Abstract
The colloidal behavior of aqueous dispersions of functionalized multiwall carbon nanotubes (F-CNTS) formed via carboxylation and polymer wrapping with polyvinyl pyrrolidone (PVP) is presented. The presence of polymer on the nanotube surface provided steric stabilization, and the aggregation behavior of the colloidal system was quite different from its covalently functionalized analog. Based on hydrophobicity index, particle size distribution, zeta potential as well as the aggregation kinetics studied using time-resolved dynamic light scattering, the PVP wrapped CNT was somewhat less prone to agglomeration. However, its long term stability was lower, and this was attributed to the partial unwrapping of the polyvinyl pyrrolidone layer on the CNT surface.
Keywords: Carbon nanotubes, hydrophobicity, aggregation, stability
1. Introduction
As the applications of carbon nanotubes (CNTs) proliferate, mass production and widespread use of these nanocarbons will continue to rise. In addition to the likelihood of occupational exposure, there is the potential for environmental contamination. While raw, unrefined, and hydrophobic carbon nanotubes tend to settle out of aqueous media/environs into solid phases such as river sediments [1], water dispersible, F-CNTs will contaminate water resource and will also be highly bioavailable on exposure. Therefore there is a need to develop an understanding of the fate of F-CNTs in aqueous media.
Several different routes to aqueous dispersion of CNTs have been explored. The most common approach includes the use of surfactants, and the covalent functionalization of CNT surface using different oxidizing agents [2, 3]. In addition, non-covalent interactions with different groups have been utilized to generate water dispersible CNTs. Well established approaches include DNA wrapping [4–7] and composite formation with polymers such as polyvinyl pyrrolidone (PVP), polyvinyl alcohol (PVA), polyethylene glycol (PEG) and poly(ethylene oxide)-b-poly [2-(N,N-dimethylamino)ethyl methacrylate] (PEO-b-PDMA) [8–10].
The colloidal behavior of CNT dispersions is important in understanding their fate and transport in the aquatic environment, and several publications have addressed this issue [11–13]. Aggregation kinetics of CNTs has been studied using time-resolved dynamic light scattering (TRDLS), Raman spectroscopy, zeta-potential measurements, and UV-Visible spectroscopy [14, 15]. Precipitation of the carboxylated CNTs under the influence of different salts has shown that [16, 17] precipitation is dependent on the charge on the cations, and in general these nanoparticles have followed the well established Derjaguin-Landau-Verwey-Overbeek (DLVO) theory [18, 19].
The polymer-wrapped CNTs were prepared from purified MWCNTs which had no surface modification and therefore hydrophobic in nature. In an aqueous solution they tend to agglomerate via van der waals interactions. The grafting of the polymer on the surface eliminates these forces. Polyvinyl pyrrolidone is a highly water soluble polymer which has been non-covalently grafted onto the CNTs surface. The polymer is attached to the CNT surface at one end, and the long chains float in water repelling each other to prevent aggregation. In an aqueous environment, these hydrophilic molecules prefer to interact with water. Therefore, the presence of PVP on the CNT surface provides steric stabilization and the aggregation behavior of the colloidal system may be quite different from the carboxylated CNTs. Since the stabilization is not caused by the electric double layer, Zeta potential data for such systems provide limited information. It has been shown that while systems stabilized with ionic polymers tend to obey the DLVO theory, non-ionic polymeric systems with low zeta potential may exhibit high stability [20]. Therefore, the aggregation behavior of oxidized CNTs and those wrapped with a polymer are expected to differ due to their modes of stabilization. The water dispersibility of covalently functionalized CNTs originates from electrostatic repulsive forces between negative surface charges, whereas a polymer wrapped CNT is stabilized sterically by the presence of the hydrophilic polymer on the surface.
The objective of this paper is to investigate the aggregation behavior of polymer modified CNTs in an ionic environment and see how it differs from its carboxylated analog. Of particular interest is poly(vinylpyrrolidone) (PVP) wrapped MWCNTs, which have been used extensively as aqueous dispersible CNTs.
2. Experimental
2.1 Chemicals
Multiwall carbon nanotubes (OD 20–30nm, Purity 95%) were purchased from Cheap Tubes Inc., and all other chemicals were purchased from Sigma Aldrich with purity higher than 95%.
2.2 Preparation of the F-CNTs
Carboxylated multiwalled carbon nanotubes (MWCNT-COOH) was functionalized in a Microwave Accelerated Reaction System (Mode: CEM Mars) fitted with internal temperature and pressure controls according to an experimental procedures previously published by our laboratory [21, 22]. Pre-weighed amounts of purified MWCNT was treated with a mixture of concentrated H2SO4 and HNO3 solution by subjecting them to microwave radiation at 140°C for 20 min. This led to the formation of carboxylic groups on the surface leading to high aqueous dispersibility. The resulting solid was filtered through a 10µm membrane filter, washed with water to a neutral pH and dried under vacuum at 80°C to a constant weight.
MWCNT-PVP was prepared according to previously reported procedures [23]. Purified MWCNT was dispersed in deionized water at a concentration of 50mg l−1 with the aid of 1% sodium dodecyl sulfate (SDS) and 1% by weight of PVP was added to the mixture, which then was incubated at 50°C for 12 hours. The carbon nanotubes were then filtered through a 10µm membrane filter, washed with deionized water and this was followed by three cycles of ultrasonic redispersion in deionized water to remove any residual SDS. The sample was filtered and dried under vacuum at room temperature to a constant weight.
