Abstract
Preterm birth is the most important direct cause of neonatal mortality and remains a major challenge for obstetrics and global health. Intrauterine infection causes approximately 50% of early preterm births. Animal models using pregnant mice, rabbits, or sheep, demonstrate the key link between infection and premature birth, but differ in mechanisms of parturition and placental structure from humans. The nonhuman primate (NHP) is a powerful model which emulates many features of human placentation and parturition. The contributions of the NHP model to preterm birth research are reviewed emphasizing the role of infections, and potential development of preventative and therapeutic strategies.
Preterm birth, defined as birth before 37 weeks of gestation, remains a major challenge in obstetrics and is the most important direct cause of neonatal mortality, accounting worldwide for more than 1 million neonatal deaths annually1. Of the four million neonatal deaths each year preterm birth is a risk factor in 50%.2 Further, the preterm birth rate has been increasing in many countries. Preterm birth now occurs in nearly 1 in every 8 pregnancies with an annual economic burden of 26 billion dollars in the United States.3 A large body of evidence suggests that up to 50% of premature births less than 30 weeks gestation where neonatal morbidity and mortality are highest, are caused by intrauterine infections, including chorioamnionitis.4
An ascending infection from the lower genital tract is thought to be the source of most intrauterine infections. This premise is supported by observations that bacteria recovered from the amniotic fluid are indigenous to the vagina including gram-negative (e.g. E. coli, Gardnerella vaginalis), gram-positive (Group B Streptococcus; GBS), and anaerobic (Mycoplasma hominis) bacteria.5-7 Factors mediating or inhibiting bacterial traffic from the vagina into the lower uterine segment and the amniotic fluid are unknown. Once bacteria are in contact with placental tissues, a pro-inflammatory response can be initiated that leads to preterm labor (PTL) and fetal injury (e.g. white matter and lung). The inflammatory mediators implicated in preterm birth include interleukin-1 beta (IL-1β)8, interleukin-6 (IL-6)9, interleukin-8 (IL-8)10, and tumor necrosis factor (TNF-α)11. A role for pro-inflammatory cytokines/chemokines in the development of PTL is based upon the following observations: (a) elevated amniotic fluid cytokines in humans and rabbits with IAI and PTL,12-14 (b) bacterial products stimulate production of cytokines by human amniotic epithelium, decidua, and trophoblast,15, 16 (c) these cytokines stimulate prostaglandin production in decidual explants and amniotic epithelial cell lines in vitro,17, 18 and (d) recombinant IL-1β induces PTL in pregnant nonhuman primates (NHP) or mice (systemic or intrauterine).19, 20 Other important downstream inflammatory mediators of infection-induced PTL include prostaglandins and matrix metalloproteinases, which enhance myometrial contractility and weaken the collagen structure of the membranes, respectively. Human studies in pregnant women have not been adequate to clarify the temporal relationships between these inflammatory mediators, which would allow study of the pathophysiology of PTL and lead to opportunities for preventative and therapeutic discovery.
Although a number of barriers have prevented discovery of an effective therapy for PTL, a major reason is the lack of a widely available and inexpensive animal model that closely emulates human disease. Animal models using pregnant mice, rabbits, or sheep have strengthened the causal link between intrauterine infection and premature birth, but differ sufficiently from women in both placentation and the hormonal events surrounding parturition to limit application to human PTL (Table 1). Generally, these studies have restricted their design to cross-sectional observations at the time of delivery, like human studies. Consequently, they have not clearly defined the sequence of mechanisms by which intrauterine infection causes premature contractions, cervical change and ultimately preterm delivery. To more closely emulate human PTL, we and others21 have used a chronically instrumented NHP model with either pregnant rhesus or pigtail macaques, in which both placentation and the endocrinology of pregnancy are similar to humans. These advantages must be tempered against the limited availability and expense of non-human primates. Nevertheless, the NHP model has provided a useful means to study in vivo the temporal relationships between infection, the immune system, and uterine contractility even in the relatively inaccessible intrauterine and intra-amniotic compartments. The ability to correlate information from maternal, fetal and amniotic fluid samples with uterine activity and fetal tissues allows one to answer questions such as: 1) why is infection-induced PTL refractory to antibiotics and tocolysis?, and 2) what are the critical mediators of PTL and fetal injury, and how can they be inhibited? Answering these questions is critical to developing rational and efficacious strategies to prevent preterm birth. If interventions to prevent preterm birth and fetal injury are to become realistic goals, then the pathways that are activated in the uterus, placenta and fetus in response to infection and inflammation need to be elucidated in a model which emulates human disease. This model and lessons learned are outlined below.
