Abstract
Background
Right ventricular (RV) systolic dysfunction is a strong predictor of adverse outcomes in heart failure, yet quantitatively assessing the impact of therapy on this condition is difficult. Our objective was to compare the clinical significance of changes in RV echocardiographic indices in response to intensive medical treatment in patients admitted to the hospital with acute decompensated heart failure (ADHF).
Methods and Results
Serial comprehensive echocardiography was performed in 62 consecutive patients with ADHF, and adverse events (death, cardiac transplantation, assist device, heart failure rehospitalization) were prospectively documented. RV peak systolic strain was assessed using speckle-tracking longitudinal strain analysis as the average of the basal, mid-, and apical segment of the RV free wall. Other conventional parameters of RV function (RV fractional area change, RV myocardial performance index, tricuspid annular peak systolic excursion, and tissue Doppler peak tricuspid annular systolic velocity) were measured for comparison. In our study cohort [left ventricular ejection fraction, 26±10%; cardiac index, 2.0±0.6 L/(min · m2)], overall mean RV peak systolic strain was –14±4% at baseline and –15±4% at 48 to 72 hours (P=0.27). Among all the RV functional indices measured, only RV peak systolic strain at 48 to 72 hours was associated with adverse events (P=0.02). In particular, improvement in RV peak systolic strain after intensive medical treatment was associated with lower adverse events in this patient population (26% versus 78%; hazard ratio, 0.13; 95% CI, 0.02 to 0.84; P=0.02).
Conclusion
Dynamic improvement in RV mechanics in response to intensive medical therapy was associated with lower long-term adverse events in patients with ADHF than in patients not showing improvement.
Keywords: echocardiography, heart failure, hemodynamics, prognosis, right ventricle
Right ventricular (RV) systolic dysfunction is widely recognized as a strong and independent predictor of adverse outcomes in patients with heart failure.1–6 Particularly in patients with advanced heart failure, a reduction of RV systolic function more closely predicts impaired exercise capacity and poor survival than does left ventricular (LV) systolic function.2,4 The ability to track improvements in RV function with therapy may therefore be clinically relevant.
Conventional echocardiographic techniques, such as RV fractional area change (RVFAC), RV myocardial performance index (RVMPI), tricuspid annular plane systolic excursion (TAPSE), and tissue Doppler-derived peak systolic velocity measured at the tricuspid annulus (RV-Sa) all have been demonstrated to be reliable indicators for RV systolic dysfunction in stable patients with chronic heart failure.7–10 However, these measurements often are often limited by the complex RV geometry, insufficient endocardial border delineation, or marked dependence on sampling angle, and few studies have been conducted in the acute decompensated heart failure (ADHF) setting.11,12 In addition, their potential ability to identify and track patients amenable to improvement after medical therapy has not been examined.
One of the newer echocardiographic techniques to quantify regional myocardial function in a simple and angle-independent manner is 2D strain imaging. By providing a more direct assessment of RV mechanics, RV strain imaging has the potential to better track responses to therapy. The primary goal of this study was to examine the prognostic value of changes in RV systolic performance assessed by 2D strain echocardiography, particularly RV peak systolic strain (RVPSS), compared with other conventional RV echocardiographic indices in subjects with ADHF admitted to the hospital.
Methods
Study Population
We prospectively identified consecutive patients aged ≥18 years who were admitted to the hospital for intensive medical therapy for ADHF. Subjects were eligible if they met the following inclusion criteria: (1) impaired systolic function defined by an LV ejection fraction ≤35% for at least 6 months, (2) elevated filling pressures defined by a pulmonary capillary wedge pressure >18 mm Hg or a central venous pressure >8 mm Hg, and (3) New York Heart Association functional class III to IV symptoms. The exclusion criteria were (1) mechanical ventilation, (2) renal replacement therapy, (3) post cardiac transplantation, and (4) post tricuspid valve surgery. The Cleveland Clinic Institutional Review Board approved the study project, and oral and written informed consent was obtained from all subjects.