2.3 Characterization of The F-CNTs
The materials were characterized by scanning electron microscope (SEM), thermogravimetric analysis (TGA), and Fourier Transform Infrared spectroscopy (FTIR). SEM Data was collected on a LEO 1530 VP Scanning Electron Microscope equipped with an energy-dispersive X-ray analyzer. TGA was performed using a Pyris 1 TGA from Perkin-Elmer Inc from 30°C to 900°C under a flow of air at 10mL/min, at a heating rate of 10°C per min. FTIR measurements were carried out in purified KBr pellets using a Perkin-Elmer (Spectrum One) instrument.
2.4 Hydrophobicity and Stability Measurements
50mg l−1 stock solutions of MWCNT-COOH and MWCNT-PVP were prepared by sonicating pre weighed amounts of the F-CNTs in MilliQ water. Various concentrations of F-CNTs used in this study were then prepared by diluting the stock solution by ultrasonication. 400 mM stock solutions of sodium chloride (NaCl), sodium acetate (NaOAc) and magnesium chloride (MgCl2) were prepared by dissolving weighed amounts of salt in MilliQ water and dilution was carried out on a as needed basis.
Hydrophobicity of MWCNT-COOH and MWCNT-PVP were determined in MilliQ water, and in the presence of 25mM and 100mM salt solutions by measuring the UV absorbance at 252nm before and after extraction with 1-octanol. The stability of 25mg l−1 dispersions of the F-CNTs over a 24-hour period was determined in the presence of 25mM solutions of sodium chloride, sodium acetate and magnesium chloride using UV absorbance at 252nm. This was measured at 0, 1, 2, 4, 6, and 24hrs. Concentration of carbon nanotubes at each point in time was determined based on calibration standards.
2.5 Dynamic Light Scattering and Zeta Potential Measurements
The hydrodynamic diameter of 1mg L−1 dispersions of the F-CNTs were measured as a function of salt concentration at 25 °C using dynamic light scattering (Beckman Coulter N4 Plus submicron particle size analyzer, operated at 90° detector angle), where the salt concentrations ranged from 10 to 200 mM. Zeta potential of the F-CNT dispersions were measured at a concentration of 5 mg l−1 at 25 °C using a Malvern Instrument (Zetasizer nano ZS90). Measurements were made in MilliQ water and as a function of electrolyte concentration. Data on CNT aggregation was collected using a 1mg l−1 dispersions of MWCNT-COOH and MWCNT-PVP in the presence of electrolyte solutions whose concentrations ranged between 10 and 200 mM. The measurements were performed for a time period ranging from 180 s to 3 h.
3 Results and Discussions
3.1 Characterization
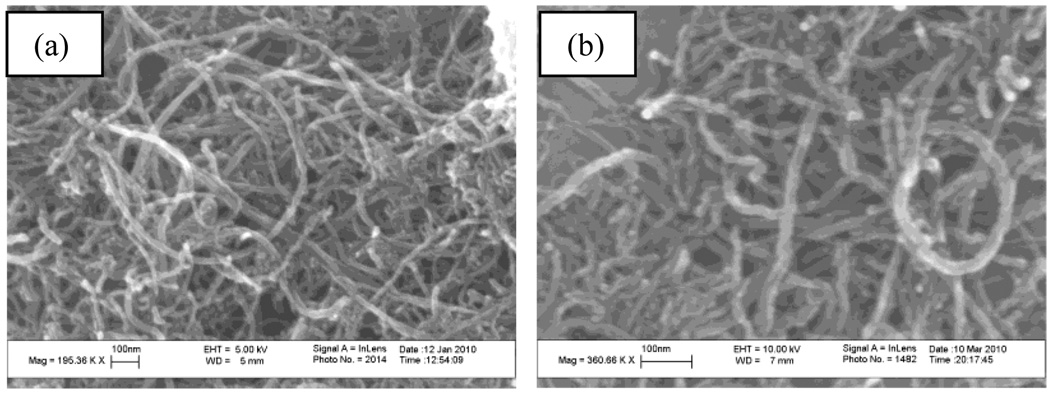
SEM images of MWCNT-COOH and MWCNT-PVP are presented in figure 1a and 1b. These show that the CNTs remained intact with minimal visible tube damage.
Figure 1.
