVD from a single camera view to allow subsequent playback and scoring from video records. The microcamera was positioned ventral to the pup along the midline, providing a clear view of all four limbs and the head and mouth. In all experiments, facial wiping responses and general motor activity were scored during the entire 2- or 3-min experimental session.
Experimental Design and Data Analysis
In Experiments 3 and 4, where subjects were pretreated with an opioid antagonist or a vehicle control injection, video recording and behavioral testing began 5-min after the injection. Previous studies have documented that 5-min is sufficient for IP injections of opioid antagonists to exert full effects on behavior in fetal rats (Korthank & Robinson, 1998; Smotherman & Robinson, 1992a). In all experiments, sessions included a 1-min baseline period before any exposure to chemosensory stimuli and concluded 1-min after presentation of the test solution of lemon. Multiple pups were tested from each pregnancy in each experiment. In experiments 2, 3, and 4, subjects from the same pregnancy were assigned to different treatment groups to avoid confounding treatment effects with litter effects (Holson & Pearce, 1992). Experiment 1 comprised multiple subjects tested in the two experimental conditions to allow for a more complete description of variability of responses among all subjects. However, in the present report, the mean response was calculated for each posture condition within each litter before statistical analysis. The order of assignment in all the different experimental conditions in each experiment was counterbalanced across pregnancies. During testing, some subjects were replaced (n = 6) due to experimental error or malfunction during infusion.
Behavioral observations of the different behavioral categories were scored using EventCoder software (version 1.0b6; written by M. Goldstein, Cornell University, 2006) that allowed for real-time coding of behavior using digital video files created from the DVD recordings. Files containing time-stamped behavioral data from these coding sessions were imported into Microsoft Excel files to create summaries for statistical analysis.
Experiment 1: Posture effects on facial wiping
Robinson & Smotherman (1992a) documented the importance of posture in the expression of facial wiping in perinatal rats. They reported that pups tested 24-hr after birth (P1) immersed in water in either a prone or a supine posture show facial wiping responses in both postures. In comparison, by 3 days after birth (P3) pups showed more facial wiping when suspended in a prone posture. The posture effect was even more pronounced when pups were observed 5 days after birth (P5). Previous studies of fetal rats that have reported milk or AF effects on sensory responsiveness have tested fetuses in a supine posture (Korthank & Robinson, 1998; Smotherman & Robinson, 1992a). Because posture is a potentially important influence on facial wiping in neonatal rats, Experiment 1 was performed to identify if a specific posture was optimal for pups to show more pronounced facial wiping in the present experimental context.
Methods
To establish an optimal protocol for the following experiments, the facial wiping response of P1 rat pups to lemon infusion was observed while pups were placed in one of two different body positions. A total of 52 rat pups from 8 litters was assigned to one of two postural conditions: (a) supine (n = 26) or, (b) prone (n = 26). In both postures, pups were secured in place with a strap across the back or abdomen, which helped to maintain the correct posture and maintain the pup in view of the video camera. In the prone posture, pups were suspended from a horizontal bar. Thus, in both postures, pups did not experience constraint of the limbs that might inhibit expression of facial wiping responses (Smotherman & Robinson, 1989). Test sessions were 2 min in length, which comprised a 1-min baseline, and 1 min after the 20 µl infusion of lemon for observation of behavioral responses. Because more than one pup from each litter was assigned to the same postural condition, data initially were reduced by calculating the mean response of all pups in the same condition within each litter. These litter means then were used in subsequent statistical analyses.
Results and discussion
To analyze facial wiping responses, litter means were calculated in each condition and compared in an unpaired t-test. Subjects in the supine condition expressed more facial wiping strokes, both unilateral, t (14) = 3.15, p < .01, and bilateral, t (14) = 2.81, p < .01 (Figure 1, left). The total number of wiping strokes (calculated as Unilateral Left + Unilateral Right + (Bilateral × 2)) also was greater in the supine posture, t (14) = 2.81, p < .01. Analysis of the percentage of wiping strokes that were bilateral suggested that a higher proportion of strokes were bilateral in the supine posture, but this difference was not statistically significant (Figure 1, right).
Figure 1.
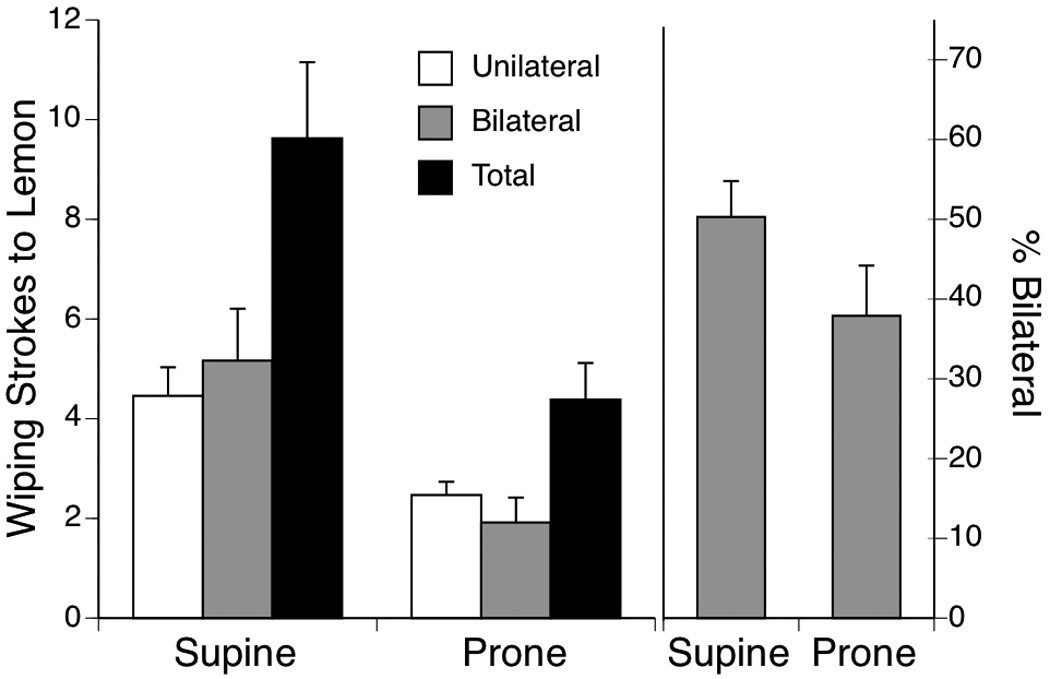
Mean number of unilateral and bilateral facial wiping strokes and total number of wipes after lemon infusion in two testing conditions (supine or prone) in Experiment 1. The right side of the figure depicts the percentage of wiping strokes that were bilateral in supine or prone. Vertical lines show S.E.M.
General activity measures were compared in a series of 2-factor ANOVAs (2 Postures × 8 15-s intervals), with the time factor treated as a repeated measure. The data are summarized in Figure 2. For forelimb activity, there was a main effect of Time, F (7, 98) = 33.69, p < .001, but no significant main or interaction effect involving Posture. Post hoc comparisons (Fischer PLSD) indicated that forelimb activity increased significantly during the 30 s immediately after lemon infusion (Figure 2, top left). Analysis of hindlimb activity indicated a main effect of Time, F (7, 98) = 10.94, p < .001, and Posture, F (1, 14) = 4.72, p < .05, but the interaction of Posture and Time was not significant. Hindlimb movements were higher in the supine posture than prone, and increased significantly after lemon infusion (Figure 2, top right). Head activity showed a similar pattern of results, with the significant main effect of Posture, F (1, 14) = 5.16, p < .05, and Time, F (7, 98) = 8.26, p < .001, but no interaction. Head movements were more frequent in the Supine posture, and increased after lemon infusion (Figure 2, bottom left). Finally, mouthing activity varied significantly with Time, F (7, 98) = 9.81, p < .001, but there was no significant main effect of Posture or an interaction of the two factors. Mouthing showed an increased after lemon infusion, predominantly during the last 30 s of the session (Figure 2, bottom right).
Figure 2.
