Abstract
Background
IgE plays an essential role in type I allergy, however there is less information about the relationship between other immunoglobulins (IgA and IgG) and atopic phenotypes in early childhood.
Hypothesis
We hypothesized that levels of circulating IgA in early childhood would be inversely related to the number of respiratory infections and the risk of becoming sensitized to allergens.
Methods
Immunoglobulin levels were analyzed (ELISA) in plasma samples (IgG, IgA), and in nasal secretions (IgA) from children participating in a high-risk birth cohort study. Samples were available from 264 children at age 2 years and 257 children at age 4 years, and results were compared to rates of respiratory illnesses, allergic sensitization, atopic dermatitis, and asthma.
Results
Children who were sensitized to allergens had higher rather than lower levels of circulating IgA. A subgroup analysis showed that IgA levels were increased in relationship to foods sensitization (58 vs. 50 mg/dL, p = 0.003) but not aeroallergen sensitization (52 vs. 53 mg/dL, p = 0.11). IgA levels in the plasma correlated with levels of IgE levels (rs=0.19, p=0.003). Levels of IgE, but not IgG or IgA, were positively correlated with rates of respiratory illnesses, atopic dermatitis, and the risk of developing asthma. Finally, there were no significant relationships between IgA in nasal secretions and infectious outcomes.
Conclusions
In conclusion, low-normal concentrations of plasma IgA are associated with a reduced prevalence of allergic sensitization in infancy. Further, levels of IgA and IgG in plasma within the range of normal, and IgA in nasal secretions, do not appear to influence the risk of subsequent respiratory illnesses. Further studies to define relationships between IgA and allergic sensitization are likely to provide new insights into the pathogenesis of allergic diseases in infancy.
Keywords: IgA, IgE, respiratory illnesses, allergy, infants
INTRODUCTION
Immunoglobulin E (IgE) antibody, found on the surface of the plasma membrane of basophils and mast cells, is necessary for Type I hypersensitivity reactions. Less is known about the relationships of allergy to immunoglobulin A (IgA) and immunoglobulin G (IgG), which are better known for their roles in host defense. There is some evidence that IgA deficiency promotes allergic sensitization. Although the mechanism is not well established, IgA may competitively bind to allergens, thereby preventing the allergen from encountering other immunologically active factors. Therefore, deficiency of allergen-specific IgA could promote allergen-specific immune activation, and in susceptible individuals lead to allergic sensitization and clinical allergy. This concept is controversial, as different groups have found evidence in support of (1–6) and against this theory (7–9).
Absence (e.g. X-linked agammaglobulinemia) or functional impairment (e.g. common variable immunodeficiency) of IgG and IgA is associated with a markedly increased risk of infection. On the other hand, the main tenet of the original “hygiene hypothesis” is that exposure to frequent infections in early childhood has beneficial effects on the developing immune system to promote health later in childhood (10). This theory raises the possibility that early respiratory infections could accelerate immune development by boosting immunoglobulin synthesis. There are few studies, however, that have tested for developmental relationships between individual variations in IgA or IgG levels and the risk of respiratory infections.
The goals of this study are to explore the possibility of an immunological interplay between IgA and IgG levels in early childhood, allergic sensitization, and the incidence of respiratory infections. We hypothesized that levels of circulating IgA in early childhood would be inversely related to the number of respiratory infections and the risk of becoming sensitized to allergens. To address these questions, we compared plasma IgG and IgE levels and both plasma and nasal IgA levels to respiratory infections and atopic outcomes in a prospective birth cohort (the Childhood Origins of Asthma, or “COAST” study group), which includes children at increased risk for allergies and asthma on the basis of parental history.
METHODS
Study population and experimental design
After obtaining informed consent, 289 newborns were enrolled from November 1998 through May 2000 in the COAST study, a prospective study of children at high risk for developing asthma/allergies. Of these children, 281 were followed prospectively for at least 2 years, and 265 were followed for 4 years. To be eligible, at least one parent was required to have respiratory allergies (defined as 1 or more positive aeroallergen skin test) and/or a history of physician-diagnosed asthma, be delivered at ≥37 weeks gestation, and lack significant respiratory difficulties or congenital anomalies. Details of the study population and design have been described previously (11). The University of Wisconsin Human Subjects Committee approved this study.