Scanning Electron microscope images of (a) MWCNT-COOH and (b) MWCNT-PVP
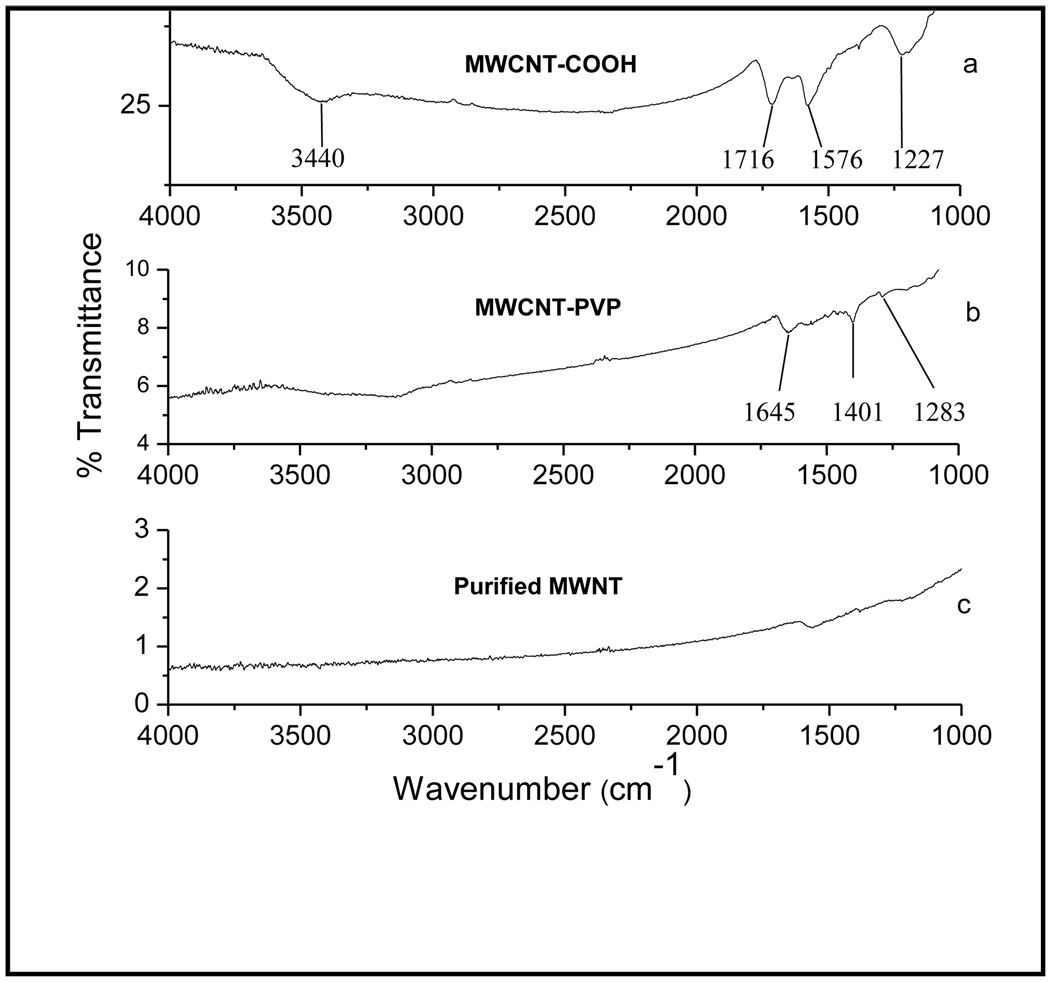
The IR spectrum (Figure 2) confirmed the presence of functional groups in the purified MWCNT, MWCNT-COOH and MWCNT-PVP. The carboxylic stretching frequency in MWCNT-COOH occurred at 1716 cm−1. The stretching (O-H) vibration occurred at 3440 cm−1 in the MWCNT-COOH spectrum (fig. 2a), which was clearly absent from the purified MWCNT spectrum (fig. 2c). The carbonyl stretching frequency from the amide group in PVP occurred at 1645 cm−1 (fig. 2b). In all the samples, the peak around 1576 cm−1 was assigned to the C=C stretching of the carbon skeleton.
Figure 2.
FTIR spectra of (a) MWCNT-COOH, (b) MWCNT-PVP and (c) purified MWCNT
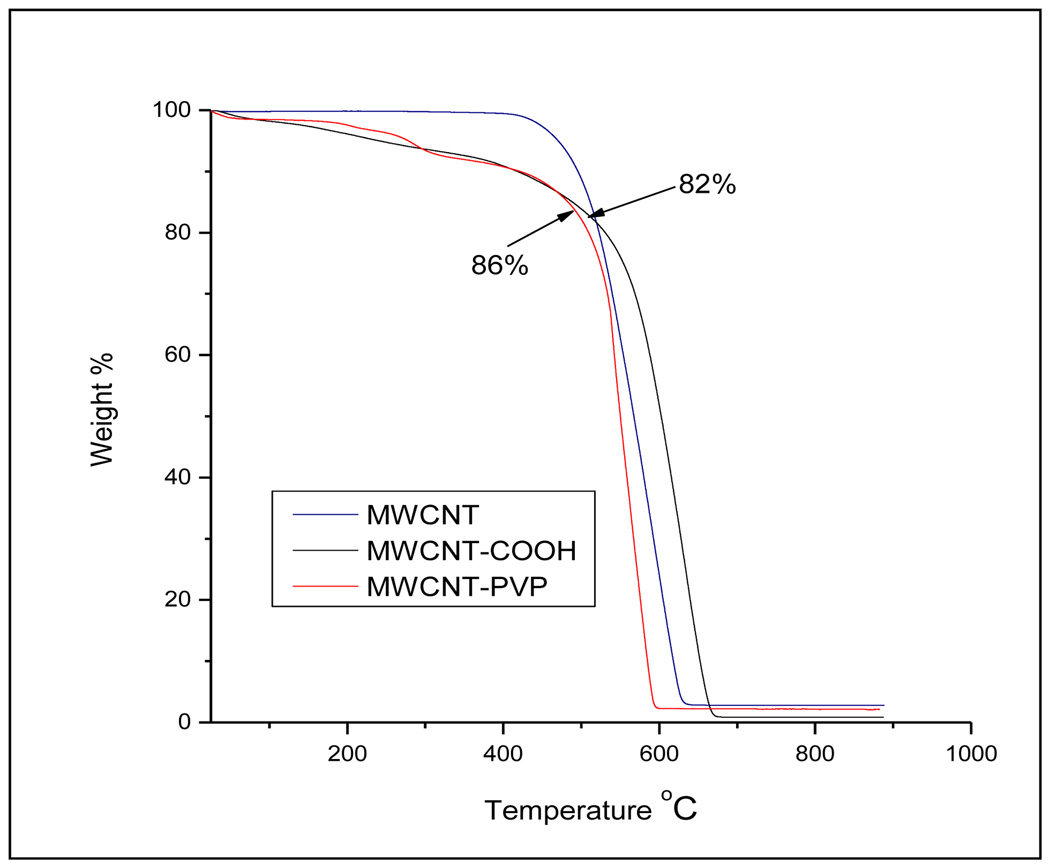
The TGA of MWCNT-PVP is presented in figure 3. It shows weight loss in the 200°C to 350°C region. This was attributed to the decomposition of the PVP wrapping on the CNT. A similar weight loss from 150°C to 400°C was observed in the MWCNT-COOH profile due to the loss of the carboxylic groups introduced through acid functionalization.