Table 1.
Comparison of animal models focusing on characteristics of gestation, parturition, and relative advantages and disadvantages of each model.
| Characteristic | Human | Monkey | Sheep | Guinea Pig | Mouse |
|---|---|---|---|---|---|
| Gestational length in days (mean ± SD | 280 ± 14 | 167 ± 7 (M. mulatta) 172 ± 7 (M. nemestrina) |
144-151 | 60-70 | 19-21 |
| Litter size (mean or range) | 1 | 1 | 1-3 | 2-4 | 10-12 (strain specific) |
| Placenta | Hemomonochorial, villous, discoid | Hemomonochorial, villous, bi-discoid | Epithelial-chorial, cotyledonary | Hemomonochorial, labyrinthine, discoid | Hemotrichorial, labyrinthine |
| Uterus | Unicornuate | Unicornuate | Bicornuate | Duplex uterus (2 uterine horns, 2 cervices) | |
| Induction of preterm birth | Prostaglandin, anti-progestin, oxytocin | Inoculation of bacteria, LPS, or cytokines (IL-1β) | Fetal ACTH, glucocorticoid, anti-progestin | Prostaglandin, anti-progestin, oxytocin | Anti-progestin, ovariectomy |
| Advantages/Disadvantages | Directly translational, but limited to cross-sectional analysis | Directly translational, but expensive and limited in availability | Chronically catheterized model possible | Similar placenta to humans, but spontaneous preterm birth can occur | Low cost, small size |
Abbreviations:
ACTH: adrenocorticotropin releasing hormone
SD: standard deviation
Technical Approach and Setup of the Nonhuman Primate Model
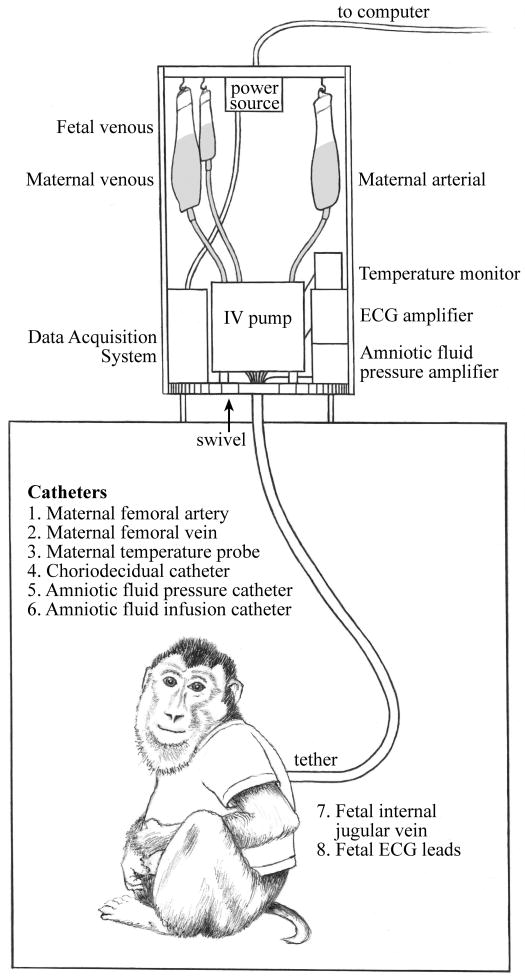
Nonhuman primate pregnancy emulates human pregnancy based on multiple similar features including uterine anatomy, singleton gestation, hemochorial placentation, hormonal control of parturition (initiation of labor), and microbial communities within the vagina. In our non-human primate model, we have utilized both Macaca mulatta (rhesus macaques) and Macaca nemestrina (pigtail macaque) to study intrauterine infection and the pathogenesis of PTL and neonatal sequelae. Pregnant monkeys with timed mated gestations are conditioned to a jacket-tether system for several weeks prior to catheterization surgery to allow acclimatization (Figure 1). The tether system allows the animal 360 degrees of movement within the cage and the animal retains the same mobility within the cage as was possible without the jacket-tether.21, 22 On pregnancy day 118-125 (term 167-172 days; approximately 28-29 wks human gestation), intrauterine surgery is performed with implantation of fetal ECG electrodes, amniotic fluid catheters, a maternal temperature probe, maternal and fetal vascular catheters, and a catheter into choriodecidual space.23 All catheters are tunneled under the animal's skin and exit from the upper back into a metal, flexible tether, which protects the catheters. For the next 10 days, the animal recovers from the surgery. Analgesics, cefazolin, and terbutaline sulfate are administered for up to 7 days to control post-operative pain and uterine irritability. Cefazolin and terbutaline are discontinued at least 72 hours prior to inoculation of bacteria in order to insure an adequate drug washout period.