Collection of blood samples
Peripheral blood samples (2.5–10 mL) were collected at age 2 and 4 years. The blood was collected in sterile heparinized tubes, kept at room temperature, and then processed on the day of collection. During processing, the plasma was stored at −80°C in labeled microcentrifuge tubes. 238 participants in the COAST study had a 2 year blood sample and 220 had both a 2 and 4 year blood sample.
Collection of nasopharyngeal mucus samples
Nasopharyngeal mucus samples were collected during the participants regularly scheduled 24 month clinical visit. The collection and handling methods of these samples have been described previously (12). Only samples from children with no reported illnesses during these scheduled clinical visits were used for analysis.
Nasal samples were also collected during periods of illnesses, and subjected to analysis with viral diagnostics, as previously reported (13;14). The viral recovery rate during respiratory illnesses ranged from 70–90%, depending on age, season, and illness severity. The viruses most commonly detected were rhinoviruses, respiratory syncytial virus, and parainfluenzaviruses.
IgG and IgA
Concentrations of IgA and IgG in plasma and secretory IgA in nasal wash were measured by ELISA (Human IgA/IgG Enzyme Linked Immune Sorbent Assays, Bethyl Laboratories, Montgomery, TX). The coating antibody for IgA is an anti-human antibody directed at the alpha chain, enabling detection of all IgA, including monomeric, polymeric and secretory IgA. Two dilutions were prepared for each sample (1:1000, 1:10,000 in sample diluent for IgA and 1:10,000 and 1:100,000 in sample diluent for IgG) in order to fall within the range of detection for the standards (7.8–500 ng/mL); duplicate wells were run for each sample and mean values were reported. For measurement of secretory IgA, the nasal lavage samples were centrifuged (13,400 rpm for 10 min at room temperature) to remove debris, and two dilutions (1:100 and 1:1000) were analyzed for each sample.
Total and allergen-specific IgE
Fluoroenzyme immunoassays (FEIAs) were performed using an automated instrument (Unicap® 100, Pharmacia Diagnostics) to determine total and allergen-specific IgE levels in plasma. Allergen-specific IgE levels were determined for 2 species of dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), egg white, milk, peanut, Alternaria alternata, cat dander and dog epithelium. The sensitivity for detection of specific IgE was 0.35 kU/L and values ≥0.35 kU/L were considered positive. The sensitivity for detection of total IgE was 2 kU/L.
Respiratory illnesses
Parents were instructed to contact one of the study coordinators when their child developed a respiratory illness and a symptom scorecard (maximum score 31) was completed.(12) Symptoms were scored on the basis of the following scoring system: fever (≥37.7°C [100°F]) = 1 point; cough, mild = 1 point, moderate = 2 points, severe = 3 points; rhinorrhea, mild = 1 point, moderate to severe = 2 points; hoarseness = 1 point; duration of illness > 4 days =1 point; apnea = 3 points; wheezing = 5 points; retractions = 5 points; tachypnea = 5 points; cyanosis = 5 points. Illnesses with symptom scores ≥ 5 were classified as moderate to severe colds. A wheezing respiratory illness was defined as meeting one or more of the following criteria: 1) physician-diagnosed wheezing at an office visit; 2) an illness for which the child was prescribed short or long-acting β-agonists, and/or inhaled or oral corticosteroids, and/or long term controller medications such as cromolyn sodium or leukotriene inhibitors; or 3) an illness given the following specific diagnoses: bronchiolitis, wheezing illness, reactive airway disease, asthma, or asthma exacerbation. Illnesses in which no wheezing was reported but oral corticosteroids were administered for “croup” or a “barky cough” reported by the parent, were not considered to be wheezing illnesses.