Figure 3.
TGA of the F-CNTs.
3.2 Hydrophobicity
1-octanol/water partitioning was used to establish a hydrophobicity index (HI) for the two MWCNT derivatives. This index was calculated based on UV-Visible absorption of the F-CNT dispersion (at 252 nm) in the original water and after 1-octanol extraction. The HI was computed as:
where, Ao is the UV-Visible absorbance of the F-CNTs before 1-octanol extraction and Ai is the absorbance after extraction (23). Visual inspection of the phase separation revealed that the tubes were homogeneously dispersed in the aqueous layer in both cases, and this is shown in figure 4. The hydrophilicity of both MWCNT-COOH and MWCNT-PVP agreed with the relatively high negative surface charge, and the zeta-potentials in water were −43.7 and −42.2 eV respectively. Although the zeta potential values of the aqueous dispersions of the two F-CNTs were similar, their HI were quite diverse (Table 1), making them fundamentally different materials. The results showed that both MWCNT-COOH and MWCNT-PVP had relatively low HI in deionized water, preferring to partition in the aqueous phase. Among the two, MWCNT-PVP was more hydrophilic.
Figure 4.
1-octanol extraction of MWCNT-COOH from A; deionized water, B; 25mM NaCl, C; 25mM NaOAc and D; 25mM MgCl2.6H2O.
Table 1.
Hydrophobicity Index
| Hydrophobicity Index % | ||||||
|---|---|---|---|---|---|---|
| DI Water | NaCl | NaOAc | MgCl2 | |||
| 25mM | 100mM | 25mM | 100mM | 25mM | ||
| MWCNT-COOH | −4.15 | 38.29 | 73.39 | 47.38 | 74.68 | 100 |
| MWCNT-PVP | −62.57 | 26.31 | 10.27 | 40.64 | 39.38 | 100 |
Table 1 compares the relative HI of the F-CNTs in deionized water, NaCl, NaOAc and MgCl2. HI indices for the F-CNTs in the presence of the divalent Mg salt were higher than in the monovalent Na which was consistent with the Schulze-Hardy Rule [13], where the critical coagulation concentration (CCC) depends upon the counterion valence z (ranges from −2 to −6). While NaCl is neutral in an aqueous environment (pKa 6.7–7.3), sodium acetate is basic (pKb 9.25). However MWCNT-COOH is expected to be dissociated completely at both pH values accounting for the similarity in their HI. The HI of the MWCNT-COOH in the monovalent Na salt solutions was generally higher than those of MWCNT-PVP. This was attributed to the presence of relatively weaker charges on the MWCNT-PVP surface for neutralization by the metal ions. On the other hand it is possible that PVP may partially unwrap [24] from the tube surface during 1-octanol extraction making the tubes hydrophobic, which may make them relatively insensitive to ionic strength.
It is known that the addition of an electrolyte to water changes the hydrogen-bonded structure of water. The effects of salt species in a polymer wrapped CNT depends on the interaction of the polymer with the solvent, the binding of water molecules to the polymer, changes in the hydrogen bonding and the alteration in the hydration sheath due to the added salt. PVP contains N-C=O units on the lactam rings, and these polar groups are involved in the association with water molecules by hydrogen bonding. The addition of salts into aqueous dispersion of MWCNT-PVP changed the association and/or hydration, and could disrupt the highly oriented water molecules surrounding the polymer leading to higher hydrophobicity [25]. MWCNT-PVP had a lower HI in the presence of sodium chloride than sodium acetate, which is consistent with previous reports where acetate ions were shown to be more effective in salting out PVP than the chloride [25].