Figure 1.
Jacket-tether system used most recently at the Washington National Primate Research Center, allowing the animal 360° of motion. As the animal turns, the equipment at the top of the cage spins on a swivel to prevent tangling of the catheters. ECG, electrocardiogram; IV, intravenous.
After the animal has recovered from surgery, the experimental phase begins. Many different experimental protocols have been used in this model to induce or simulate an intrauterine infection and PTL. Inoculation of bacteria into the amniotic fluid is meant to replicate the end-stage of an intrauterine infection. More recently, experiments to inoculate bacteria into the choriodecidual space have attempted to replicate earlier events in the pathogenesis of PTL. The choriodecidual space is a potential space between the myometrium and the fetal membranes; in the lower uterine segment, this space represents the first placental site in contact with bacteria trafficking from the lower genital tract into the uterus. In addition to bacteria, bacterial products (lipopolysaccharide), cytokines, and immunomodulators have been infused into either the amniotic fluid or choriodecidual space in past experiments. Following inoculation, amniotic fluid, maternal blood and fetal blood are serially sampled. Uterine contractions, fetal heart rate, and maternal temperature are continuously monitored. Cesarean section may then be performed at a pre-determined time or once labor begins. Performing a Cesarean section is important to recover myometrial biopsies as well as placenta, which is often ingested by the animal following birth. Several types of fetal tissues (e.g. fetal lung and brain) are also recovered and rapidly preserved enabling a variety of analyses to characterize fetal injury. At the time of Cesarean section, the catheters are removed. Analgesics and antibiotics (if necessary) are administered for 7-14 days until the animal recovers and can be returned to the colony.
Induction of PTL
The NHP model of infection-induced PTL was originally characterized in rhesus macaques with Group B Streptococcus (GBS), a common pathogen in both maternal and neonatal infections.23 In this model, intense PTL occurred on average 28 hours after intra-amniotic infusion of 1×106 colony forming units (CFU) of GBS (range 14 to 40 hr). In all animals, this uterine activity led to progressive cervical effacement or dilatation. In contrast, control animals undergoing only surgery and catheter implantation delivered approximately 30 days later, near term (∼160 days with term being 167 days in rhesus macaques). Amniotic fluid concentrations of cytokines (IL-1β, IL-6, TNF-α increased dramatically, followed by increases in prostaglandins (PGE2, PGF2α) and finally, uterine contractility. At fetal necropsy, bacteria were cultured from the fetal lungs and meninges. Neonatal pneumonia was diagnosed based on neutrophil infiltration into the fetal pulmonary alveoli. This study represented the first work to establish the temporal relationship between activation of the cytokine-prostaglandin cascade and PTL.
The intra-amniotic infection/inflammation (IAI) model is limited by replicating only the end stage of what is presumed to be the result of an ascending infection from the lower genital tract. Infection-associated preterm birth is thought to occur from an ascending infection from micro-organisms arising from the lower genital tract. This is supported by clinical observations in women that bacteria recovered from amniotic fluid and extraplacental membranes are also commonly found in the lower genital tract.5, 24 We therefore modified our model to induce experimental choriodecidual infection by direct inoculation of GBS via an indwelling catheter placed in the choriodecidual space, between the myometrium and the fetal membranes in the lower uterine segment.25 Unexpectedly, inoculation with a low dose of GBS (102 – 104 CFU/milliliter) did not result in PTL, and the organisms were cleared. In contrast, inoculation with higher doses (104 – 106 CFU/ml) consistently led to amniotic fluid infection and PTL. These data provided evidence that choriodecidual inflammation is a transitional stage of ascending infection and dependent upon bacterial inoculum and host response.