Statistical analysis
Immunoglobulin levels are summarized by listing the median and 1st and 3rd quartiles, and respiratory illness frequencies are summarized with mean and standard deviation. Dichotomous parameters are summarized as percentages. Plasma immunoglobulins (IgA and IgG) were compared between year 2 and year 4 with paired t-tests. The relationships between plasma immunoglobulins and asthma, atopic dermatitis, and allergic sensitization were examined using analysis of variance (ANOVA) models. Immunoglobulin levels were log-transformed for these analyses. The relationships between immunoglobulins and sensitization were also examined by comparing sensitization rates among immunoglobulin quartiles using logistic regression models. Nasopharyngeal mucosal IgA was compared to allergic sensitization with the chi-square test for association. Associations between plasma immunoglobulins (IgA, IgG and IgE) and the frequency of respiratory illnesses were assessed using the Spearman rank correlation coefficient (rs). Respiratory illness frequency and nasal pharyngeal mucosal IgA were compared with the Kruskal-Wallis rank sum test. A 2-sided p-value of less than 0.05 was regarded as statistically significant.
RESULTS
Immunologic development in the first four years of life
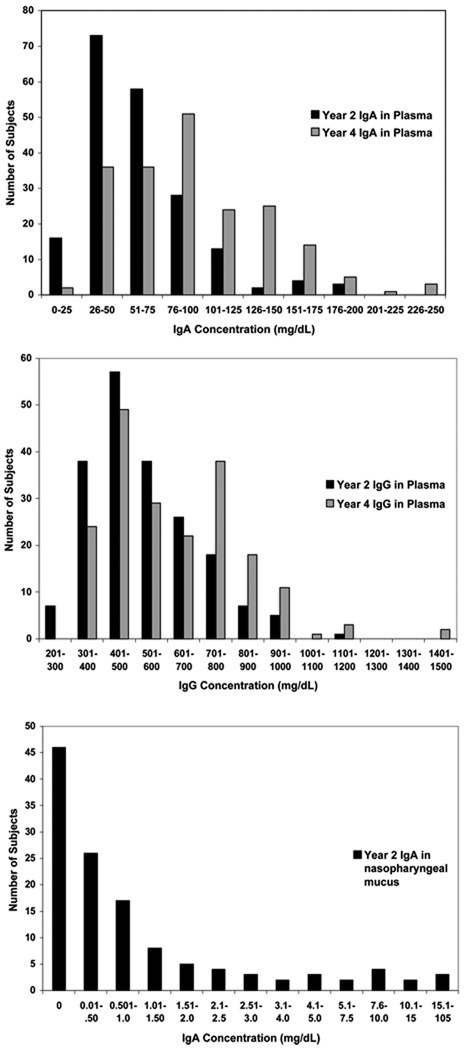
Total IgA was detected in all plasma samples, and median values increased (p<0.0001) from 53 [37,76] mg/dL at age 2 years to 90 [65,128] mg/dL at age 4 years (Fig 1A). Median plasma IgG increased (p<0.0001) from 492 [405, 617] mg/dL at age 2 years to 597 [440, 776] mg/dL at age 4 years (Fig 1B). IgA was below detection in 46 of the 127 nasopharyngeal mucus samples (Fig 1C), and the median value was 0.21 mg/dL. At age 2, there was a correlation between plasma total IgE and IgA (r = 0.19, p = 0.003), and a trend for a similar relationship between IgE and IgG (r = 0.12, p = 0.07).
Figure 1.
Distribution of immunoglobulin levels. These graphs depict histograms of plasma IgA (A), plasma IgG (B), and nasal lavage IgA (C) concentrations at 2 and 4 years of age. There were 238 plasma samples at age 2 years, 220 plasma samples at age 4 years, and 127 samples of nasal wash fluid.
Immunoglobulins in plasma and allergic sensitization
At age 2, children sensitized to any allergen had slightly elevated IgA levels (median 53 [44,80] vs. 51 [35,73] mg/dL, p=0.02, Fig 2A). Further analysis showed that sensitization to foods (58 [45,83] vs. 50 [35,73] mg/dL, p = 0.003), but not aeroallergens (52 [42,87] vs 53 [36,73] mg/dL, p = 0.11), was associated with elevated IgA levels (Fig 2B,C). Furthermore, children with the lowest quartile (Q) of IgA levels were least likely to be sensitized to foods (food sensitization rates 13%, 36%, 31% and 33% for Q1-4 respectively, p≤0.02 vs. quartile 1), but not aeroallergens (Table I). IgG levels at age 2 years were not related to allergic sensitization (Table I).