Study Design
Echocardiographic data were collected within 12 hours of admission (baseline) and at 48 to 72 hours after intensive medical therapy. The hemodynamic goals and pharmacological approach to IV therapy in the specialized heart failure intensive care unit have been described previously.13 Briefly, optimal hemodynamic response was defined as a decrease in pulmonary capillary wedge pressure to ≤18 mm Hg, decrease in central venous pressure to ≤8 mm Hg, and improvement in cardiac index to ≥2.2 L/(min · m2) while maintaining mean arterial pressure >65 mm Hg. To achieve the hemodynamic goals, most patients were treated with intravenous loop diuretics in combination with vasodilators (ie, sodium nitroprusside) or inotropic agents (milrinone or dobutamine) while continuing or intensifying previous therapies with angiotensin-converting enzyme inhibitors, antiadrenergic blockers, aldosterone receptor antagonists, and other vasodilators as indicated and as tolerated. A prespecified end point was defined as the combined end point of all-cause mortality, heart transplantation, or first rehospitalization for worsening heart failure. All-cause mortality was determined using data documented in the electronic medical record and confirmed by the Social Security Death Index.
Transthoracic Echocardiography
Comprehensive 2D echocardiography was performed at bedside with a commercially available system. Standard 2D and Doppler echocardiography images were acquired in the left lateral decubitus position using a phased-array transducer in the parasternal and apical views. Three consecutive cardiac cycles were recorded and stored for subsequent offline analysis by 2 independent investigators experienced with echocardiographic measurements (A.B., D.V.). Both investigators were unaware of the time of registration, identity of subjects, or potential hemodynamic response to therapy.
Assessment of RV Function
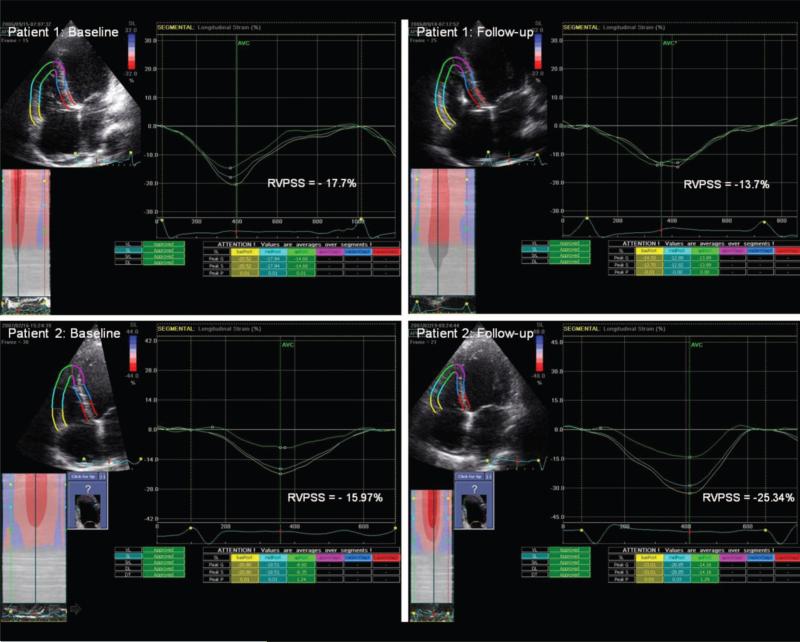
RV function was assessed by several methods. RV area was measured in end systole and end diastole in 4-chamber view, and RVFAC was calculated as the ratio of systolic RV area change to diastolic RV area. Maximal longitudinal tricuspid annular motion was measured with M-mode imaging (TAPSE). Pulsed-wave tissue Doppler measurements were recorded with the sample volume at the lateral tricuspid annulus, aligning the ultrasound beam as parallel as possible with the RV free wall, and RV-Sa was measured. The RVMPI (Tei index) was measured as the ratio of the isovolumic time intervals to RV ejection time.14 Finally, RV strain analysis to measure RVPSS was performed with 2D strain software (EchoPAC) using high frame-rate acquisitions (60 to 90 frames/s) of the RV free wall in the apical 4-chamber view. After tracing the endocardial contour of the RV free wall, the width of the region of interest was adjusted to fit the RV myocardial wall thickness. Natural acoustic markers, or speckles, were tracked throughout systole starting from tricuspid valve closure until end systole, which was defined by pulmonic valve closure. Peak longitudinal systolic strain was obtained for 3 RV wall segments (basal, mid, and apical). To ensure optimal accuracy and to get a more global assessment of RVPSS in subjects with advanced heart failure and possible segmental wall motion abnormalities, all analyses provided are based on the average of the 3 segments (Figure 1). RVPSS was measured in a control group of 44 subjects (22 men; mean age, 47±12 years) with no history of cardiovascular disease, hypertension, or diabetes; no cardiac medications; and a normal ECG to define normal values of this index.