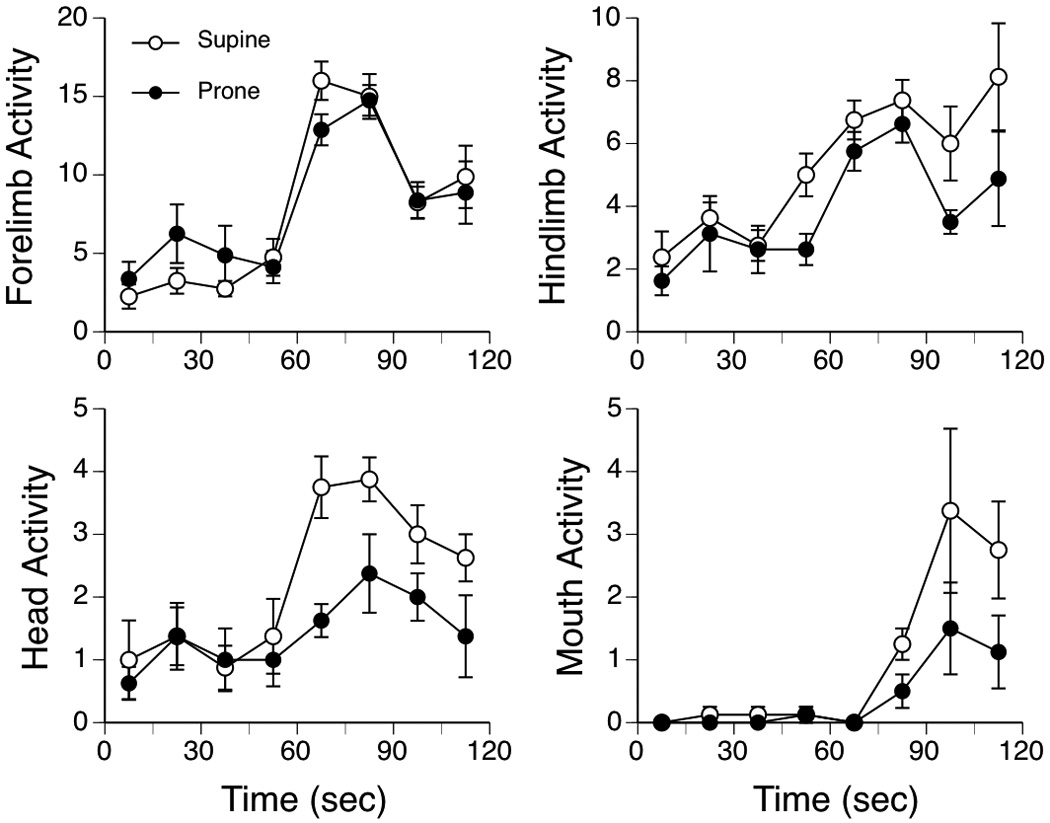
Changes in forelimb, hindlimb, head and mouth activity during the 2-min test session of Experiment 1. Pups were tested in two posture conditions: Supine and Prone. Points depict mean frequencies; vertical lines show S.E.M.
The findings of Experiment 1 confirmed the hypothesis that posture plays an important role in the expression of the facial wiping response to novel chemosensory stimulation in neonatal rats. Overall, subjects expressed more facial wiping as well as more general activity in the supine posture after the infusion, with the exception of mouth and forelimb activity that did not reveal differences between conditions. These results suggest that although chemosensory exposure is what triggers the wiping response, whether pups are placed in one posture or the other will affect the expression of this behavior. Our results are consistent with previous findings about the postnatal expression of facial wiping. For example, Smotherman and Robinson (1989) reported that pups tested in a prone posture on a hard surface show little or no facial wiping in response to lemon infusion. However, this reduction of the postnatal expression of facial wiping can be reversed if pups are suspended in water. In another study, Robinson and Smotherman (1992a) reported that pups show more facial wiping in a supine posture on P1, but this expression changes to more wiping in prone, if pups are suspended in air, when tested on P3 or P5. This change to prone seems to be related to the postnatal emergence of righting responses elicited by chemosensory stimulation (Robinson & Smotherman, 1992b).
Results in the general movement categories were consistent with behavioral reactions to aversive stimuli, which include mouthing activity such as open or closed mouth and gaping in both neonatal rats (Ganchrow, Steiner & Canetto, 1986) and human neonates (Ganchrow, Steiner, & Daher, 1983; Rosenstein & Oster, 1988), and forelimb flailing in infant rats (Ganchrow, Steiner & Canetto, 1986; Johanson & Shapiro, 1986) and infant primates (Steiner, Glaser, Hawilo, & Berridge, 2001). Mouth activity is a component of responses that could be considered as having either a negative (aversive) or positive (appetitive) hedonic value (Berridge, 2000; Berridge & Grill, 1983), whereas forelimb flailing has been described as an aversive response in rats similar to facial wiping (also called face washing) (Grill & Norgren, 1978).
In summary, the findings of Experiment 1 revealed that facial wiping was more frequent when pups were tested in the supine posture. Following these results, all pups in subsequent experiments were tested in a supine posture.
Experiment 2: Milk effects on the facial wiping response to lemon in the P1 rat
Previous studies have described that milk can exert effects on sensory responsiveness in the prenatal rat. For instance, rat fetuses not only can discriminate milk from other stimuli, such as an infusion of lemon, but they also express a stretch response to an infusion of milk (Robinson & Smotherman, 1992c, Smotherman & Robinson, 1987). In addition, an infusion of a small amount of milk in the fetal rat reduces responsiveness to a tactile stimulus or chemosensory stimulus (Korthank & Robinson, 1998; Smotherman & Robinson, 1992a). The behavioral effects of milk in the fetus are reversed by blockade of kappa opioid receptors with the selective antagonist nor-binaltorphimine. However, the effects of milk on facial wiping to an infusion of lemon have not been described in the neonatal rat. In Experiment 2, P1 rat pups were infused with a small volume of milk before evoking facial wiping through an infusion of the testing solution of lemon. The purpose of Experiment 2 was to describe if milk would exert a similar effect on the facial wiping response of the P1 rat pup by reducing the number of wiping strokes evoked by lemon infusion.
Methods
Forty-eight P1 rat pups, including one male and one female pup from each litter, from a total of 8 litters, were assigned as subjects to one of six conditions that resulted from the combination of Pretreatment and Exposure infusion. In pretreatment, subjects were assigned to one of three conditions: (a) an intraperitoneal (IP) injection of 50 µl of naltrexone (1.0 mg/kg), a nonselective opioid antagonist, (b) an IP injection of the vehicle control of isotonic saline (50 µl), or (c) no injection (NI). Naltrexone was selected as the opioid receptor antagonist in this experiment for its high affinity for both mu and kappa receptors (Powell & Holtzman, 1999) and its successful application to block opioid effects in previous studies with neonatal rats (Blass & Fitzgerald, 1988; Shide & Blass, 1989). All injections were administered 5-min before testing and were performed using a 30-ga hypodermic needle. In addition, pups received an infusion of milk or DW. Thus, 6 treatment groups (n = 8 pups per group) resulted from the combination of the pretreatment condition (Naltrexone, Saline, or NI) and the exposure infusion (Milk or DW). Pups were tested in a 3-min testing session that started 5-min after pretreatment with naltrexone, saline or NI. Testing included a 1-min baseline before the 20 µl exposure infusion of either milk or DW, 1-min after the exposure infusion, and 1-min after the 20 µl test infusion of lemon. Behavioral observations involved scoring of facial wiping throughout the 3-min experimental session.
Results and discussion
Analysis of facial wiping was performed in 2-factor analysis of variance (3 Pretreatments × 2 Exposures), with the Time factor treated as a repeated measure. Results showed that there was no significant expression of facial wiping across the groups during the 1-min baseline or after the exposure infusion of milk or DW. Facial wiping after the lemon infusion varied significantly among the Pretreatment groups, F (2, 41) = 3.24, p < .05. In addition, there was a significant interaction between Pretreatment and Exposure infusion, F (2, 41) = 4.58, p < .01 (Figure 3). To confirm the behavioral effects of milk, an initial planned comparison of pups in the NI condition was conducted with a t test. This comparison revealed that pups infused with DW showed significantly higher levels of facial wiping compared to those infused with milk, t (14) = 2.25, p < .05. Following this comparison, a series of one-way ANOVAs were conducted to assess the simple main effect of Pretreatment in each of the Exposure conditions. In the DW groups, there were no significant differences in the wiping response evoked by lemon (p > .50). In contrast, there was significant effect of pretreatment injection among the groups that were infused milk, F (2, 20) = 11.46, p < .001. Post hoc comparisons of means by the method of Fisher PLSD indicated significantly more wiping strokes among subjects pretreated with naltrexone than the Saline-injected or NI subjects (ps < .05). These analyses revealed that subjects that received no pretreatment injection before testing and were infused with water showed greater wiping responses to lemon than subjects infused with milk. In other words, oral exposure to milk was effective in reducing lemon-evoked facial wiping in the P1 rat pup. Additionally, the results from the Naltrexone and Saline pretreatment conditions revealed that the behavioral effect of milk is mediated by the endogenous opioid system. Subjects that were pretreated with naltrexone and infused with milk showed higher levels of facial wiping than control pups injected with Saline, indicating that naltrexone was effective in blocking opioid receptors and reinstating the facial wiping response.