Purified cytokines have also been inoculated into this model to try to clarify the functional role of individual cytokines, because cytokine antagonists exist and certain immunomodulators can suppress specific cytokines. Significant elevations of the pro-inflammatory cytokines IL-1β, TNF-α, IL-6 and IL-8 have been reported in the setting of IAI in women, NHP and other animals.8-11, 23 There is some evidence that IL-1β and TNF-α may play a greater role in PTL than IL-6. In genetically altered mice, both IL-1β and TNF-α receptors are necessary for infection-induced preterm birth.20 In contrast, intrauterine infusions of IL-6 did not induce preterm birth and the absence of IL-6 (knockout mouse) did not prevent preterm birth induced by heat-killed E. coli.26 To compare relative ability of individual cytokines to stimulate PTL in NHP without the confounding influences of infection, we infused single pro-inflammatory cytokines (10 μg recombinant IL1β, 10-100 μg recombinant human or rhesus TNF-α, or 20 μg human IL-6 or IL-8 twice a day until delivery) directly into the amniotic fluid.19 Increases in uterine contractility and PTL occurred only following IAI infusion of IL-1β (100% of animals) or TNF-α (40% of animals). No animals receiving intra-amniotic IL-6 or IL-8 infusions experienced PTL, despite significant and sustained increased in amniotic fluid concentrations of these cytokines to levels seen in IAI. Whether these cytokine effects might be gestational-age specific could not be assessed as the animals were similar in gestational age at the time of cytokine infusion. These data provide direct evidence of the role of IL-1β and TNF-α in the pathogenesis of infection-associated PTL, and confirm observations in genetically altered mice.
Response to Antibiotics and Immunomodulators
The observations summarized above demonstrated the temporal relationships among infection (either intra-amniotic or choriodecidual), pro-inflammatory cytokines, prostaglandins, and preterm contractions, and specifically have established a critical role for IL-1β and TNF-α. We now turn to address the important question of why antibiotics and tocolytics do not prolong gestation in the setting of infection. The failure of antibiotics to delay or prevent infection-induced birth is likely multifactorial, but one major reason may be a failure to inhibit the pro-inflammatory mediators that play a critical role in the initiation of labor. To investigate this hypothesis, we treated monkeys following experimental infection-induced PTL with antibiotics alone or antibiotics and immunosuppressants.27 Then, we compared the time from the onset of PTL to delivery with animals that were similarly infected but not treated (Figure 2). Immunosuppressants used in this study included dexamethasone, a non-specific anti-inflammatory drug, and indomethacin, an inhibitor of prostaglandin synthesis. The average time from the onset of uterine contractions until delivery was 33 hours after GBS IAI and no treatment, which did not significantly differ from animals treated with antibiotics alone (81 hr). In contrast, the pregnancies among animals treated with both antibiotics and immunosuppressants were significantly prolonged and delivered on average at 213 hours; four of five animals had a prolongation of pregnancy greater than 10 days and delivered near term. While antibiotic therapy alone eradicated or reduced bacteria from amniotic fluid and fetal compartments as detected by microbial culture, amniotic fluid cytokine and prostaglandin concentrations remained significantly elevated until delivery. In contrast, treatment with antibiotics and immunosuppressants resulted in prompt reductions in cytokines and prostaglandins until near delivery. Interestingly, the up-regulation of matrix metalloproteinases seen in the setting of infection-induced PTL was not affected by any treatment, although premature rupture did not occur in any animal. While these observations provide a basis for future human trials utilizing combined antibiotics and immunosuppressants, caution must be exercised. Further study is required to demonstrate fetal safety as well as efficacy in animal models.
Figure 2.