Figure 1.
Representative example of changes in segmental RV free wall RVPSS in 2 patients with ADHF from baseline (worsened RVPSS at follow-up) (top) to follow-up (improved RVPSS at follow-up) (bottom).
Other Measurements
LV volumes and ejection fraction were calculated using Simpson's biplane method according to the guidelines of the American Society of Echocardiography.15 LV peak systolic global strain (LVPSS) was calculated with the same 2D strain software by averaging segmental (basal, mid, and apical) peak longitudinal strain values using high frame-rate acquisitions (60 to 90 frames/s) of the apical 4-chamber, 2-chamber, and long-axis views. The severity of mitral regurgitation and tricuspid regurgitation (TR) were graded semiquantitatively on a scale of 0 to 4 using the recommended integrative approach and quantitatively by measuring the width of the vena contracta.16 Interobserver variability for measuring RVPSS was tested in 46 studies (23 at baseline and 23 at 48 to 72 hours), and intraobserver variability was tested in 18 studies. Reproducibility was expressed as the SD of the difference between 2 paired measurements and as a percentage of variability (SD divided by the average value of the variable).
Statistical Analysis
All data are expressed as mean±SD for continuous data and as a ratio for categorical data. Univariable comparisons of these variables were performed between subjects with and without adverse events using paired and unpaired t tests for continuous data and Fisher exact test for categorical data. The strength of the relationship between 2 continuous variables was tested by Spearman rank correlation. The predictive value of RV functional indices (including RVPSS at baseline and follow-up and the difference between baseline and follow-up) was assessed by Cox proportional regression analysis, adjusting for age, ejection fraction, and severity of TR. Kaplan–Meier survival curves were constructed after dichotomizing the subjects based on the value corresponding to a point of maximum sensitivity-specificity product on a receiver operating characteristic curve. Statistical significance was set at a 2-tailed probability level of <0.05. All analyses were performed using SPSS for Windows, release 13.0. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
Baseline Characteristics
Of the 73 subjects who fulfilled the inclusion criteria and were prospectively approached, 5 refused to participate and 3 were unable to tolerate a comprehensive echocardiographic study due to their critical clinical state. The final data set included 65 subjects. Baseline characteristics, including medical therapy during hospital admission, are summarized in Table 1. The mean LVEF was 26±10%, and a high proportion of subjects received vasoactive medications as part of their medical regimen. Comprehensive baseline echocardio-graphic studies were performed in all 65 subjects at baseline. Studies at 48 to 72 hours could not be obtained in 3 subjects (1 subject withdrew consent, 1 subject underwent urgent cardiac transplantation, and 1 subject died between baseline and follow-up).
Table 1.
Baseline Characteristics and Vital Status (n=65)
| Parameter | Value |
|---|---|
| Age, y | 56±13 |
| Male, % | 79 |
| Weight, kg | 85±21 |
| Medical history, % | |
| Hypertension | 48 |
| Diabetes mellitus | 36 |
| Etiology of heart failure, % | |
| Idiopathic dilated | 48 |
| Ischemic | 52 |
| Baseline medical therapy, % | |
| ACE inhibitors/ARB | 74 |
| β-adrenergic blockers | 70 |
| Aldosterone antagonists | 45 |
| Loop diuretics | 95 |
| Digoxin | 45 |
| Hydralazine | 22 |
| Isosorbide dinitrate | 30 |
| Medical intervention at admission, % | |
| Milrinone | 39 |
| Dobutamine | 27 |
| Sodium nitroprusside | 58 |
| Intraaortic balloon pump | 5 |
Data are presented as mean±SD unless otherwise indicated.