Figure 2.
IgA levels vs. patterns of allergic sensitization. Any food includes the allergens: egg white, milk and peanut. Any aeroallergen includes: cat dander, dog epithelium, alternaria alternate, d. farinae and d. pterynyssinus. The data were examined using ANOVA models.
Table I.
Allergic Sensitization at Age 2 Years According to Quartile of Serum IgA and IgG Levels
| IgA (mg/dL) |
n | Ag- Specific IgE |
P-value* vs. Lowest Quartile |
IgG | n | Ag- Specific IgE |
P-value vs. Lowest Quartile |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Any | Q1† | 12.0–37.0 | 61 | 23% | — | Q1 | 239.0–405.0 | 60 | 33% | — |
| Q2 | 37.1–52.5 | 58 | 48% | 0.005 | Q2 | 405.1–492.0 | 60 | 35% | 0.85 | |
| Q3 | 52.6–75.8 | 59 | 32% | 0.26 | Q3 | 492.1–616.5 | 58 | 24% | 0.27 | |
| Q4 | 75.9–186.0 | 60 | 40% | 0.05 | Q4 | 616.6–1140.0 | 60 | 50% | 0.07 | |
| Any Food | Q1 | 12.0–37.0 | 61 | 13% | — | Q1 | 239.0–405.0 | 60 | 28% | — |
| Q2 | 37.1–52.5 | 58 | 36% | 0.005 | Q2 | 405.1–492.0 | 60 | 28% | 1.00 | |
| Q3 | 52.6–75.8 | 59 | 31% | 0.02 | Q3 | 492.1–616.5 | 58 | 19% | 0.23 | |
| Q4 | 75.9–186.0 | 60 | 33% | 0.01 | Q4 | 616.6–1140.0 | 60 | 37% | 0.33 | |
| Any Aero-allergen | Q1 | 12.0–37.0 | 61 | 16% | — | Q1 | 239.0–405.0 | 60 | 15% | — |
| Q2 | 37.1–52.5 | 58 | 24% | 0.30 | Q2 | 405.1–492.0 | 60 | 20% | 0.47 | |
| Q3 | 52.6–75.8 | 59 | 12% | 0.48 | Q3 | 492.1–616.5 | 58 | 14% | 0.85 | |
| Q4 | 75.9–186.0 | 60 | 25% | 0.25 | Q4 | 616.6–1140.0 | 60 | 28% | 0.08 | |
| Egg | Q1 | 12.0–37.0 | 61 | 7% | — | Q1 | 239.0–405.0 | 60 | 17% | — |
| Q2 | 37.1–52.5 | 58 | 21% | 0.03 | Q2 | 405.1–492.0 | 60 | 22% | 0.49 | |
| Q3 | 52.6–75.8 | 59 | 24% | 0.01 | Q3 | 492.1–616.5 | 58 | 14% | 0.67 | |
| Q4 | 75.9–186.0 | 60 | 20% | 0.04 | Q4 | 616.6–1140.0 | 60 | 18% | 0.81 |
Logistic regression analysis
Abbreviation: Q, quartile.
Nasopharyngeal mucus samples from asymptomatic participants at the 24 month clinical visit were available for 127 subjects. At age 2 there were no significant associations between nasal IgA levels and allergic sensitization (Table II).
Table II.