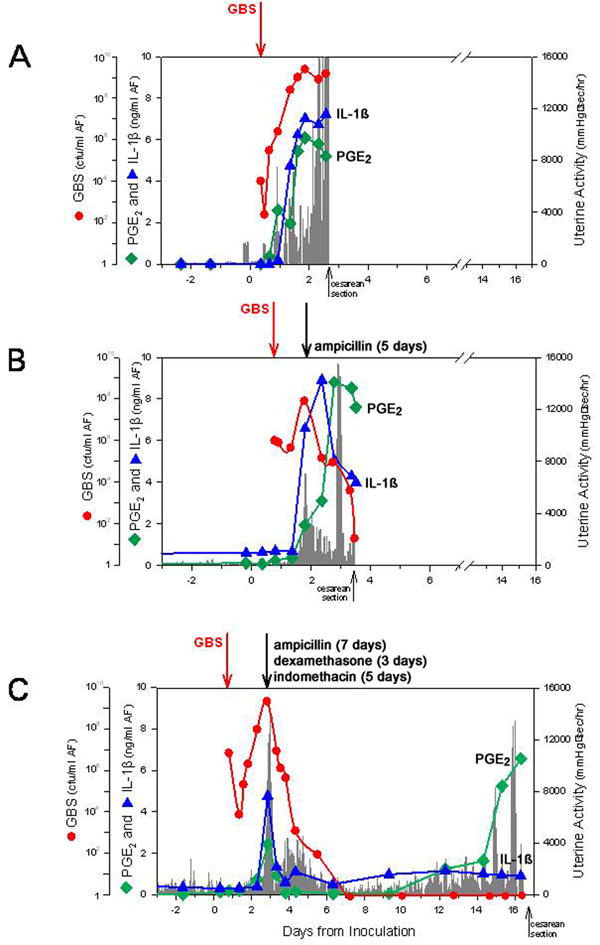
Serial changes after experimental intra-amniotic group B Streptococcus (GBS) inoculation in uterine contractility, GBS [colony-forming units (cfu)/ml], pro-inflammatory cytokines and prostaglandins for single representative animals in each treatment group: (A) control; (B) ampicillin alone; (C) ampicillin, dexamethasone and indomethacin. The x-axis represents the gestational age in days. The y-axis is the hourly contraction area, or the quantity of GBS (cfu/ml; red line), amniotic fluid interleukin-1β (IL-1β; blue line) or prostaglandin E2 (PGE2; green line). The length of time that a particular drug was administered is indicated above (B) and (C). Reprinted from the American Journal of Obstetrics and Gynaecology, with permission of Elsevier, and modified.27
The reason that the cytokine-prostaglandin inflammatory response remained elevated despite eradication of bacteria by antibiotics is likely due to continued recognition of pathogenic ligands created by bacteriolysis by the innate immune system through toll-like receptors (TLR). TLR are a family of pattern recognition receptors, which act as the principal sensors of bacterial pathogens to activate the innate and adaptive immune system.28 Activation of TLR 2, 3, and 4 have been associated with PTL in murine and NHP animal models.29-31 Pathogen particles derived from GBS signal through TLR1, 2, and 6, which would be expected to induce cytokines and augment PTL.32 Cytokines are temporarily suppressed with antibiotics and immunosuppressants in our GBS model, but whether in utero fetal injury might occur due to some degree of TLR activation and cytokine production during prolongation of pregnancy is unknown.27 We therefore reasoned that blockade of pathogen recognition by TLR antagonists may be more efficient at delaying preterm birth than blockade of downstream effectors such as cytokines or prostaglandins. This concept was tested in our nonhuman primate model by blocking a single TLR to a specific pathogen particle.29 TLR4 recognizes lipopolysaccharide (LPS), the major component of the outer membrane of gram-negative bacteria, and results in pro-inflammatory cytokine gene expression.33-35 Many pathogens commonly associated with IAI contain LPS and are thought to signal using TLR4 including E. coli, Gardnerella vaginalis, Fusobacterium36, and Bacteroides.37 TLR4 is also highly expressed by many placental tissues including amniotic epithelium, immune cells within the membranes and decidua, and chorionic villi.38, 39 TLR4 signaling is likely an early event during gram-negative bacterial infection in the fetal membranes and is required for LPS-induced preterm birth.29, 30, 38