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Echocardiographic and Hemodynamic Measurements
Reliable measures for RV-Sa, TAPSE, RVFAC, RVMPI, RVPSS, and LVPSS could be obtained at baseline in 63 (97%), 63 (97%), 61 (94%), 61 (93%), 57 (86%), and 60 (92%) subjects, respectively. RVPSS and LVPSS could be measured both at baseline and 48 to 72 hours later in 49 and 56 patients of the 62, respectively, in whom both studies were performed (79% and 90%, respectively). Unobtainable RVPSS or LVPSS data were primarily due to visually unsatisfactory strain curves mostly caused by suboptimal 2D image quality.
Table 2 presents the clinical, hemodynamic, and standard echocardiographic measurements at baseline assessment stratified according to the presence or absence of adverse events during follow-up. Hemodynamic derangements, indices of LV remodeling, biochemical markers, and medication use were similar at baseline. Use of intracardiac defibrillator therapy was similar for both groups. Compared with subjects without events, subjects who experienced adverse events had greater severity of TR at baseline (P<0.001).
Table 2.
Baseline Clinical, Hemodynamic, and General Echocardiographic Variables
| Variable | No Adverse Events (n=29) | With Adverse Events (n=36) | P |
|---|---|---|---|
| Male, % | 79 | 80 | NS |
| Mean age, y | 57±14 | 55±12 | NS |
| ICD/CRT, % | 25/3.5 | 32/3 | NS |
| Serum creatinine, mg/dL | 1.36±0.7 | 1.45±0.7 | NS |
| NT-proBNP, median pg/mL (IQR) | 4214 (2322–6438) | 4582 (2164–10565) | NS |
| hsCRP, mg/dL | 23.9±25 | 19.4±21.7 | NS |
| Hemodynamic indices | |||
| HR, bpm | 83±16 | 80±16 | NS |
| Systolic BP, mm Hg | 107±15 | 106±23 | NS |
| Systolic PAP, mm Hg | 49±12 | 48±16 | NS |
| CVP, mm Hg | 13±5 | 13±6 | NS |
| PCWP, mm Hg | 21±7 | 21±8 | NS |
| PVR, dynes/cm5 | 240±128 | 198±106 | NS |
| Cardiac index, L/(min · m2) | 2.0±0.5 | 2.0±0.8 | NS |
| Echocardiographic indices | |||
| LVEDV, mL | 220±87 | 246±126 | NS |
| LV ejection fraction, % | 25±11 | 27±10 | NS |
| Tricuspid valve regurgitation grade, n | <0.001 | ||
| 0 | 11 | 4 | |
| 1 | 9 | 5 | |
| 2 | 7 | 11 | |
| 3 | 2 | 7 | |
| 4 | 0 | 9 | |
| Tricuspid regurgitation VC, mm | 2.24 | 5.04 | <0.001 |
| Mitral valve regurgitation grade, n | NS | ||
| 0 | 7 | 5 | |
| 1 | 8 | 10 | |
| 2 | 6 | 11 | |
| 3 | 3 | 6 | |
| 4 | 5 | 4 | |
| Mitral regurgitation VC, mm | 3.47 | 3.88 | NS |
Data are presented as mean±SD unless otherwise indicated. BP indicates systemic arterial pressure; CVP, central venous pressure; HR, heart rate; hsCRP, high-sensitivity C-reactive protein; ICD/CRT, intracardiac defibrillator/cardiac resynchronization therapy; IQR, interquartile range; LVEDV, left ventricular end-diastolic volume; NT-proBNP, N-terminal pro-brain natriuretic peptide; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonic vascular resistance; VC, vena contracta width.