3.3 Aggregation of F-CNTs
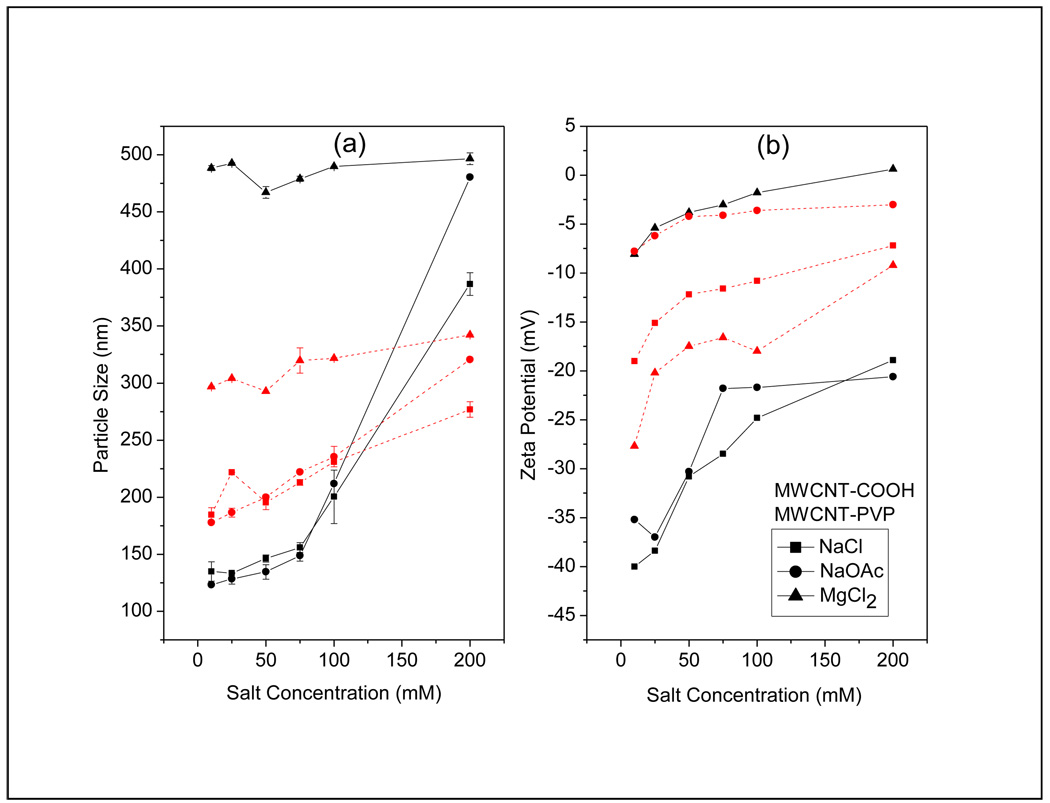
Figure 5a shows the particle size distribution of MWCNT-COOH and MWCNT-PVP in water as a function of salt concentration. Agglomeration led to large particle size, which increased with salt concentration. The two functional forms showed markedly different behavior. The water dispersibility of MWCNT-COOH originates from the negatively charged oxygen-containing groups on the CNT surface. In aqueous phase, the electrostatic repulsive forces between negative surface charges of the oxygen-containing groups may lead to stability of the MWCNT-COOH in water. In the presence of electrolyte solutions, positively charged metal ions neutralized the surface charges on the MWCNT-COOH surface, and compressed the double layer of the dispersed nanotubes, which lead to agglomeration. On the other hand, the CNTs in the MWCNT-PVP dispersion were sterically stabilized by the presence of the hydrophilic polymer on the surface. The diameter of the MWCNT-COOH increased gradually at low monovalent salt concentration, but the size increased rapidly past a concentration of 100mM (figure 5 a). This was not observed in the case of PVP wrapped CNTs, where the size increase was gradual even at high salt concentrations. The anions did not appear to have any effect on the stability of the dispersions because the agglomeration in presence of NaOAc was similar to that of NaCl. The presence of acetate did not lead to any common ion effect in the case of MWCNT-COOH. It is interesting to note that in the case of MWCNT-COOH, as compared to the monovalent Na salt, the divalent Mg led to a marked increase in the particle size (nearly 4 times). The difference between the mono and the divalent salts was not as pronounced in the case of MWCNT-PVP, implying that the aggregation behavior was not as dependent on counterion valence in accordance to the Schulze-Hardy Rule [13]. In general, these observations were consistent with the zeta potential of the F-CNTs dispersions (figure 5b), where an increase in salt concentration led to less negative values.
Figure 5.
(a)Particle size distribution and (b) zeta potential of the F-CNTs as a function of salt concentration
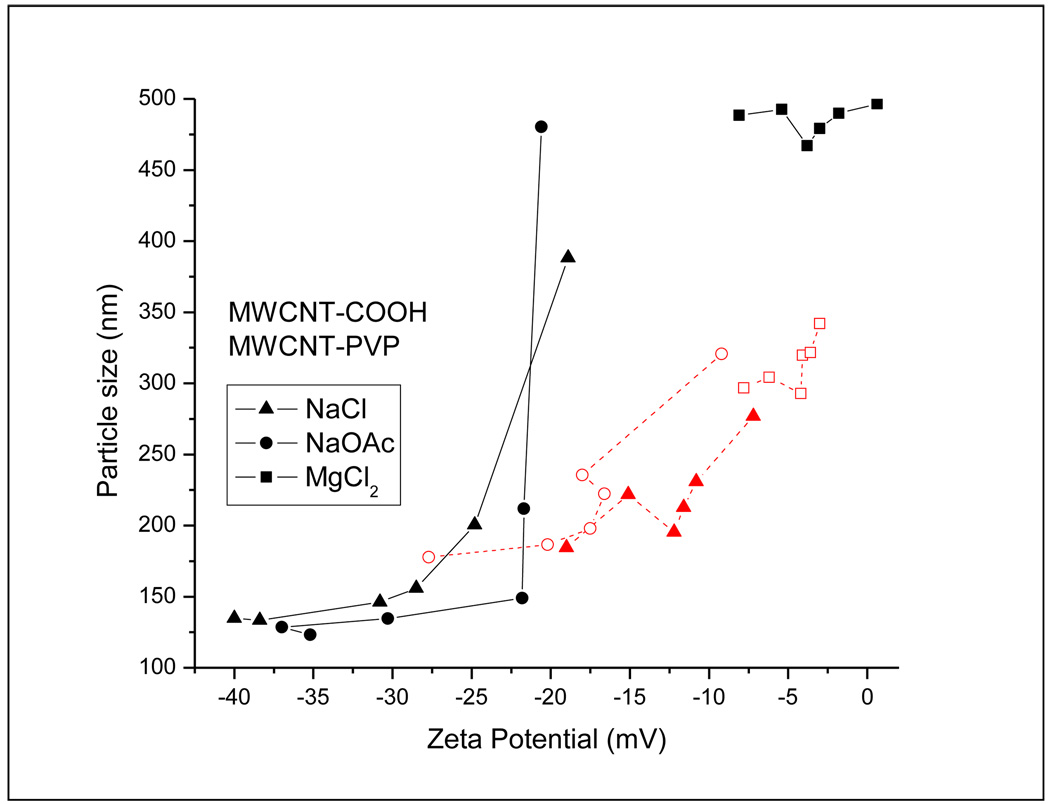
Figure 6 shows the particle size distribution as a function of zeta potential for the F-CNTs in the electrolyte media. It was observed that up to a point, the particle size of the F-CNTs generally increased as their zeta potential became less negative with increasing salt concentration. The size of the MWCNT-COOH showed a strong dependence on zeta potential in the presence of the sodium salts, where the particle size increased first linearly and then asymptotically. The effect was less pronounced in the case of MWCNT-PVP and significantly lesser in the presence of the Mg salt.