Figure 3.
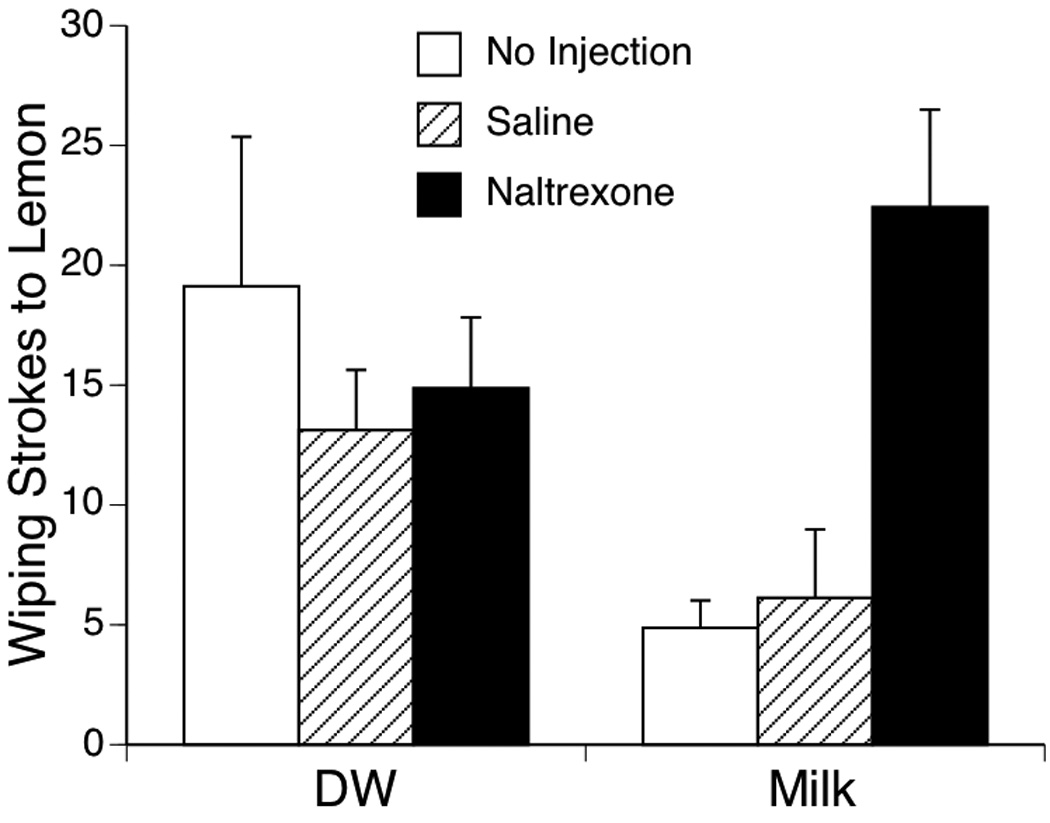
Number of facial wiping strokes evoked by lemon after oral exposure to distilled water (DW) or milk in Experiment 2. Pups were pretreated by IP injection of isotonic saline or the non-selective opioid antagonist naltrexone (1.0 mg/kg), or no injection, 5 min before oral exposure. Bars show mean number of wiping strokes; vertical lines depict S.E.M.
Analyses of general activity were performed in a series of three-factor ANOVAs (3 Pretreatment groups × 2 Exposure groups × 3 1-min intervals), with the Time factor treated as a repeated measure. Results showed a significant main effect of Time across the groups on forelimb movements, F (2, 82) = 18.60, p < .0001. Post hoc tests indicated a significant increase of forelimb activity across each min of the test session: baseline (mean ± SEM = 15.0 ± 1.6), min after milk exposure (21.8 ± 1.9), min after lemon infusion (31.1 ± 2.1). A significant effect of Time also was evident for hindlimbs, F (2, 82) = 5.35, p < .01, with no change from min 1 (12.1 ± 2.1) to min 2 (13.7 ± 1.4) and elevated activity at min 3 (19.6 ± 1.9) after the infusion of lemon (p < .05). In addition, there was significant main effect of Exposure infusion on hindlimb activity, F (2, 41) = 4.98, p < .05, with more hindlimb movements expressed by DW-infused pups. Head movements also showed a significant main effect of time, F (2, 82) = 5.28, p < .01, with head activity increasing from min 1 (4.5 ± 0.9) to min 2 (7.6 ± 1.0) and remaining at similar levels in min 3 (8.7 ± 0.9) after the lemon infusion (p < .05). Finally, mouth movements showed a significant effect of time, F (2, 82) = 17.70, p < .0001, with significant higher levels of activity at min 3 (4.9 ± 0.8) after the lemon infusion compared to either min 1 (1.0 ± 0.4) or min 2 (1.1 ± 0.3). Overall, the analyses of general activity suggested a general increase in activity after the infusion of lemon.
The findings of Experiment 2 are comparable to those described by Korthank and Robinson (1998), who reported that E20 rat fetuses that were infused with milk showed lower levels of facial wiping to lemon. In addition, the results of the present study confirm that milk effects on facial wiping are mediated by the endogenous opioid system in the newborn rat. Taken together, these findings and those reported by Korthank and Robinson (1998) suggest a continuum in the effects of milk before and after birth. Although this experiment did not evaluate which opioid receptors mediate the effects of milk on facial wiping, it has been reported before that milk evokes a kappa opioid response in the E20 rat fetus (Korthank & Robinson, 1998; Smotherman & Robinson, 1992a). Similarly, Korthank & Robinson also reported that AF reduced facial wiping in a manner similar to milk in fetuses and that this response also was mediated by the kappa opioid system. Since the effects of AF on facial wiping in the P1 rat have not previously been evaluated, Experiments 3 and 4 were conducted to determine whether AF can reduce facial wiping to a lemon infusion in the neonatal rat. Because measures of general activity indicated that pups were responsive to infusions, but did not differentiate among the pretreatment or exposure conditions, subsequent experiments report behavioral data only for facial wiping responses.
Experiment 3: AF effects on facial wiping
Previous experiments performed with rat fetuses reported that AF has the capacity to reduce the behavioral expression of facial wiping after a lemon infusion (Korthank & Robinson, 1998). Several other reports have provided evidence of the postnatal effects of AF expressed in newborn rats (Hepper, 1987), lambs (Schaal, Orgeur et al., 1995), and humans (Marlier et al., 1998a, 1998b; Schaal, Marlier et al., 1995) with emphasis on the preference response and the capacity of neonates to recognize AF after birth. Currently, there is no evidence of the effects of AF on the expression of a behavioral response to chemosensory stimulation after birth. The aim of Experiment 2 was to evaluate if AF can exert an effect on the facial wiping response of the neonatal rat that is similar to effects reported previously for rat fetuses.
Methods
Forty-eight P1 rat pups, including one male and one female pup from each litter, from a total of 9 litters, were assigned as subjects to one of three experimental groups in Experiment 2. Subjects were tested in 3-min sessions. At the end of a 1-min baseline period, pups received a 20 µl exposure infusion of AF (n = 9 litters) or DW (n = 8), or no infusion (group designated as NI; n = 9). After a delay of 1-min after the exposure infusion, all pups received a 20 µl test infusion of lemon to evoke facial wiping and were observed for one final minute.
Results and discussion
Preliminary analysis examined the effect of gender on facial wiping responses of pups after exposure to AF, and no differences were found between male and female subjects (ps > .05). As a consequence, litter means were calculated for male and female pups assigned to the same experimental condition; these litter means were used in subsequent data analyses in this experiment. There was no significant expression of facial wiping across the three groups during the 1-min baseline or during the 1-min after the exposure infusion of AF or DW. A one-factor ANOVA indicated that facial wiping to the lemon infusion varied significantly among the three exposure conditions, F (2, 22) = 11.87, p < .0005 (Figure 4). Post-hoc analysis (Fisher PLSD) showed that facial wiping did not differ between NI and DW pups (p > .05). In contrast, a significant reduction in facial wiping was found in the AF group relative to both other conditions (p < .05).
Figure 4.