Indices of RV Function and Outcomes
Overall, LV or RV function assessed at baseline by different echocardiographic indices was similar in subjects with adverse events versus without (Table 3). The mean RVPSS for the whole study population was –14±4% at baseline and –15±4% at 48 to 72 hours (P=0.27). Intra- and interobserver variability for RVPSS in this cohort were 0.51±1.97 (–3±16%) and 0.15±1.04 (–1± 8%), respectively. In the control group, RVPSS was –28.8±2.5%. Mean LVPSS for the whole study cohort was –6.4±2.7% at baseline and showed a small, but significant absolute increase to –7.2±3% at 48 to 72 hours (P=0.005).
Table 3.
RV and LV Echocardiographic Indices at Baseline and Follow-Up
| Baseline |
Follow-Up |
|||||
|---|---|---|---|---|---|---|
| Variable | No Adverse Events (n=29) | With Adverse Events (n=36) | P | No Adverse Events (n=28) | With Adverse Events (n= 34) | P |
| RVPSS, % | –15±4 | –14±5 | 0.57 | –17±4 | –13.5±5 | 0.02 |
| RVFAC, % | 31±8 | 30±8 | 0.5 | 32±8 | 29±7 | 0.75 |
| TAPSE, mm | 14±4 | 14±4 | 0.9 | 15±4 | 14±5 | 0.6 |
| RV-Sa, cm/s | 10±4 | 10±4 | 0.46 | 11±4 | 10±3 | 0.86 |
| RV-MPI | 0.9 | 1.07 | 0.1 | 1.03 | 1.02 | 0.94 |
| LVPSS, % | –6.9±2.5 | –5.9±2.7 | 0.15 | –7.5±2.3 | –6.9±3.4 | 0.44 |
Data are presented as mean±SD.
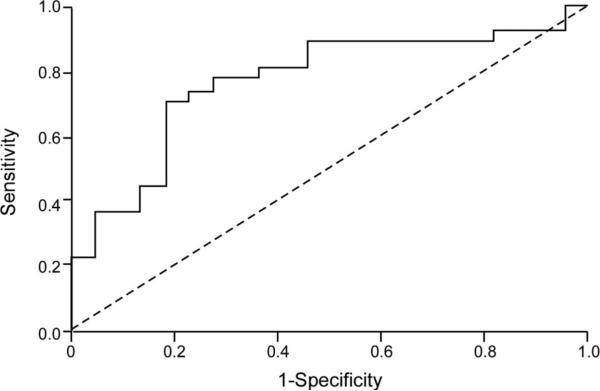
At the end of the follow-up period (mean follow-up duration 7.5±6 months), 12 (18.5%) subjects died, 13 (20%) underwent cardiac transplantation, and 13 (20%) were rehospitalized for recurrent decompensated heart failure. As illustrated in Table 3, only RVPSS at 48 to 72 hours was significantly associated with adverse outcome (–13.5±5 versus –17±4% for subjects with and without adverse events, respectively; P=0.02). In particular, when baseline RVPSS was compared with RVPSS at 48 to 72 hours, we found that subjects who demonstrated an absolute increase in RVPSS at 48 to 72 hours had a better outcome compared with those without improved RVPSS at 48 to 72 hours, even after adjusting for age, ischemic etiology, and severity of TR (26% versus 78%; hazard ratio, 0.13; 95% confidence interval 0.02 to 0.84; P=0.03) (Figure 2). In contrast, changes of the more conventional RV echocardiographic indices (TAPSE, RV-Sa, RVMPI, and RVFAC) were not related with adverse events (Table 4). In 13 patients, RVPSS could not be analyzed either at baseline or at follow-up because of technical difficulties. Of these 13 patients, 4 died and 2 underwent heart transplantation. The average duration of follow-up in the groups with and without improved RVPSS at follow-up were 299±229 days and 197±184 days, respectively. Baseline characteristics (including use of inotropic and vasodilator therapy) were not significantly different between patients with or without improved RVPSS at 48 to 72 hours follow-up. Using receiver operating characteristic curve analysis, we observed that both RVPSS at 48 to 72 hours (area under curve, 0.690; P=0.004) and change of RVPSS from baseline (area under curve, 0.769; P<0.001) predicted adverse events. To look at it in a different manner, worsening of RVPSS at 48 to 72 hours had a 70% sensitivity and 82% specificity to predict adverse events (Figure 3). Spearman rank correlation did not show a correlation between any of the RV functional indices at baseline or follow-up and the severity of TR. Figure 1 illustrates a patient with worsened RVPSS (top) and another with improved RVPSS (bottom) at follow-up.