Nasopharyngeal Mucus IgA and at Age 2 Years
| Year 2 Nasopharyngeal Mucus IgA |
n | Year 2 Allergen- Specific IgE (%positive) |
P value* |
|---|---|---|---|
| Any allergen | 0.63 | ||
| Undetectable | 46 | 18 (39%) | |
| Detectable, ≤ 1 mg/dL | 43 | 13 (30%) | |
| Detectable, > 1 mg/dL | 38 | 12 (32%) | |
| Any food | 0.62 | ||
| Undetectable | 46 | 15 (33%) | |
| Detectable, ≤ 1 mg/dL | 43 | 11 (26%) | |
| Detectable, > 1 mg/dL | 38 | 9 (24%) | |
| Any aero | 0.86 | ||
| Undetectable | 46 | 8 (17%) | |
| Detectable, ≤ 1 mg/dL | 43 | 7 (16%) | |
| Detectable, > 1 mg/dL | 38 | 5 (13%) |
Chi-square test
Immunoglobulins and atopic outcomes
We next compared serum immunoglobulin levels to the presence of active atopic dermatitis (AD) at ages 2 and 4 years, and to asthma at age 6 years. Neither IgA nor IgG levels at age 2 differed in children according to asthma status at age 6 or atopic dermatitis at 2 ad 4 years of age (data not shown). In contrast, IgE levels at age 2 were significantly increased in toddlers with AD at age 2 years (AD, 40 [20, 84]; no AD, 29 [11, 60]; p = 0.02), and in those who were later diagnosed with asthma at age 6 years (asthma, 58 [15, 141]; no asthma, 30 [13, 55]; p = 0.006).
Immunoglobulins and respiratory illnesses
To determine whether illnesses influenced immunoglobulin levels at age 2 years, we compared the frequency of illnesses from birth until age 2 years to plasma IgG and IgA, and to IgA in nasal wash. There were no significant associations between either moderate to severe respiratory illnesses or wheezing illnesses, and immunoglobulin levels at age 2 years (Table III).
Table III.
Correlations* Between Respiratory Illness Frequency and Immunoglobulin Levels
| Year 2 | Year 4 | ||||||
|---|---|---|---|---|---|---|---|
| IgA | IgG | IgE | IgA | IgG | |||
| Age 0–2 Years | |||||||
| MSI | 0.02 | 0.03 | −0.01 | ||||
| Wheezing Illnesses | 0.02 | 0.04 | 0.08 | ||||
| Age 2–4 Years | |||||||
| MSI | −0.01 | −0.08 | 0.11† | −0.05 | −0.08 | ||
| Wheezing Illnesses | 0.04 | 0.01 | 0.20‡ | 0.07 | 0.01 | ||
Abbreviation: MSI, moderate-to-severe illnesses
Spearman rank correlation coefficients
p = 0.07,
p = 0.002
Next, we tested whether immunoglobulin levels at age 2 were predictive for the frequency of illnesses in the next two years. IgG and IgA levels were not associated with subsequent moderate to severe respiratory illnesses, or wheezing illnesses. In contrast, age 2 year IgE levels were associated with increased numbers of subsequent wheezing illnesses in the third and fourth years of life (rs=0.20, p=0.002), and there was a trend for a similar relationship with moderate to severe respiratory illnesses (rs=0.08, p=0.07, Table III). Finally, age 2 year nasopharyngeal mucosal IgA concentrations were associated with neither illnesses from age 0–2 years, nor illnesses from age 2 to 4 years (Table IV).
Table IV.
Nasal Wash IgA at Age 2 Years and Symptomatic Outcomes
| IgA in Nasal Wash Fluid at Age 2 Years | |||||
|---|---|---|---|---|---|
| Undetectable (n=46) |
0.01 – 1 mg/dL (n=43) |
> 1 mg/dL (n=38) |
P Value† |
||
| Age 0–2 Years | |||||
| # of MSI* | 4.3 ± 3.1 | 3.6 ± 2.6 | 3.9 ± 2.5 | 0.49 | |
| Wheezing illness | 20 (43%) | 18 (42%) | 13_ (34%) | 0.66 | |
| Age 2–4 Years | |||||
| # of MSI | 1.7 ± 2.1 | 2.1 ± 3.5 | 1.5 ± 1.9 | 0.56 | |
| Wheezing illness | 15_ (33%) | 9 (21%) | 8_ (21%) | 0.35 | |
Abbreviation: MSI, moderate-to-severe illnesses. MSI values represent mean ± sd
Kruskal-Wallis rank sum test
DISCUSSION
This study was conducted to determine the relationship between concentrations of immunoglobulins and the development of allergic sensitization and respiratory illnesses in early childhood. Information regarding immune development and clinical outcomes was gathered prospectively from birth; enabling evaluation of the impact of past respiratory illnesses on immune development, and to also test whether concentrations of immunoglobulins predict the frequency of subsequent respiratory illnesses. Unexpectedly, our results indicate that low-normal concentrations of plasma IgA are associated with a reduced prevalence of allergic sensitization in infancy. Furthermore, respiratory illnesses prior to two years of age did not influence levels of immunoglobulins (IgA, IgG and IgE) at two years of age, and only IgE predicted wheezing illnesses in subsequent years.