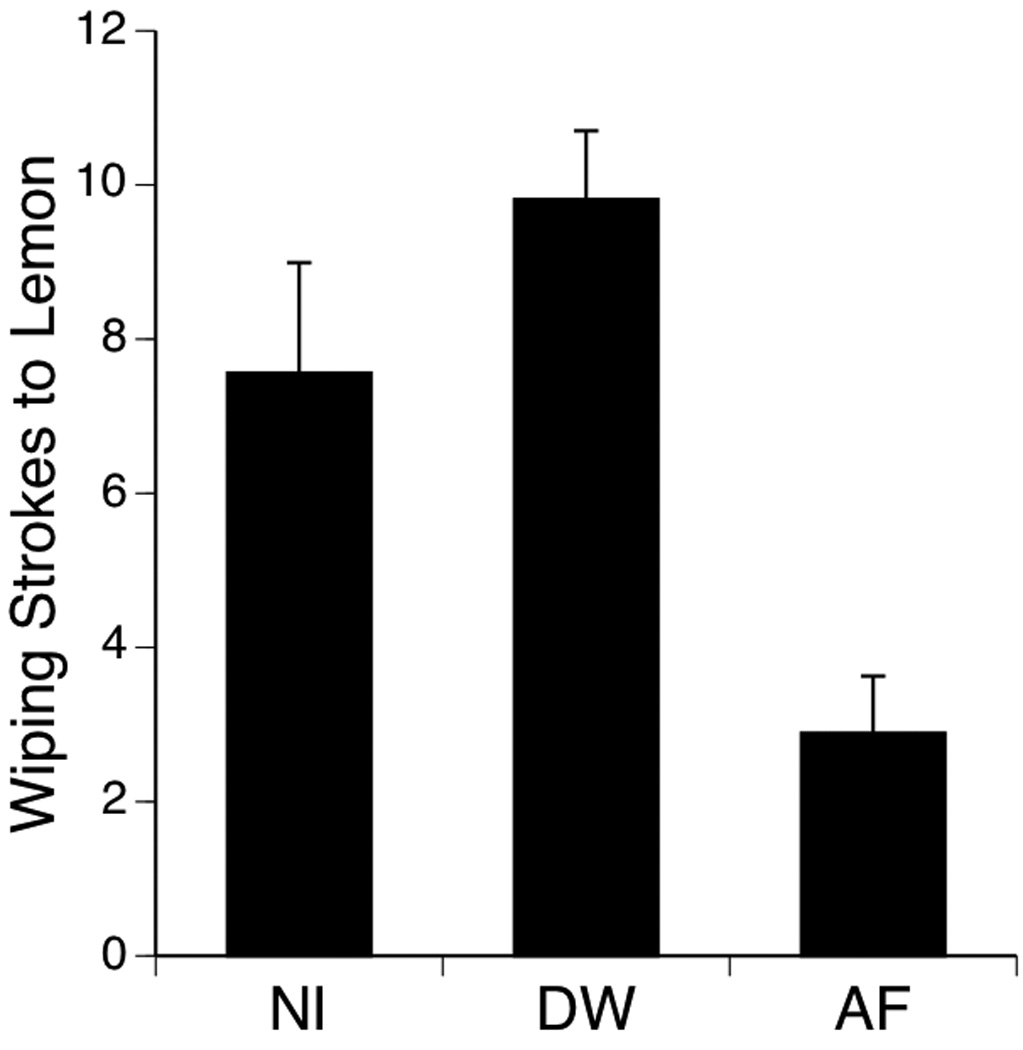
Number of facial wiping strokes evoked by lemon after oral exposure to one of three fluids: amniotic fluid (AF), a control stimulus of DW, or no infusion (NI). Bars depict mean number of wiping strokes; vertical lines show S.E.M.
To summarize, these results provide evidence that AF is effective in reducing the facial wiping response to lemon in neonatal rats. These findings are concordant with similar findings from work with rat fetuses (Korthank & Robinson, 1998) and suggest a behavioral continuity of the effects of AF between the prenatal and postnatal periods.
Experiment 4: Opioid Mediation of AF effects
Previous studies have documented that some of the behavioral responses to chemosensory stimulation in fetal rats are influenced by the endogenous opioid system. Korthank and Robinson (1998) reported that the effect of AF on chemosensory responses of fetal rats is opioid mediated. In contrast, studies that have reported the impact of AF on postnatal behavior in rats, lambs and humans have not evaluated if AF effects are mediated by the endogenous opioid system. The aim of Experiment 4 was to determine if the behavioral effect of AF on facial wiping in the neonatal rat is mediated by the endogenous opioid system.
Methods
Subjects were 40 P1 rat pups from 10 litters that were assigned to one of two pretreatment conditions: (a) an intraperitoneal (IP) injection of 50 µl of naltrexone (1.0 mg/kg), a nonselective opioid antagonist, or (b) an IP injection of the vehicle control of isotonic saline (50 µl). All injections were administered 5-min before testing and were performed using a 30-ga hypodermic needle. As in the previous experiment, pups in Experiment 4 received an infusion of AF or DW after the 1-min baseline. Thus, four treatment groups (n = 10 pups per group) resulted from the combination of the pretreatment condition (Saline or Naltrexone) and the exposure infusion (AF or DW). During the 3-min test session, 1-min after the exposure infusion, all subjects received a 20 µl test infusion of the lemon solution. Behavioral scoring included only the 3-min session comprising baseline, exposure and test periods.
Results and discussion
Facial wiping was not expressed by any of the subjects during the Baseline period or after the Exposure infusion of AF or DW. Wiping responses to the Test infusion of lemon were compared in a 2-factor ANOVA (2 Pretreatments × 2 Exposure conditions). This analysis indicated a main effect of Pretreatment, F (1, 36) = 4.23, p < .05, but no effect of Exposure (p >.05). The interaction of Pretreatment and Exposure also was significant, F (1, 36) = 4.92, p < .05, which indicated that subjects pretreated with Saline and exposed to AF showed reduced wiping responses compared to subjects exposed to DW, and that pretreatment with naltrexone reinstated higher levels of wiping response after AF exposure (Figure 5).
Figure 5.
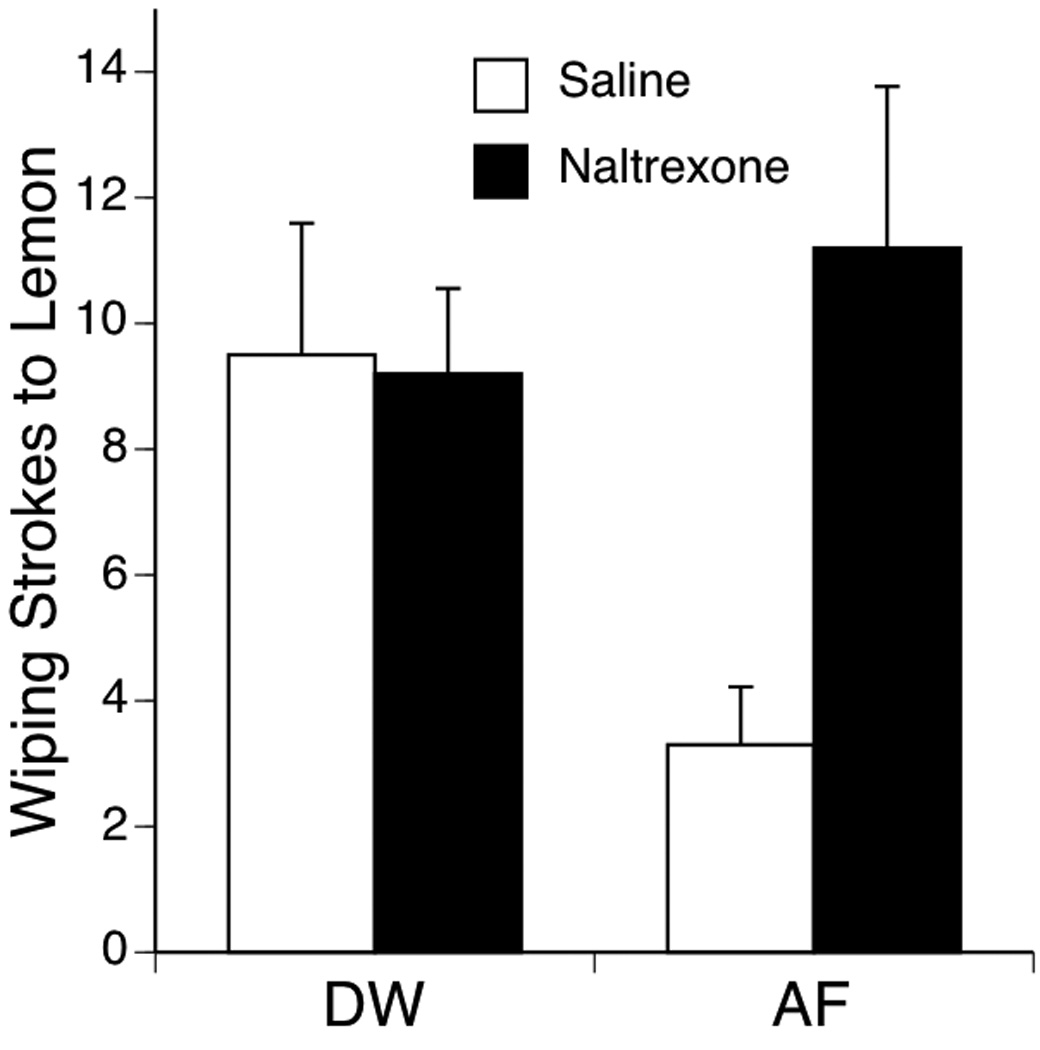
Number of facial wiping strokes evoked by lemon in Experiment 4 after exposure to DW or AF. Subjects were pretreated by IP injection of the saline vehicle or the non-selective opioid antagonist naltrexone (1.0 mg/kg) 5 min before receiving the oral infusion of DW or AF. Bars depict mean number of wiping strokes; vertical lines show S.E.M.