Figure 6.
Particle size distribution as a function of zeta potential in the presence of (a) sodium chloride, (b) sodium acetate and (c) magnesium chloride.
The initial aggregation kinetics of the F-CNTs was investigated using time resolved dynamic light scattering. The initial rate of change in particle size (rh) is proportional to kno where k is the initial aggregation rate constant and no is the initial concentration of the MWCNTs [26]. The reciprocal of stability ratio 1/W or the attachment efficiency α for suspensions with the same particle concentration were computed as:
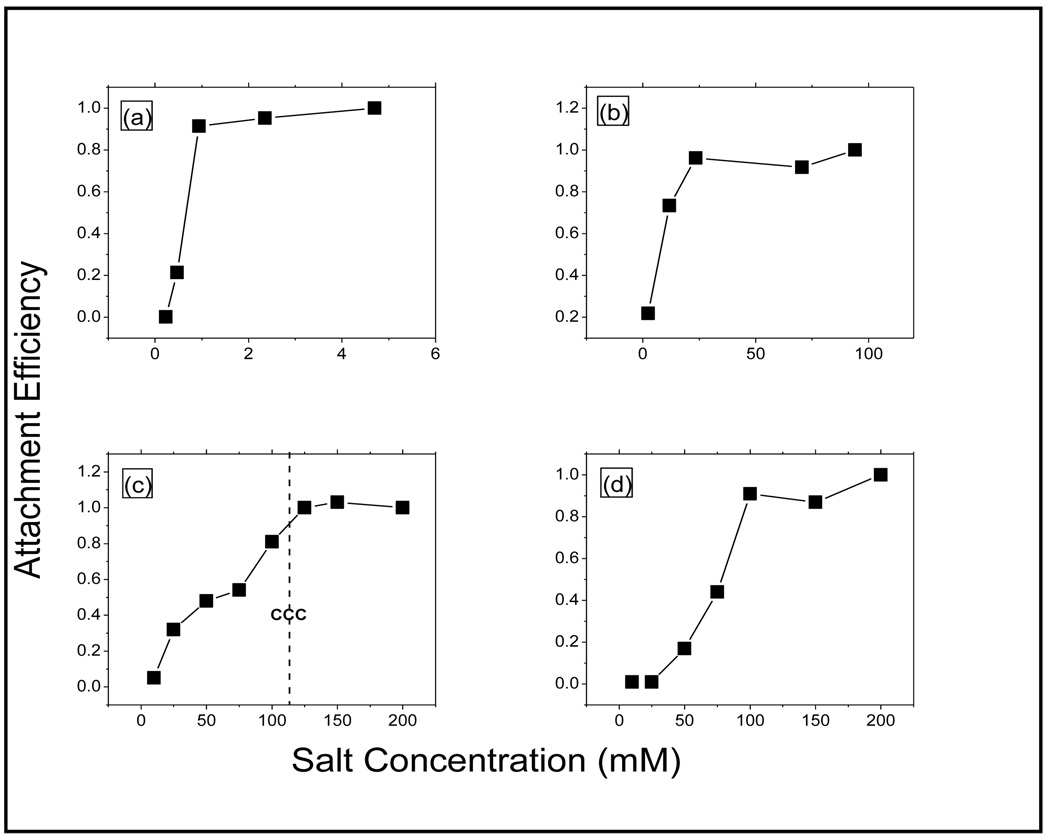
where represent the slow and fast aggregation regimes respectively [12, 26]. The attachment efficiency, which is a measure of the ratio of the initial slope of the aggregation profile in a given electrolyte system to the slope obtained under fast aggregation conditions are shown in Figure 7. This shows the attachment efficiencies of the F-CNTs in NaCl and MgCl2 as a function of salt concentration. The data for NaOAc was similar to that for NaCl, and is not presented here for brevity.
Figure 7.
Attachment effeciency of the F-CNTs are presented as a function of Electrolyte concentration. (a) MWCNT-COOH in MgCl2, (b) MWCNT-PVP in MgCl2, (c) MWCNT-COOH in NaCl, and (d) MWCNT-PVP in NaCl.