We expected IgA to be inversely associated with the development of allergic sensitization; however low-normal concentrations of plasma IgA were associated with a reduced prevalence of allergic sensitization in the first 2 years of life. While IgA in plasma was positively related to allergic sensitization in general, food allergens appeared to drive this trend. These findings raise the question as to why low levels of IgA would be associated with lower, instead of higher rates of sensitization. It has been proposed that local inflammation, perhaps due to a reduction in local tolerance mechanisms and/or reduced barrier function, could promote the synthesis of food-specific IgE. Since B lymphocytes in Peyer’s patches and within the mucosal epithelium are important sources for the synthesis of both IgA and IgE (15), inflammatory responses to foods in the gut could serve to activate B cells committed to the IgA lineage through a bystander effect. Notably, total plasma IgE levels were more closely correlated with IgA (r = 0.19, p = 0.003) than with IgG (r = 0.12, p = 0.07).
Aeroallergen sensitivity was not associated with increased IgA. This could be because aeroallergen sensitivity is less common in early childhood. Alternatively, since there is quantitatively less lymphoid tissue in the respiratory tract compared to the GI tract, allergic inflammation of the respiratory tract may be less likely to affect systemic levels of IgA.
In contrast to our findings, Ludviksson et al reported that the frequency and severity of allergic manifestations in a cohort of 179 Icelandic children between 18–23 months of age were increased in children with low normal serum IgA (lowest quartile) (3). Although their findings seem to conflict with our own, there are substantial differences in the outcome measures. Ludviksson calculated a global allergy score from a structured questionnaire to obtain information on the frequency of wheezing attacks, the severity or persistence of atopic eczema, history of allergic reaction to food, urticara and the results from a skin prick test. In contrast, our main outcome was allergic sensitization as defined by the presence of allergen-specific IgE, and we considered wheezing illnesses and AD, which may or may not be manifestations of atopy in this age group, separately.
In addition, we found no evidence that respiratory illness history prior to and after 2 years of age influenced, or is influenced by, circulating IgA at age 2 years. Since IgA is the primary isotype of immunoglobulin at mucosal surfaces (16), we also tested for relationships between secretory IgA levels and frequency of illnesses. No relationships were found between 2 year nasopharyngeal mucus samples and either wheezing or respiratory illnesses between years 0 and 4 years. These findings imply that the quantity of IgA, within the range of normal, has little influence on the frequency of respiratory illnesses.
The COAST study was designed to evaluate how interactions between immune response and viral infections in early life affect the development of wheezing illnesses and eventually asthma, and the experimental design has several strengths, and some limitations, in this regard. The strengths of the study include its prospective nature, which enabled the measurement of immune response before and after viral infections and wheezing episodes, identification of specific viral pathogens, large sample size, and excellent retention (91.7% at year 4). One of the limitations of the study is that specimens for analysis of secretory IgA were obtained by nasal wash, and this procedure introduces a measurement error due to the uncertain dilution factor. In addition, this cohort includes only children from families in which at least one parent has allergies or asthma. Some previous studies have demonstrated immunologic differences between healthy infants born to atopic versus nonatopic parents (17–19). Therefore, our data must be interpreted keeping in mind that the healthy children in the COAST study, who serve as our comparator group, could be immunologically different than healthy children of parents without allergies or asthma. Nevertheless, determining risk factors for childhood allergy and asthma is perhaps of greatest interest to affected parents and high-risk families.
In conclusion, low-normal concentrations of plasma IgA are associated with a reduced prevalence of allergic sensitization in infancy. Further, levels of IgA and IgG in plasma within the range of normal, as well as IgA in nasal secretions, do not appear to influence the risk of subsequent respiratory illnesses. Further studies to define relationships between IgA and allergic sensitization are likely to provide new insights into the pathogenesis of allergic diseases in infancy.