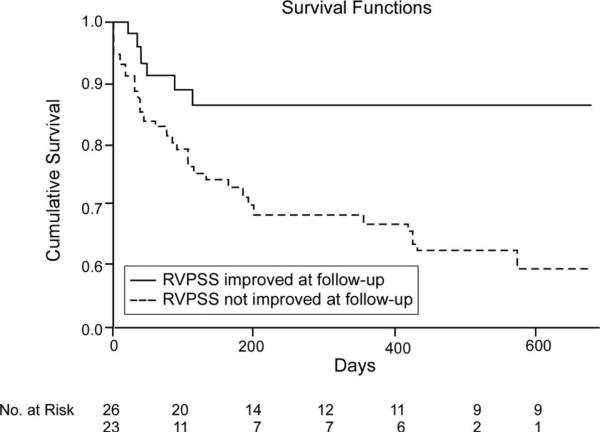
Figure 2.
Kaplan–Meier analysis for changes in RVPSS in ADHF stratified by subjects with improved (n=26) versus worsened or unchanged (n=23) RVPSS (log-rank P=0.004).
Table 4.
Multivariable Cox Proportional Regression Analysis of Changes in RV and LV Echocardiographic Indices After Intensive Medical Treatment
| Hazard Ratio* | 95 % CI | P | |
|---|---|---|---|
| Changes in RVPSS | 0.14 | 0.02–0.9 | 0.04 |
| Changes in RVFAC | 0.38 | 0.03–3.3 | 0.4 |
| Changes in TAPSE | 0.66 | 0.15–3 | 0.8 |
| Changes in RV-Sa | 0.86 | 0.1–6.9 | 0.88 |
| Changes in RV-MPI | 1.45 | 0.25–10 | 0.7 |
| Changes in LVPSS | 1.88 | 0.23–14 | 0.54 |
Adjusted for age, ischemic etiology, and severity of tricuspid regurgitation.
Figure 3.
Receiver operator curve showing lack of improvement in RVPSS, despite intensive medical therapy, to be highly associated with adverse events during follow-up (death, cardiac transplantation, and heart failure rehospitalization).
Discussion
The primary finding from this prospective evaluation of RV responses to intensive medical therapy is that the ability to improve RV performance assessed by an integral physiological assessment of RV mechanics portends a better prognosis in the setting of ADHF. These observations also highlight the important contributions of RV function in maintaining clinical compensation.
Our data provide direct evidence to support the notion that the ability of RV function to improve in response to therapy may directly affect the clinical course for patients with ADHF. Diuretics and vasodilator therapy play a central role in the treatment of patients with ADHF.13 Particularly in those with dilated cardiac chambers, an acute vasodilator response can provide not only relief in venous congestion through reduction in filling pressures, but also an indirect increase in cardiac output by reducing LV forward impedance and backward valvular regurgitation.13,17 Most research has focused on improving LV performance, and the return to a more normal hemodynamic profile has been attributed to a restoration of optimal LV filling pressure and cardiac output, which also was demonstrated by an increase in LVPSS observed in this study. However, as RV systolic function can be highly sensitive to changes in RV afterload, it is conceivable that the response to ADHF therapy might indirectly improve RV loading conditions, and this ultimately may lead to an increase in RV systolic performance and has been the premise for effective therapies for end-stage pulmonary hypertension. Our findings are concordant with previous investigations that demonstrated that the lack of response of RV function to dobutamine is associated with worse outcomes in patients with severe chronic heart failure.18,19 Clearly, the underlying mechanisms are complex. A suboptimal RV free wall systolic response to therapy may be derived from either intrinsic RV systolic dysfunction (from previous infarction or ischemia or in the context of idiopathic cardiomyopathy) or an overall suboptimal initial hemodynamic response to the medical therapy. This may even be independent of baseline RV dysfunction or hemodynamic derangements at the height of congestion, as we observed in our series. Contrary to previous reports, none of the RV function indices evaluated at baseline (including RVPSS) were associated with adverse outcome in our study population, a finding that may be attributed to the advanced disease state and the decompensated nature of the population studied.