Distinct unfavorable and favorable aggregation kinetics regimes, demarcated by the critical coagulation concentration (CCC) (figure 7) were observed. This indicated that electrostatic DLVO type interactions to be the dominant mechanism for stabilization [27]. At low ionic strengths, an increase in salt concentration led to a corresponding increase in attachment efficiency. This is in line with Figure 5b, where an increase in electrolyte concentration led to less negative zeta potential. At high concentrations, the attachment efficiency was no longer a function of concentration and remained constant. This was similar to what had been reported previously [12]. Higher attachment efficiencies were observed in the presence of the divalent Mg ion (figure 7a and 7b) which was also consistent with the zeta potential, where the latter became much less negative with even small increase in Mg concentration.
The CCC values were estimated from the plot of the attachment efficiencies against the salt concentration which shows the slow and fast aggregation regimes (figure 7). The CCC values were obtained from the intersection of the interpolated lines through the slow and fast regimes. MWCNT-COOH showed higher attachment efficiencies than MWCNT-PVP (figure 7c and d), which was evident from the CCC values. For example, the CCC values were 0.6mM and 18mM of MgCl2 for MWCNT-COOH and MWCNT-PVP respectively, and the corresponding values in NaCl were 125Mm and 100Mm. The difference between MgCl2 and NaCl was also quite apparent. With the exception of MWCNT-COOH in MgCl2, previously reported CCC values are significantly lower than the present data which deals with highly water dispersible MWCNT [12].
3.4 Long Term Stability of the F-CNTs
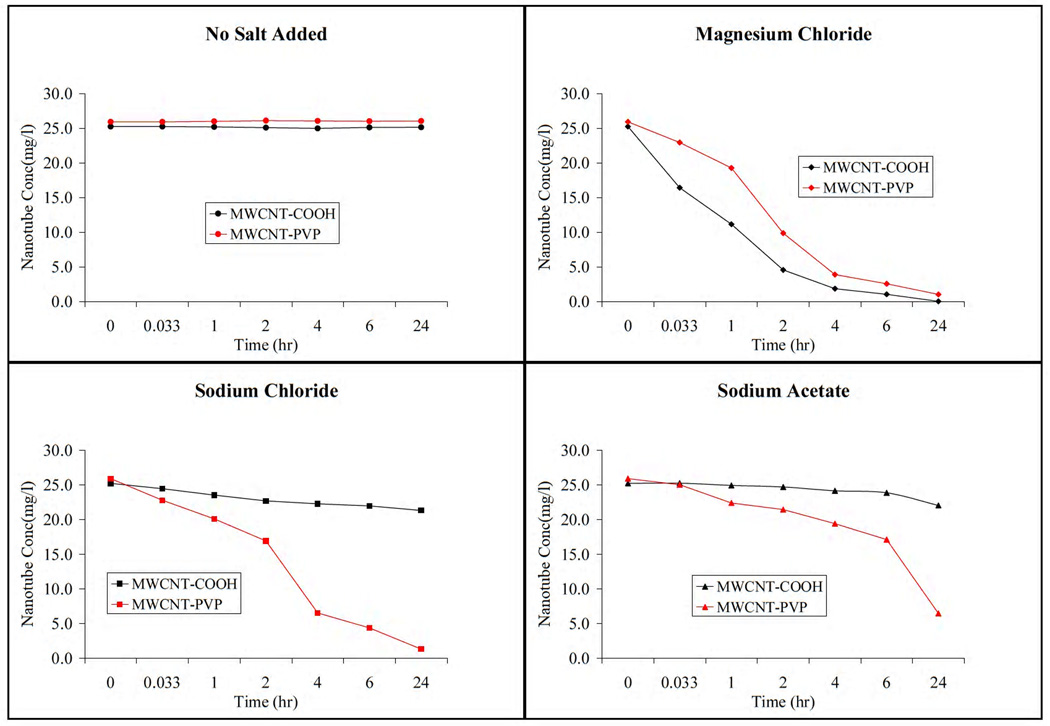
The time dependent stability of the nanoparticles dispersions are presented in figure 8. It was observed that both MWCNT-COOH and MWCNT-PVP dispersions in deionized water were stable over the 24 hour measurement period. Aggregation and subsequent deposition of the nanoparticles was observed in the electrolytic environment. The higher the hydrophobic indexes of the F-CNTs, the less stable they became in aqueous solutions. Therefore the decreased long-term stability of the F-CNTs was in agreement with their hydrophobicity indexes (table 1) in deionized water and electrolyte solution. Stability of MWCNT-COOH was better in the presence of the monovalent Na ions than the divalent Mg ions. This was consistent with the Schulze-Hardy Rule [13] where CCC is said to be dependent on counterion valence.
Figure 8.
Colloidal stability as a function of time measured by UV absorbance at 252nm
MWCNT-PVP showed lower stability in the presence of electrolyte than MWCNT-COOH over the 24-hour period. This was attributed to the long term behavior of the PVP wrapped carbon nanotubes in the electrolytes. Wrapping of carbon nanotubes by water soluble polymers are driven largely by a thermodynamic drive to eliminate the hydrophobic interface between the tubes and their aqueous environment, therefore changing the solvent system to remove the strong hydrophobic thermodynamic penalty would induce the polymer-CNT complexes to dissociate [24]. It is therefore possible that the PVP wrapping on the carbon nanotubes were partially removed after prolonged exposure to the electrolyte solution causing them to lose their hydrophilicity and subsequently they deposited out of the solution.