The finding that blockade of opioid receptors with naltrexone is effective in reinstating higher levels of facial wiping confirms that the effect of AF on this behavioral response to novel chemosensory stimulation is mediated by the endogenous opioid system. These results are consistent with previous evidence of similar experimental manipulation with fetuses (Korthank & Robinson, 1998), suggesting that both behavioral manifestations before and after birth have the same neural substrates.
Experiment 5: Kappa opioid system mediation of AF effects
The previous report by Korthank and Robinson (1998) found that behavioral effects of AF in the rat fetus are mediated by the kappa subclass of opioid receptors. In Experiment 3, naltrexone was shown to be effective in reinstating higher levels of facial wiping after pups were exposed to AF, suggesting that the behavioral effects of AF are mediated by the endogenous opioid system in infant rats as well. The aim of Experiment 5 was to identify if the effects of AF on behavior of the neonatal rat are mediated by the kappa or mu subclass of opioid receptors. (Previous reports have documented that delta opioid receptors undergo rapid developmental change during the postnatal period in both brain and spinal cord in the rat (Attali, Saya, & Vogel, 1990; Petrillo, Tavani, Verotta, Robson, & Kosterlitz, 1987), but delta opioid activity is not involved in sensory-evoked opioid responses in the fetal rat (Smotherman et al., 1994). For these reasons, this experiment did not include selective antagonism of delta opioid receptors.) Pretreatment with a selective opioid antagonist such as BNI for kappa opioid receptors or CTOP for mu receptors should be effective to reinstate a higher facial wiping response to lemon after exposure to AF.
Methods
Subjects were 24 P1 rat pups collected from 8 litters that were assigned to one of three pretreatment conditions: (a) an intraperitoneal (IP) injection of 50 µl of the selective kappa antagonist nor-binaltorphimine di-HCl (BNI, 9.0 mg/kg), (b) the mu antagonist [Cys2, Tyr3, Orn5, Pen7]-Amide (CTOP, 6.0 mg/kg), (c) or the isotonic saline vehicle (SAL). Opioid receptor antagonists and the vehicle control were administered using a 30-ga hypodermic needle. Dosages of both CTOP and BNI were determined from previous dose-response curves of the effectiveness of mu and kappa antagonists to block the behavioral effects of selective mu or kappa agonist drugs (Smotherman & Robinson, 1992a, Smotherman, Simonik, Andersen, & Robinson, 1993). As in Experiment 3, pups were pretreated 5 min before testing. After the 1-min baseline, all subjects received an infusion of AF followed 1 min later by an infusion of the lemon solution. The 3-min session comprised the baseline, the exposure infusion, and the test infusion of lemon. Behavioral scoring was conducted through the entire 3-min test session.
Results and discussion
As in the previous experiments, facial wiping was not expressed by any of the subjects during the Baseline period or after the Exposure infusion of AF. After delivery of the lemon infusion, facial wiping responses varied significantly among the three pretreatment conditions, F (2, 21) = 4.32, p < .05 (Figure 6). Post-hoc analysis (Fisher PLSD) showed that facial wiping was expressed significantly more by subjects in the BNI condition than by pups injected with CTOP (p < .05) or Saline (p < .05). Facial wiping did not differ between Saline and CTOP conditions (p > .05).
Figure 6.
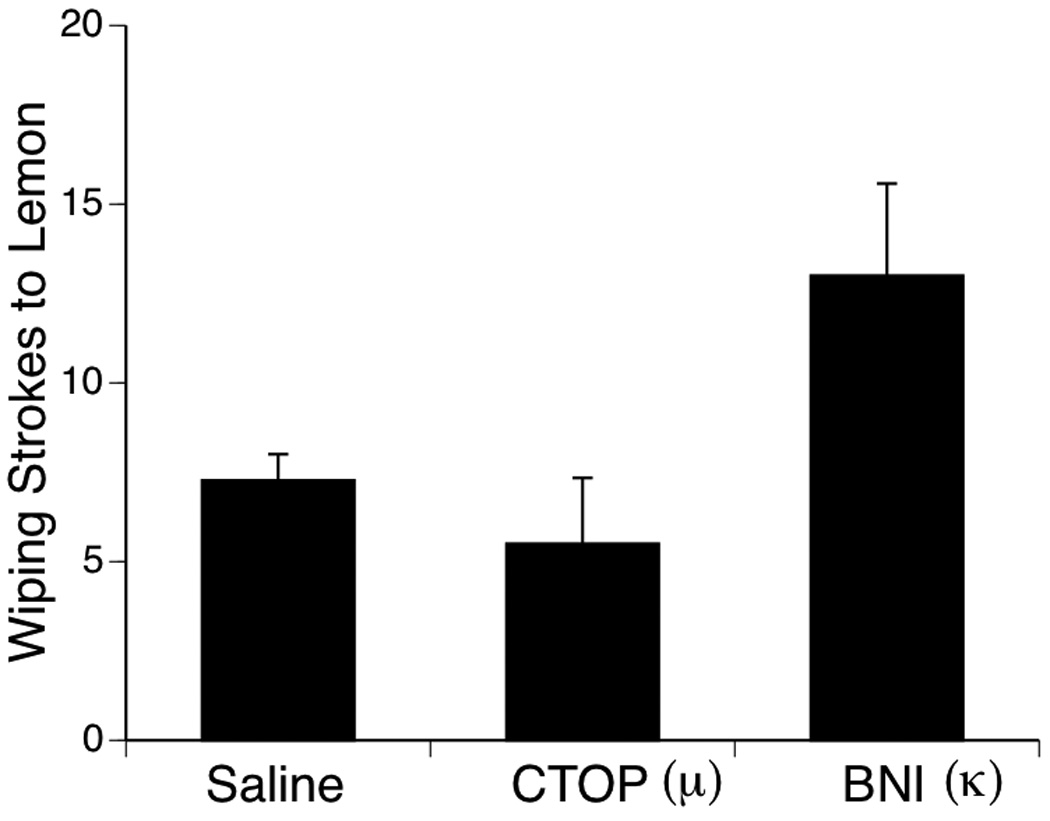
Number of facial wiping strokes evoked by lemon in Experiment 5. Subjects were pretreated by IP injection of the saline vehicle, the selective m opioid antagonist CTOP (6.0 mg/kg), or the selective k opioid antagonist BNI (9.0 mg/kg) 5 min before receiving an oral infusion AF. Bars depict mean number of wiping strokes; vertical lines show S.E.M.
To summarize, results of Experiment 5 demonstrated that subjects pretreated with the selective kappa receptor antagonist BNI showed higher levels of facial wiping in response to the test infusion of lemon after exposure to AF. These findings confirm and extend the findings of Experiment 4 that indicated the involvement of the endogenous opioid system in the behavioral effects of AF on neonatal facial wiping. More specifically, since BNI was effective in reinstating the facial wiping response, it suggests that exposure to AF results in activity at the kappa subclass of opioid receptors. These results are consistent with those reported by Korthank and Robinson (1998) in rat fetuses and provide more evidence for developmental continuity in the behavioral effects of AF during the perinatal period.