References
- 1.Aghayan-Ugurluoglu R, Ball T, Vrtala S, Schweiger C, Kraft D, Valenta R. Dissociation of allergen-specific IgE and IgA responses in sera and tears of pollen-allergic patients: a study performed with purified recombinant pollen allergens. J Allergy Clin Immunol. 2000;105(4):803–813. doi: 10.1067/mai.2000.104782. [DOI] [PubMed] [Google Scholar]
- 2.Van Asperen PP, Gleeson M, Kemp AS, Cripps AW, Geraghty SB, Mellis CM, Clancy RL. The relationship between atopy and salivary IgA deficiency in infancy. Clin Exp Immunol. 1985;62(3):753–757. [PMC free article] [PubMed] [Google Scholar]
- 3.Ludviksson BR, Eiriksson TH, Ardal B, Sigfusson A, Valdimarsson H. Correlation between serum immunoglobulin A concentrations and allergic manifestations in infants. J Pediatr. 1992;121(1):23–27. doi: 10.1016/s0022-3476(05)82535-1. [DOI] [PubMed] [Google Scholar]
- 4.Thorarinsdottir HK, Ludviksson BR, Vikingsdottir T, Leopoldsdottir MO, Ardal B, Jonsson T, Valdimarsson H, Arason GJ. Childhood levels of immunoglobulins and mannan-binding lectin in relation to infections and allergy. Scand.J Immunol. 2005;61(5):466–474. doi: 10.1111/j.1365-3083.2005.01588.x. [DOI] [PubMed] [Google Scholar]
- 5.Payette K, Weiss NS. Salivary IgA levels in atopic children. Ann Allergy. 1977;39(5):328–331. [PubMed] [Google Scholar]
- 6.Stokes CR, Taylor B, Turner MW. Association of house-dust and grass-pollen allergies with specific IgA antibody deficiency. Lancet. 1974;2(7879):485–488. doi: 10.1016/s0140-6736(74)92014-5. [DOI] [PubMed] [Google Scholar]
- 7.Martino DJ, Currie H, Taylor A, Conway P, Prescott SL. Relationship between early intestinal colonization, mucosal immunoglobulin A production and systemic immune development. Clin Exp Allergy. 2008;38(1):69–78. doi: 10.1111/j.1365-2222.2007.02856.x. [DOI] [PubMed] [Google Scholar]
- 8.Bottcher MF, Haggstrom P, Bjorksten B, Jenmalm MC. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin Exp Allergy. 2002;32(9):1293–1298. doi: 10.1046/j.1365-2222.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 9.Gleeson M, Clancy RL, Hensley MJ, Cripps AW, Henry RL, Wlodarczyk JH, Gibson PG. Development of bronchial hyperreactivity following transient absence of salivary IgA. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1785–1789. doi: 10.1164/ajrccm.153.6.8665035. [DOI] [PubMed] [Google Scholar]
- 10.Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000;55 Suppl 1:S2–S10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr.Allergy Immunol. 2002 Suppl 15:38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 12.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, Adler K, Gilbertson-White S, Hamilton R, Shult PA, et al. Relationships among specific viral pathogens, virus-induced interleukin- 8, and respiratory symptoms in infancy. Pediatr.Allergy Immunol. 2002;13(6):386–393. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 13.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans ME, Li Z, Shult P, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J.Allergy Clin.Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am.J.Respir.Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagarasan S. Evolution, development, mechanism and function of IgA in the gut. Curr Opin Immunol. 2008;20(2):170–177. doi: 10.1016/j.coi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208(2):270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 17.Miles EA, Warner JA, Lane AC, Jones AC, Colwell BM, Warner JO. Altered T lymphocyte phenotype at birth in babies born to atopic parents. Pediatr Allergy Immunol. 1994;5(4):202–208. doi: 10.1111/j.1399-3038.1994.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 18.Gold DR, Bloomberg GR, Cruikshank WW, Visness CM, Schwarz J, Kattan M, O'Connor GT, Wood RA, Burger MS, Wright RJ, et al. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. J Allergy Clin Immunol. 2009;124(5):1078–1087. doi: 10.1016/j.jaci.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353(9148):196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]