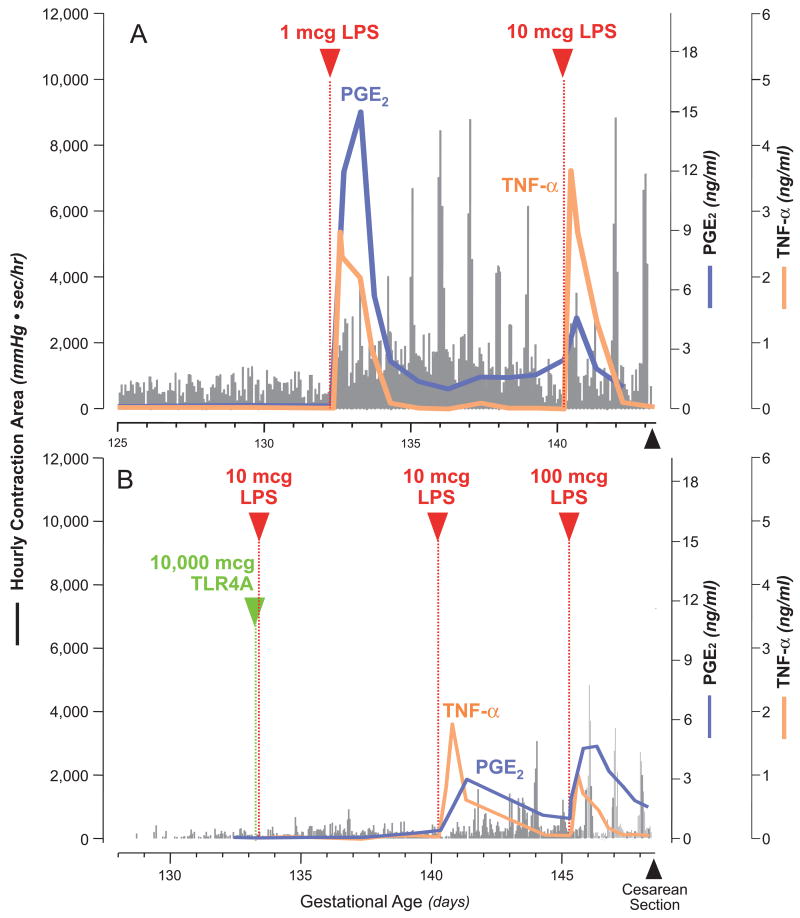
Natural TLR4 antagonists exist and represent minor structural variations in the toxic portion of LPS, called lipid A.40, 41 We chose to test the ability of a TLR4 antagonist (TLR4A) to inhibit LPS-induced PTL by pre-treating the amniotic cavity for one hour before LPS inoculation; drug failure in this circumstance would have suggested drug degradation, binding or transplacental transfer. Gestational length was compared in three groups of M. mulatta receiving either intra-amniotic LPS or a TLR4A followed by LPS 1 hour later (Figure 3).29 LPS inoculation was associated with significant increases in uterine contractility with peak uterine activity achieved within 4 to 6 days. Pre-treatment with a TLR4 antagonist largely ablated the increase in uterine contractility observed in LPS only animals; uterine contractility in the TLR4A group did not differ significantly from saline controls. In animals treated with a TLR4A, a repeat LPS challenge one week later was associated with only modest increases in uterine activity, but in one animal it triggered PTL. Pre-treatment with a TLR4A was also associated with decreases in IL-1β and significant reductions in TNF-α, IL-8, and PGE2 compared to LPS infusion alone. Amniotic fluid leukocytes followed a similar pattern to that of cytokines and prostaglandins and were significantly increased with LPS infusion alone versus saline controls and reduced by TLR4A pre-treatment. Overall, the TLR4 antagonist was associated with the most profound suppression of LPS or infection-induced prostaglandins (PGE2, PGF2α) and cytokines (IL-1β, TNF-α) in comparison with all other immunomodulators (i.e. dexamethasone, cytokine antagonists, indomethacin) previously tested in our model.
Figure 3.
Temporal relationships between lipopolysaccharide (LPS) inoculation, uterine activity, and amniotic fluid cytokines and prostaglandins in representative animals from each experimental group receiving LPS only (A) and intra-amniotic Toll-like receptor 4 antagonist (TLR4A; green arrowhead) 1 hour prior to intra-amniotic LPS (B). The x-axis represents the gestational age in days, ranging from vascular implantation surgery until caesarean delivery. The y-axis is the hourly contraction area, or the level of amniotic fluid tumour necrosis factor-α (TNF-α; orange line) or prostaglandin E2 (PGE2; blue line). Reprinted with permission.46 2007 American Chemical Society.