General Discussion
The present study provides important information about the effects of posture and oral exposure to two biologically important fluids—amniotic fluid (AF) and milk—on chemosensory responsiveness in the newborn rat. Specifically, facial wiping behavior in response to infusion of lemon extract was used as a behavioral index of sensory responsiveness in the infant rat. Because an initial experiment found that wiping responses were more pronounced when pups were tested in a supine posture, all subsequent experiments tested pups in this position. Moreover, previous studies have suggested that chemosensory stimulation can evoke righting responses that interfere with facial wiping when older pups (P3-P5) were tested in a supine posture (Smotherman & Robinson, 1989; Robinson & Smotherman, 1992a). Measures of head and limb activity were low during the baseline period in Experiment 1, and general activity was not indicative of competing responses, such as righting (Robinson & Smotherman, 1992b), when P1 pups were tested in a supine posture. For these reasons, the supine posture was judged optimal for testing facial wiping responses in P1 rat pups.
The next four experiments assessed the behavioral effects of oral exposure to milk or AF. Experiment 2 confirmed that exposure to milk resulted in a reduced facial wiping response, measured as fewer wiping strokes, after an infusion of the test solution of lemon, and blockade of opioid receptors with the non-selective opioid antagonist naltrexone was effective in reinstating higher levels of facial wiping after exposure to milk, suggesting mediation by the endogenous opioid system. In Experiments 3 and 4, oral exposure to a small amount of AF collected on E20 also resulted in a diminished facial wiping response to lemon, and blockade of opioid receptors with naltrexone was effective in reinstating higher levels of facial wiping after exposure to AF. Finally, Experiment 5 demonstrated that pretreatment with the kappa-selective opioid antagonist BNI, but not the mu antagonist CTOP, also reversed the effects of exposure to AF and resulted in higher levels of facial wiping to lemon.
From a psychobiological perspective, this study reports a number of findings that are important for understanding the developmental transition from prenatal to the postnatal life. First, the finding that the facial wiping response of infant rats is influenced by the posture of the pup at the time of testing provides further evidence for the importance of controlling environmental context when evaluating behavior in developing animals. Although the rationale for Experiment 1 was to determine the most appropriate posture for testing pups in subsequent experiments, it also affirmed the importance of controlling behavioral context, such as posture, in assessing other behavioral capacities in neonatal subjects. There are other well-known examples of the influence of posture on motor performance in both human and animal infants. In humans, the development of reaching is intrinsically related to the development of postural control (Rochat, 1992; Rochat & Goubet, 1995). For instance, previous work in this area suggests that postural control is important for infants to control the trajectory of arm movements toward a target (Thelen & Spencer, 1998). In newborn rats, the initial posture of the pup exerts a strong influence on the motor systems activated, and thus the patterns of movement expressed, during righting responses on a surface (Pellis, Pellis & Teitelbaum, 1991). Facial wiping specifically can be disrupted when older rat pups are tested in postures that evoke conflicting motor responses such as righting, or which require limb support for postural maintenance (Robinson & Smotherman, 1992b). These examples illustrate how one behavior may serve as scaffolding for another. Pups do not simply express a response to sensory stimulation in a vacuum. Their behavior is shaped by the physical and behavioral context in which the exposure takes place.
The principal finding of this study is the apparent effectiveness of milk and AF to modulate a response to sensory stimulation in the neonatal rat. Previous work has documented similar effects on sensory responsiveness when perinatal rats are exposed to milk. For example, 2–10 day old rats exhibit an increased latency to withdraw a forepaw from a thermal stimulus after oral exposure to milk (Blass & Fitzgerald, 1988). The ability of milk to reduce responsiveness to nociceptive stimuli also is evident at the time of birth in Caesarean-derived pups that lack suckling experience (Blass, Jackson and Smotherman, 1991). Smotherman and Robinson (1992a) reported that rat fetuses tested the day before birth (E21), after oral exposure to milk, showed significantly less facial wiping to an oral infusion of lemon than controls exposed to saline. The same study reported that wiping responses of E20 or E21 fetal rats elicited by a perioral tactile probe were virtually eliminated after oral exposure to milk.
Moreover, parallels between AF and milk also are reflected in the neural mechanisms that mediate these behavioral effects. Previous reports have demonstrated that a variety of behavioral effects evoked by milk in the fetal or neonatal rat can be blocked by antagonism of the endogenous opioid system. For example, the greater latency to respond to a thermal stimulus after oral exposure to milk was reversed when pups were pretreated with the nonspecific opioid antagonist naloxone (Blass et al., 1991). Direct injection of naloxone or a selective kappa opioid antagonist into the cerebral ventricles also caused newborn pups to show a greater latency to attach to a surrogate nipple that provided milk, and markedly reduced time on the nipple and milk intake (Petrov, Varlinskaya, & Smotherman, 1998; 2000). Diminished responses of fetal rats to a perioral stimulus after milk exposure also were prevented by blockade of the opioid system: both naloxone and the selective kappa antagonist nor-binaltorphimine (BNI) blocked the effect of milk and reinstated high levels of wiping in response to perioral stimulation in E20 rat fetuses (Smotherman & Robinson, 1992a, 1992c). However, antagonism of mu or delta opioid receptors was not effective in blocking the milk effect (Smotherman et al., 1994). Other behavioral responses elicited by milk also appear to be affected by activity in the endogenous opioid system. Rat fetuses pretreated with naloxone and infused with milk did not show the stretch response typically evoked by this stimulus (Smotherman & Robinson, 1992b), and classical conditioning supported by milk as an unconditioned stimulus was blocked by antagonism of opioid receptors (Robinson, Arnold, Spear & Smotherman, 1993). Taken together, these results suggest that milk exposure not only produces similar behavioral effects in the fetal and neonatal rat, but that these effects are supported by similar neural substrates.
The findings of Experiments 4 and 5 of the present study confirm the functionality of the opioid system only a few hours after birth, and corroborate the involvement of the endogenous opioid system in the behavioral effect of AF on facial wiping. Specifically, Experiment 5 provided evidence that the kappa subclass of opioid receptors, but not mu, mediate the behavioral effects of AF in the newborn pup. The same pattern of results was reported for fetal rats by Korthank and Robinson (1998): E20 rat fetuses showed diminished facial wiping to lemon after oral exposure to AF, and the effects of AF on fetal responsiveness was effectively blocked by a nonspecific or selective kappa opioid antagonist. Knowing that AF can exert a behavioral response in neonates similar to that previously described in fetuses not only provides evidence for developmental continuity in the behavioral effects of AF, but also suggests that the same neural mechanisms govern responses to AF and milk before and after birth.
Other authors have suggested that AF and milk represent points along a continuum of predictable environmental features that may serve to bridge the perinate in the transition from prenatal to perinatal life. Marlier, Schaal and Soussignan (1997) reported that 3 days after birth, human neonates showed an olfactory preference for maternal lacteal secretion when paired with AF. Before that age, newborns did not distinguish between AF and colostrum; they showed a positive approach to both stimuli when they were presented simultaneously (Marlier et al., 1998b). At later ages, the postnatal olfactory response was specific to mature (post-colostrum) human milk (Marlier & Schaal, 2005). A parallel series of studies has suggested a similar pattern of developmental continuity in the responses of fetal and newborn lambs, rabbits and piglets to the odors of AF and milk (Coureaud, Schaal, Hudson, Orgeur, & Coudert, 2002; Parfet & Gonyou, 1991; Schaal & Orgeur, 1992). These studies suggest that preferences for the odors of biologically relevant natural fluids shifts continuously from AF to colostrum to milk during the perinatal period in a number of mammalian neonates (Schaal, 2005). Of course, none of the studies of newborn human or lamb olfactory preferences specifically implicate neural systems that may mediate infant responses. Therefore, the present study may aid in filling this gap by implicating a common mechanism underlying the behavioral effects of milk and AF in fetal and newborn rats: both fluids appear to activate the kappa subclass of opioid receptors in fetuses (milk: Smotherman & Robinson, 1992c; AF: Korthank & Robinson, 1998) and newborns (milk: Petrov et al., 2000, this study; AF: this study).
In the same manner that current research has concentrated efforts on understanding clinical, biological, and nutritive aspects of AF regulation in the human fetus (Ross & Brace, 2001; Underwood, Gilbert, & Sherman, 2005), the findings of this study emphasize the importance of understanding the role of AF in behavioral development. Because this study was conducted with rat neonates, it will be important in future research to determine if behavioral effects of AF reported in other mammals, including human newborns, are also opioid mediated. Previous studies have documented that AF can provide a potent cue for guiding the behavior of the newborn rat, lamb and human (Marlier et al., 1998a, 1998b; Porter, Winberg, & Varendi, 2005; Schaal, 2005; Schaal, Marlier et al., 1995; Schaal, Orgeur et al., 1995). But further research will be needed to determine whether the postnatal effects of AF on other aspects of neonatal behavior are related to endogenous opioid activity during perinatal development (Korthank & Robinson, 1998; Smotherman & Robinson, 1992c).