4 Conclusion
The colloidal behavior of carboxylated and polymer wrapped CNTs were markedly different in terms of hydrophobicity, particle size distribution and zeta potential. Aggregation of the polymer modified CNT was lower compared to the carboxylated CNTs, with orders of magnitude higher CCC. Since dispersion in presence of NaOAc and NaCl followed similar patterns, it is concluded that the anions did not have any significant effect on stability. Both F-CNTs were highly stable in pure water, while MWCNT-PVP showed lower long term stability in the presence of electrolytes. Our results suggest that these highly dispersible F-CNTs can be relatively stable in typical aquatic environments, and their behavior will depend upon the presence of other species.
Acknowledgments
This work was funded by the National Institute of Environmental Health Sciences (NIEHS) under Grant Number RC2 ES018810. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NIEHS. Partial support for this work was also provided by the Schlumberger Foundation Faculty for the Future Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin DH, Xing B. Environ. Sci. Technol. 2008;42(15):5917–5923. doi: 10.1021/es800329c. [DOI] [PubMed] [Google Scholar]
- 2.Aitchison TJ, Ginic-Markovic M, Matisons JG, Simon GP, Fredericks PM. J. Phys. Chem. 2007;111:2440–2446. [Google Scholar]
- 3.Yu J, Grossiord N, Koning CE, Loos J. Carbon. 2007;45:618–623. [Google Scholar]
- 4.Kharisov BI, Kharissova OV, Gutierrez HL, Me#ndez UO. Ind. Eng. Chem. Res. 2009;48:572–590. [Google Scholar]
- 5.Daniel S, Rao TP, Rao KS, Rani SU, Naidu GRK, Lee HY, Kawai TA. Sens. Actuators B. 2007;122(2):672–682. [Google Scholar]
- 6.Noguchi Y, Fujigaya T, Niidome Y, Nakashima N. Chem. Phys. Lett. 2008;455:249–251. [Google Scholar]
- 7.Yang QH, Gale N, Oton CJ, Li H, Nandhakumar IS, Tang ZY, Brown T, Loh WH. Carbon. 2007;45(13):2701–2703. [Google Scholar]
- 8.Wang Z, Liu Q, Zhu H, Liu H, Chen Y, Yang M. Carbon. 2007;45:285–292. [Google Scholar]
- 9.Wise EK, Park C, Siochi EJ, Harrison JS. Chem. Phys. Lett. 2004;391:207–211. [Google Scholar]
- 10.Hu C, Liao H, Li F, Xiang J, Li W, Duo S, Li M. Mat. Lett. 2008;62:2585–2588. [Google Scholar]
- 11.Yurekli K, Mitchell CA, Krishnamootri R. J. Am. Chem. Soc. 2004;126:9902–9903. doi: 10.1021/ja047451u. [DOI] [PubMed] [Google Scholar]
- 12.Saleh NB, Pfefferle LD, Elimelech M. Environ. Sci. Technol. 2008;42(21):7963–7969. doi: 10.1021/es801251c. [DOI] [PubMed] [Google Scholar]
- 13.Elimelech M, Gregory J, Jia X, Williams RA. In: Particle Deposition and Aggregation: Measurement, Modeling and Simulation. Williams RA, editor. Oxford, England: Butterworth Heinemann; 1995. [Google Scholar]
- 14.Chiang IW, Brinson BE, Smalley RE, Margrave JL, Hauge RH. J. Phys. Chem. B. 2001;105:1157–1161. [Google Scholar]
- 15.Peng X, Jia J, Gong X, Luan Z, Fan B. J. Hazard. Mater. 2009;165:1239–1242. doi: 10.1016/j.jhazmat.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Smith B, Wepasnick K, Schrote KE, Bertele AR, Ball WP, O’Melia C, Fairbrother HD. Environ. Sci. Technol. 2009;43:819–825. doi: 10.1021/es802011e. [DOI] [PubMed] [Google Scholar]
- 17.Manivannan S, Jeong IO, Ryu JH, Lee CS, Kim KS, Jang J, Park KC. J. Mater Sci: Mater Electron. 2009;20:223–229. [Google Scholar]
- 18.Derjaguin BV, Landau L. Acta Physicochimica. 1941;14:633–662. [Google Scholar]
- 19.Verwey EJ, Overbeek JTG. Theory of the Stability of Lyophobic Colloids. New York: Elsevier; 1948. [Google Scholar]
- 20.Troy DB, editor. Remington: The science and practice of pharmacy. Pennsylvania: Lippincott Williams and Wilkins Pennsylvania; 2005. [Google Scholar]
- 21.Chen Y, Iqbal Z, Mitra S. Adv. Function. Mater. 2007;17:3946–3951. [Google Scholar]
- 22.Chen Y, Mitra S. J. Nanosci. Nanotechnol. 2008;8(11):5770–5775. doi: 10.1166/jnn.2008.215. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Xia T, Addo Ntim S, Ji Z, George S, Meng H, Zhang H, Castranova V, Mitra S, Nel AE. ACS Nano. 2010;4(12):7241–7252. doi: 10.1021/nn102112b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell MJ, Boul P, Ericson LM, Huffman C, Wang Y, Haroz E, Kuper C, Your J, Ausman KD, Smalley RE. Chem. Phys. Lett. 2001;342:265–271. [Google Scholar]
- 25.Schudel M, Behrens SH, Holthoff H, Kretzschmar R. J. Colloid Interface Sci. 1997;196:241–253. doi: 10.1006/jcis.1997.5207. [DOI] [PubMed] [Google Scholar]
- 26.Gfiner A, Ataman M. Colloid Polym. Sci. 1994;272:175–180. [Google Scholar]
- 27.Rector DR, Bunker BC. PNL-10761. 1995:2.4–2.5. [Google Scholar]