Systems Biology and Identification of Diagnostic Markers
Observations above suggest that early intervention in IAI with antibiotics and immunomodulators may prolong gestation. Furthermore studies in women with IAI indicate that timely intrapartum antibiotic therapy reduces the risk of neonatal sepsis when compared to therapy initiated after birth.42, 43 The early diagnosis of IAI would facilitate timely and appropriate interventions. However, the early diagnosis of IAI is difficult because clinical signs and symptoms tend to be late manifestations and adjunctive laboratory tests (e.g., maternal white blood cell count) have limited predictive value or require an invasive amniocentesis to directly examine amniotic fluid.44 Utilizing multiple proteomic approaches, we have characterized the NHP amniotic fluid and cervical-vaginal fluid proteome to identify potential early biomarkers of IAI.45, 46 We identified multiple proteins, including azurocidin, calgranulin B, and a proteolytic fragment of insulin-like growth factor binding protein-1 in the amniotic fluid that were differentially expressed as early as 12 hours after experimental IAI and before clinical signs or symptoms of infection. We then validated these biomarkers among a cohort of women in PTL with intact fetal membranes with and without IAI, with a sensitivity of 100% and a specificity of 91%.45 However, detection of these biomarkers in women requires amniocentesis, a procedure many providers are reluctant to perform in women with PTL.
To develop a non-invasive test, we next sought to determine if proteomic evaluation of biomarkers identified within amniotic fluid might also be detected in cervical-vaginal fluid (CVF) of women with intact membranes and thus, forego the need for amniocentesis. Multidimensional liquid chromatography coupled to tandem mass spectrometry identified a total of 205 unique proteins within CVF.46 Functional annotation of the CVF proteome showed that a majority of them were associated with metabolism (25%) or immune response (23%). Twenty-seven proteins were differentially abundant following experimental IAI with Ureaplasma parvum, a common isolate from amniotic fluid in the setting of infection, including those previously identified in amniotic fluid. The detection of differentially expressed proteins within CVF in this proof-of-concept study in non-human primates provided a rationale to develop non-invasive reliable tests to aid in the diagnosis in women. In a subsequent study of the CVF of 170 women in PTL with intact fetal membranes, we identified 15 differentially expressed proteins associated with subclinical IAI.47 The performance characteristics of potential biomarkers were analyzed by receiver operator characteristic curves. A four analyte immunoassay panel developed from these differentially expressed proteins including alpha-1-acid glycoprotein, insulin-like growth factor binding protein-1, calgranulin C, and cystatin A was able to correctly classify 89% of patients as infected or not infected. These data may facilitate the development of rapid, non-invasive, and reliable tests for IAI and are an excellent example of the direct translational relevance from the NHP model.
Summary
We have described how the NHP model can be utilized to understand the pathogenesis of PTL, including identification of key inflammatory pathways, which participate in this complex syndrome. The NHP model emulates human PTL, including the physiology of myometrial contractions, expression of inflammatory markers, response to antibiotics and anti-inflammatory agents, and histopathological findings of placental and fetal injury. Our experimental approach provides control of experimental conditions before, during, and after PTL and birth. Maternal and fetal samples collected during the experiments have laid the foundation for evaluating the role of the hormonal axis, myometrial physiology, maternal/fetal inflammatory responses, and early events in choriodecidual inflammation that predispose to IAI and ultimate preterm delivery. This model now creates opportunity to fully explore in more robust detail many of the mechanisms, like infection and inflammation, which are involved in the initiation of PTL. In addition, our data have begun to demonstrate the important signals that occur between mother and fetus in the early response to infection, even in the absence of overt IAI. Advantages of this animal model must also be tempered with the limitations of cost and availability to most scientists in the field. Implementation of the chronically catheterized NHP model also requires a highly experienced surgical and technical staff to set up the model and ensure proper functioning of the catheters throughout the experiment. We are now pursuing additional studies on the role of intrauterine stretch, as well as characterizing the hormonal signals that precede the initiation of PTL.
The high rates of preterm birth observed in the developing and developed world and its contribution to neonatal mortality in all countries underscores the importance of research efforts to prevent it. We need diagnostics for identifying women at risk of preterm delivery our recent observations of protein biomarkers from the NHP model using a systems biology approach appears to emulate similar findings for PTL in women. We see an exciting opportunity for extending these studies to define the important mechanisms that control normal pregnancy. These findings could help characterize what leads to PTL and delivery in different populations, such as African Americans and Native Americans who have very high rates of premature birth and infant mortality. As our understanding increases of the initiating events and complex pathways involved in PTL and delivery, we hope to predict women at risk via new diagnostics so that we can implement prevention strategies and more effective therapies to decrease the significant morbidity and mortality of the preterm infant.