Although strain is a load-dependent index of contractile function, it has an advantage over velocity and displacement-based indices of being less dependent on tethering and translational effects. These effects play a role not only in ischemic heart disease, but also in idiopathic dilated cardiomyopathy where echocardiography frequently reveals a swinging motion of the heart (also referred to as longitudinal rotation20), which may influence indices like TAPSE and RV-Sa. Problems with endocardial border delineation may have limited the reliability of RVFAC in assessing sometimes more subtle RV function changes secondary to therapy. In contrast, 2D strain is unaffected by changes in insonation angle or border delineation. Although the technical feasibility of measuring RV strain was lower compared with the more conventional indices of RV function, RVPSS may have been more sensitive in picking up time-dependent segmental systolic dysfunction through integration of contractility data from 3 different segments.
The importance of TR is also worth some discussion, as TR was shown to be significantly worse in patients with adverse events. Although TR may initially augment most RV functional parameters by causing a decrease in RV afterload, it may eventually have an opposite effect by the detrimental effects of longstanding RV volume overload, which may explain our observation of an absent correlation between indices of RV function and TR severity (in a similar manner with the lack of a tight association between LV systolic function and mitral regurgitation severity). TR is a common finding in patients with advanced systolic heart failure, and its poor prognosis in this setting has been shown repeatedly by other investigators.21,22 The correlation between TR and adverse outcomes in patients with ADHF may represent the longstanding consequences of pulmonary venous hypertension in patients with high LV filling pressures independent of reversibility of intrinsic RV function.
Study Limitations
Although our study demonstrated the feasibility of measuring RV strain and that inter- and intraobserver variability were limited, variability can still be of concern given the small changes in RVPSS between early and follow-up studies. The direct clinical application of RVPSS is limited by the requirement of good-quality recordings of the RV free wall and high enough frame rates as well the labor-intensive postprocessing analyses necessary to generate the data. However, novel software tools may eventually allow for a semiautomatic assessment of 2D strain that might overcome part of this problem. Although most subjects were monitored by a pulmonary artery catheter on hospital admission, follow-up hemodynamic data were missing in a subset of subjects due to study logistics as part of clinical care so that the relationship between changes in RVPSS and hemodynamic measures cannot be carefully examined.
Given our small sample size, heart transplantation was included as a clinical end point. Although transplantation was urgent in the majority of patients, this can be considered a limitation because the timing of transplantation often depends on factors unrelated to the patient with heart failure. The 1-year event-free survival (freedom from death, heart transplantation, or LV assist device) in our population was ≈50%. Our aim was to detect predictors associated with at least a 2-fold increase of death, heart transplantation, or LV assist device. Given a sample size of 65 subjects, we had a power of 79% to detect presence of such predictors at the double-sided P level of 0.05. Nevertheless, given the limited size of our study cohort, further studies addressing the clinical relevance of dynamic RV functional changes in patients with ADHF are needed. Future research also should determine whether an acute improvement in RV function in response to intensive medical therapy eventually is sustained at a much longer follow-up. Finally, our patients all come from a single tertiary-care center and by no means represent the full spectrum of heart failure pathology. It is interesting to speculate, however, on the importance of chronic changes in RV function in patients with less advanced disease. Other imaging modalities (cardiac MRI in particular) potentially could play important roles in tracking these long-term RV functional changes, specifically in more stable patients.
Conclusions
In patients with ADHF, the presence of dynamic improvement in RV mechanics (assessed by RVPSS using 2D strain echocardiography) in response to intensive medical therapy was associated with lower long-term adverse events than those without improvement.
Acknowledgments
Sources of Funding
This research was supported in part by an American Society of Echocardiography Sonographers’ grant (to Dr Borowski) and the National Institutes of Health Clinical and Translational Science Award (CTSA UL1-RR024989).
Footnotes
Disclosures
None.