Acknowledgments
This research was supported in part by NIH grant HD 33862 to SRR.
References
- Attali B, Saya D, Vogel Z. Pre- and postnatal development of opiate receptor subtypes in rat spinal cord. Developmental Brain Research. 1990;53:97–102. doi: 10.1016/0165-3806(90)90128-l. doi:10.1016 /0165-3806(90)90128-L. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neuroscience and Biobehavioral Reviews. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. doi:10.1016/S0149-7634(99)00072-X. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Grill HJ. Alternating ingestive and aversive consummatory responses suggest a two-dimensional analysis of palatability in rats. Behavioral Neuroscience. 1983;97:563–573. doi: 10.1037//0735-7044.97.4.563. doi:10.1037/0735-7044.97.4.563. [DOI] [PubMed] [Google Scholar]
- Blass EM, Fitzgerald E. Milk-induced analgesia and comforting in 10-day-old rats: Opioid mediation. Pharmacology Biochemistry & Behavior. 1988;29:9–13. doi: 10.1016/0091-3057(88)90266-3. doi:10.1016/0091-3057(88)90266-3. [DOI] [PubMed] [Google Scholar]
- Blass EM, Jackson AM, Smotherman WP. Milk-induced, opioid-mediated antinociception in rats at the time of cesarean delivery. Behavioral Neuroscience. 1991;105:677–686. doi: 10.1037/0735-7044.105.5.667. doi:10.1037/0735-7044.105.5.667. [DOI] [PubMed] [Google Scholar]
- Brace R. Physiology of amniotic fluid volume regulation. Clinical Obstetrics and Gynecology. 1997;40:280–289. doi: 10.1097/00003081-199706000-00005. Retrieved from http://journals.lww.com/clinicalobgyn/pages/default.aspx. [DOI] [PubMed]
- Brumley MR, Robinson SR. Facial wiping in the rat fetus: Variation of chemosensory stimulus parameters. Developmental Psychobiology. 2004;44:219–229. doi: 10.1002/dev.20005. doi:10.1002/dev.20005. [DOI] [PubMed] [Google Scholar]
- Coureaud G, Schaal B, Hudson R, Orgeur P, Coudert P. Transnatal olfactory continuity in the rabbit: Behavioral evidence and short-term consequence of its disruption. Developmental Psychobiology. 2002;40:372–390. doi: 10.1002/dev.10038. doi:10.1002/dev.10038. [DOI] [PubMed] [Google Scholar]
- Danneman PJ, Mandrell TD. Evaluation of five agents/methods for anesthesia of neonatal rats. Laboratory Animal Science. 1997;47:386–395. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/journals/833/ [PubMed]
- Ganchrow JR, Steiner JE, Canetto S. Behavioral displays to gustatory stimuli in newborn rat pups. Developmental Psychobiology. 1986;19:163–174. doi: 10.1002/dev.420190303. doi:10.1002/dev.420190303. [DOI] [PubMed] [Google Scholar]
- Ganchrow JR, Steiner JE, Daher M. Neonatal facial expressions in response to different qualities and intensities of gustatory stimuli. Infant Behavior & Development. 1983;6:473–484. doi:10.1016/S0163-6383(83)90301-6. [Google Scholar]
- Golani I, Fentress JC. Early ontogeny of face grooming in mice. Developmental Psychobiology. 1985;18:529–544. doi: 10.1002/dev.420180609. doi:10.1002/dev.420180609. [DOI] [PubMed] [Google Scholar]
- Goldstein M. EventCoder. Ithaca, NY: Cornell University; 2006. (Version 1.0b6) [Computer software] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. doi:10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Hall WG, Rosenblatt JS. Suckling behavior and intake control in the developing rat pup. Journal of Comparative and Physiological Psychology. 1977;91:1232–1247. doi:10.1037/h0077420. [Google Scholar]
- Hepper PG. The amniotic fluid: an important priming role in kin recognition. Animal Behaviour. 1987;35:1343–1346. doi:10.1016/S0003-3472(87)80006-4. [Google Scholar]
- Hepper PG. Adaptative fetal learning: Prenatal exposure to garlic affects postnatal preferences. Animal Behaviour. 1988;36:935–936. doi:10.1016/S0003-3472(88)80177-5. [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. doi:10.101 6/0892-0362(92)90020-B. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Johanson IB, Shapiro EG. Intake and behavioral responsiveness to taste stimuli in infants rats from 1 to 15 days of age. Developmental Psychobiology. 1986;19:593–606. doi: 10.1002/dev.420190610. doi:10.1002/dev.420190610. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Blass EM. Gustatory determinants of suckling in albino rats 5–20 days of age. Developmental Psychobiology. 1985;18:67–82. doi: 10.1002/dev.420180106. doi:10.1002/dev.420180106. [DOI] [PubMed] [Google Scholar]
- Korthank AJ, Robinson SR. Effects of amniotic fluid on opioid activity and fetal responses to chemosensory stimuli. Developmental Psychobiology. 1998;33:235–248. doi: 10.1002/(sici)1098-2302(199811)33:3<235::aid-dev4>3.0.co;2-s. doi:10.1002/(SICI)1098-2302(199811)33:3<235∷AID-DEV4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Marlier L, Schaal B. Human newborns prefer human milk: Conspecific milk odor is attractive without postnatal exposure. Child Development. 2005;76:155–168. doi: 10.1111/j.1467-8624.2005.00836.x. doi:10.1111/j.146 7-8624.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Marlier L, Schaal B, Soussignan R. Orientation responses to biological odours in the human newborn. Initial pattern and postnatal plasticity. Comptes Rendus De L Academie Des Sciences Serie Iii-Sciences De La Vie-Life Sciences. 1997;320:999–1005. doi: 10.1016/s0764-4469(97)82473-0. doi:10.1016/S0764-4469(97)82473-0. [DOI] [PubMed] [Google Scholar]
- Marlier L, Schaal B, Soussignan R. Bottle-fed neonates prefer an odor experienced in utero to an odor experienced postnatally in the feeding context. Developmental Psychobiology. 1998a;33:133–145. doi:10.1002/(SICI)1098-2302(199809)33:2<133∷AID-DEV4>3.0.CO;2-K. [PubMed] [Google Scholar]
- Marlier L, Schaal B, Soussignan R. Neonatal responsiveness to the odor of amniotic and lacteal fluids: a test of perinatal chemosensory continuity. Child Development. 1998b;69:611–623. doi:10.1111/j.1467-8624.1998.tb06232.x. [PubMed] [Google Scholar]
- Parfet KA, Gonyou HW. Attraction of newborn piglets to auditory, visual, olfactory and tactile stimuli. Journal of Animal Science. 1991;69:125–133. doi: 10.2527/1991.691125x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2005005. [DOI] [PubMed]
- Pellis VC, Pellis SM, Teitelbaum P. A descriptive analysis of the postnatal development of contact-righting in rats (rattus norvegicus) Developmental Psychobiology. 1991;24:237–263. doi:10.1002/dev.420240405. [Google Scholar]
- Petrillo P, Tavani A, Verotta D, Robson LE, Kosterlitz HW. Differential postnatal development of μ-, δ- and κ- opioid binding sites in rat brain. Developmental Brain Research. 1987;31:53–58. doi: 10.1016/0165-3806(87)90082-4. doi:10.1016/0165-3806(87)90082-4. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Smotherman WP. Endogenous opioids and the first suckling episode in the rat. Developmental Psychobiology. 1998;33:175–183. doi:10.1002/(SICI)1098-2302(199809)33:2<175∷AID-DEV8>3.0.CO;2-G. [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Smotherman WP. The first suckling episode in the rat: The role of endogenous activity at mu and kappa opioid receptors. Developmental Psychobiology. 2000;37:129–143. doi: 10.1002/1098-2302(200011)37:3<129::aid-dev2>3.0.co;2-p. doi:10.1002/1098-2302(200011)37:3<129∷AID-DEV2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiology & Behavior. 1986;38:887–890. doi: 10.1016/0031-9384(86)90058-2. doi:10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- Porter RH, Winberg J, Varendi H. Prenatal preparation for early postnatal olfactory learning. In: Hopkins B, Johnson SP, editors. Prenatal development of postnatal functions. volume 2. Westport, CT, US: Praeger Publishers; 2005. pp. 103–129. (Advances in Infancy Research series) [Google Scholar]
- Powell KR, Holtzman SG. Differential antagonism of the rate-decreasing effects of κ-opioid receptor agonists by naltrexone and norbinaltorphimine. European Journal of Pharmacology. 1999;377:21–28. doi: 10.1016/s0014-2999(99)00394-5. doi:10.1016/S0014-2999(99)00394-5. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Arnold HM, Spear NE, Smotherman WP. Experience with milk and an artificial nipple promotes conditioned opioid activity in the rat fetus. Developmental Psychobiology. 1993;26:375–387. doi: 10.1002/dev.420260702. doi:10.1002/dev.420260702. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. The amniotic sac as scaffolding: Prenatal ontogeny of an action pattern. Developmental Psychobiology. 1991;24:463–485. doi: 10.1002/dev.420240703. doi:10.1002/dev.420240703. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Fundamental motor patterns of the mammalian fetus. Journal of Neurobiology. 1992a;23:1574–1600. doi: 10.1002/neu.480231013. doi:10.1002/neu.480231013. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Motor competition in the prenatal ontogeny of species-typical behavior. Animal Behaviour. 1992b;44:89–99. doi:10.1016/S0003-3472(05)80 758-4. [Google Scholar]
- Robinson SR, Smotherman WP. Organization of the stretch response to milk in the rat fetus. Developmental Psychobiology. 1992c;25:33–49. doi: 10.1002/dev.420250104. doi:10.1002/dev.420250104. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Behavioral effects of milk in the rat fetus. Behavioral Neuroscience. 1994;108:1139–1149. doi:10.1037/0735-7044.108.6.1139. [PubMed] [Google Scholar]
- Rochat P. Self-sitting and reaching in 5- to 8-month-old infants: The impact of posture and its development on early eye-hand coordination. Journal of Motor Behavior. 1992;24:210–220. doi: 10.1080/00222895.1992.9941616. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14977620. [DOI] [PubMed]
- Rochat P, Goubet N. Development of sitting and reaching in 5- to 6-month-old infants. Infant Behavior and Development. 1995;18:53–68. doi:10.1016/0163-6383(95)90007-1. [Google Scholar]
- Rosenstein D, Oster H. Differential facial responses to four basic tastes in newborns. Child Development. 1988;59:1555–1568. Retrieved from http://www.jstor.org/stable/1130670. [PubMed]
- Ross MG, Brace RA. National Institute of Child Health and Development Conference summary: amniotic fluid biology-basic and clinical aspects. The Journal of Maternal-Fetal Medicine. 2001;10:2–19. doi: 10.1080/714904292. doi:10.1080/714904292. [DOI] [PubMed] [Google Scholar]
- Ross MG, Nijland MJM. Fetal swallowing: Relation to amniotic fluid regulation. Clinical Obstetrics and Gynecology. 1997;40:352–365. doi: 10.1097/00003081-199706000-00011. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9199846. [DOI] [PubMed]
- Schaal B. From amnion to colostrum to milk: Odour bridging in early developmental transitions. In: Hopkins B, Johnson SP, editors. Prenatal development of postnatal functions. volume 2. Westport, CT, US: Praeger Publishers; 2005. pp. 51–102. (Advances in Infancy Research series) [Google Scholar]
- Schaal B, Marlier L, Soussignan R. Responsiveness to the odour of amniotic fluid in the human neonate. Biology of the Neonate. 1995;67:397–406. doi: 10.1159/000244192. doi:10.1159/000244192. [DOI] [PubMed] [Google Scholar]
- Schaal B, Marlier L, Soussignan R. Olfactory function in the human fetus: Evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behavioral Neuroscience. 1998;112:1438–1449. doi: 10.1037//0735-7044.112.6.1438. doi:10.1037/0735-7044.112.6.1438. [DOI] [PubMed] [Google Scholar]
- Schaal B, Orgeur P. Olfaction in utero: can the rodent model be generalized? The Quarterly Journal of Experimental Psychology. 1992;44B:245–278. doi: 10.1080/02724999208250615. doi:10.1080/0272499 9208250615. [DOI] [PubMed] [Google Scholar]
- Schaal B, Orgeur P, Arnould C. Chemosensory preferences in newborn lambs: Possible influence of prenatal experience. Behavior. 1995;132:351–365. doi:10.1163/1568539 95x00603. [Google Scholar]
- Shide DJ, Blass EM. Opioidlike effects of intraoral infusions of corn oil and polycose on stress reactions in 10-day-old rats. Behavioral Neuroscience. 1989;103:1168–1175. doi: 10.1037//0735-7044.103.6.1168. doi:10.1037/0735-7044.103.6.1168. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Environmental determinants of behavior in the rat fetus. Animal Behaviour. 1986;34:1859–1873. doi:10.1016/S0003-3472(86)80272-X. [Google Scholar]
- Smotherman WP, Robinson SR. Prenatal expression of species-typical action patterns in the rat fetus (Rattus norvegicus) Journal of Comparative Psychology. 1987;101:190–196. doi:10.1037/0735-7036.101.2.190. [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behavioral Neuroscience. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. doi:10.1037/0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Cryptopsychobiology: the appearance of a species-typical action pattern during early development. Behavioral Neuroscience. 1989;103:246–253. doi: 10.1037//0735-7044.103.2.246. doi:10.1037/0735-7044.103.2.246. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Olfactory bulb transection alters fetal behavior after chemosensory but not tactile stimulation. Developmental Brain Research. 1990;57:175–180. doi: 10.1016/0165-3806(90)90043-x. doi:10.1016/0165-3806(90)90043-X. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Kappa opioid mediation of fetal responses to milk. Behavioral Neuroscience. 1992a;106:396–407. doi: 10.1037//0735-7044.106.2.396. doi:10.1037/0735-7044.106.2.396. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Opioid control of the fetal stretch response: Implications for the first suckling episode. Behavioral Neuroscience. 1992b;106:866–873. doi: 10.1037//0735-7044.106.5.866. doi:10.1037/0735-7044.106.5.866. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Prenatal experience with milk: Fetal behavior and endogenous opioid systems. Neuroscience and Biobehavioral Reviews. 1992c;16:351–364. doi: 10.1016/s0149-7634(05)80205-2. doi:10.1016/S0149-7634(05)80205-2. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR, Varlinskaya EI, Petrov ES, Orlowski M, de Costa BR, Rice KC. Central administration of the endopeptidase 24.15 inhibitor cFP-AAF-pAB suggests dynorphin as the endogenous ligand underlying behavioral effects of milk in the fetal rat. Pharmacology Biochemistry & Behavior. 1994;47:715–719. doi: 10.1016/0091-3057(94)90178-3. doi:10.1016/0091-3057(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Simonik DK, Andersen SL, Robinson SR. Mu and kappa opioid systems modulate responses to cutaneous perioral stimulation in the fetal rat. Physiology & Behavior. 1993;53:751–756. doi: 10.1016/0031-9384(93)90184-h. doi:10.1016/0031-9384(93)90184-H. [DOI] [PubMed] [Google Scholar]
- Spear LP, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Developmental Psychobiology. 1989;22:401–411. doi: 10.1002/dev.420220407. doi:10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neuroscience & Biobehavioral Reviews. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. doi:10.1016/S0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. doi:10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Thelen E, Spencer JP. Postural control during reaching in young infants: a dynamic systems approach. Neuroscience and Biobehavioral Reviews. 1998;22:507–514. doi: 10.1016/s0149-7634(97)00037-7. doi:10.1016/S0149-7634(97)00037-7. [DOI] [PubMed] [Google Scholar]
- Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. Journal of Perinatology. 2005;25:341–348. doi: 10.1038/sj.jp.7211290. doi:10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- Varendi H, Christensson K, Porter RH, Winberg J. Soothing effect of amniotic fluid smell in newborn infants. Early Human Development. 1998;51:47–55. doi: 10.1016/s0378-3782(97)00082-0. doi:10.1016/S03 78-3782(97)00082-0. [DOI] [PubMed] [Google Scholar]
- Varendi H, Porter RH, Winberg J. Attractiveness of amniotic fluid odor: Evidence of prenatal olfactory learning? Acta Paediatrica. 1996;85:1223–1227. doi: 10.1111/j.1651-2227.1996.tb18233.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8922088. [DOI] [PubMed]