References
- 1.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 2.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 3.Field ME, Solomon SD, Lewis EF, Kramer DB, Baughman KL, Stevenson LW, Tedrow UB. Right ventricular dysfunction and adverse outcome in patients with advanced heart failure. J Card Fail. 2006;12:616–620. doi: 10.1016/j.cardfail.2006.06.472. [DOI] [PubMed] [Google Scholar]
- 4.Gavazzi A, Berzuini C, Campana C, Inserra C, Ponzetta M, Sebastiani R, Ghio S, Recusani F. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant. 1997;16:774–785. [PubMed] [Google Scholar]
- 5.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 6.Juilliere Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–280. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 7.Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, Gavazzi A, Tavazzi L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–842. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 8.Meluzin J, Spinarova L, Hude P, Krejci J, Dusek L, Vitovec J, Panovsky R. Combined right ventricular systolic and diastolic dysfunction represents a strong determinant of poor prognosis in patients with symptomatic heart failure. Int J Cardiol. 2005;105:164–173. doi: 10.1016/j.ijcard.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Samad BA, Alam M, Jensen-Urstad K. Prognostic impact of right ventricular involvement as assessed by tricuspid annular motion in patients with acute myocardial infarction. Am J Cardiol. 2002;90:778–781. doi: 10.1016/s0002-9149(02)02612-7. [DOI] [PubMed] [Google Scholar]
- 10.Sun JP, James KB, Yang XS, Solankhi N, Shah MS, Arheart KL, Thomas JD, Stewart WJ. Comparison of mortality rates and progression of left ventricular dysfunction in patients with idiopathic dilated cardiomyopathy and dilated versus nondilated right ventricular cavities. Am J Cardiol. 1997;80:1583–1587. doi: 10.1016/s0002-9149(97)00780-7. [DOI] [PubMed] [Google Scholar]
- 11.Greil GF, Beerbaum P, Razavi R, Miller O. Imaging the right ventricle: non-invasive imaging. Heart. 2008;94:803–808. doi: 10.1136/hrt.2005.079111. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 13.Mullens W, Abrahams Z, Francis GS, Skouri HN, Starling RC, Young JB, Taylor DO, Tang WH. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol. 2008;52:200–207. doi: 10.1016/j.jacc.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 14.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–847. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson LW, Bellil D, Grover-McKay M, Brunken RC, Schwaiger M, Tillisch JH, Schelbert HR. Effects of afterload reduction (diuretics and vasodilators) on left ventricular volume and mitral regurgitation in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1987;60:654–658. doi: 10.1016/0002-9149(87)90376-6. [DOI] [PubMed] [Google Scholar]
- 18.Gorcsan J, III, Murali S, Counihan PJ, Mandarino WA, Kormos RL. Right ventricular performance and contractile reserve in patients with severe heart failure. Assessment by pressure-area relations and association with outcome. Circulation. 1996;94:3190–3197. doi: 10.1161/01.cir.94.12.3190. [DOI] [PubMed] [Google Scholar]
- 19.Otasevic P, Popovic Z, Pratali L, Vlahovic A, Vasiljevic JD, Neskovic AN. Right vs. left ventricular contractile reserve in one-year prognosis of patients with idiopathic dilated cardiomyopathy: assessment by dobutamine stress echocardiography. Eur J Echocardiogr. 2005;6:429–434. doi: 10.1016/j.euje.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Popovic ZB, Grimm RA, Ahmad A, Agler D, Favia M, Dan G, Lim P, Casas F, Greenberg NL, Thomas JD. Longitudinal rotation: an unrecognised motion pattern in patients with dilated cardiomyopathy. Heart. 2008;94:e11. doi: 10.1136/hrt.2007.122192. [DOI] [PubMed] [Google Scholar]
- 21.Hung J, Koelling T, Semigran MJ, Dec GW, Levine RA, Di Salvo TG. Usefulness of echocardiographic determined tricuspid regurgitation in predicting event-free survival in severe heart failure secondary to idiopathic-dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol. 1998;82:1301–1303. doi: 10.1016/s0002-9149(98)00624-9. [DOI] [PubMed] [Google Scholar]
- 22.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144:524–529. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]