Acknowledgments
The authors acknowledge Jan Hamanishi for assistance with graphic design.
Funding: The NHP work described has been supported by grants from the March of Dimes and National Institutes of Health HD06159, HD18185, HD 01264, HD41676, RR00163, AI42490, AI067910, and AI42490. Proteogenix, Inc. supported in part the proteomics portion of the work in the diagnostics section.
Footnotes
Drs. Adams Waldorf and Rubens have no conflicts of interest to disclose.
Disclosure of Interests: Dr. Gravett has a financial interest in ProteoGenix, Inc., which supported, in part, the proteomics work. This company may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and a management plan approved by the Oregon Health Sciences University Conflict of Interest in Research Committee and the University of Washington.
Contribution to Authorship: All authors contributed to writing and editing the manuscript.
Details of ethics approval: Study protocols were approved by the Institutional Animal Care and Utilization Committee at the Oregon and Washington National Primate Research Centers and guidelines for humane care were followed. The most recent approval of these longstanding studies is at the Washington National Primate Research Center and is #4165 (Washington National Primate Research Center, 2/11/2011).
Reference List
- 1.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006 Jun;35(3):706–18. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why. Lancet. 2005 Mar 5-11;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Berhman RE, Butler AS, editors. Committee on Understanding Premature Birth and Assuring Healthy Outcomes BoHSP, Institute of Medicine Preterm Birth: Causes, Consequences, and Prevention. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- 4.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000 May 18;342(20):1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 5.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988 Oct 13;319(15):972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989 Sep;161(3):817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 7.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):955–61. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989 May;160(5 Pt 1):1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990 May;85(5):1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989 Aug;161(2):336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi T, Matsuzaki N, Kameda T, Shimoya K, Jo T, Saji F, et al. The enhanced production of placental interleukin-1 during labor and intrauterine infection. Am J Obstet Gynecol. 1991 Jul;165(1):131–7. doi: 10.1016/0002-9378(91)90241-i. [DOI] [PubMed] [Google Scholar]
- 13.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993 Jun;81(6):941–8. [PubMed] [Google Scholar]
- 14.Davies JK, Shikes RH, Sze CI, Leslie KK, McDuffie RS, Jr, Romero R, et al. Histologic inflammation in the maternal and fetal compartments in a rabbit model of acute intra-amniotic infection. Am J Obstet Gynecol. 2000 Nov;183(5):1088–93. doi: 10.1067/mob.2000.108888. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989 Jan;37(1):13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur J Pharmacol. 1991 Jan 3;192(1):189–91. doi: 10.1016/0014-2999(91)90090-d. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Mazor M, Wu YK, Avila C, Oyarzun E, Mitchell MD. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids. 1989 Sep;37(3):183–6. doi: 10.1016/0952-3278(89)90083-5. [DOI] [PubMed] [Google Scholar]
- 18.Norwitz ER, Lopez Bernal A, Starkey PM. Tumor necrosis factor-alpha selectively stimulates prostaglandin F2 alpha production by macrophages in human term decidua. Am J Obstet Gynecol. 1992 Sep;167(3):815–20. doi: 10.1016/s0002-9378(11)91595-6. [DOI] [PubMed] [Google Scholar]
- 19.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006 Dec;195(6):1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006 May;194(5):1334–40. doi: 10.1016/j.ajog.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Bryant JM. Vest and tethering system to accommodate catheters and a temperature monitor for nonhuman primates. Lab Anim Sci. 1980 Aug;30(4 Pt 1):706–8. [PubMed] [Google Scholar]
- 22.Novy MJ, Walsh SW, Cook MJ. Chronic implantation of catheters and electrodes in pregnant nonhuman primates. Amsterdam and New York: Elsevier/North-Holland Biomedical Press; 1980. [Google Scholar]
- 23.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994 Dec;171(6):1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982 Jan;145(1):1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Grigsby PL, Novy MJ, Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reprod Sci. 2010 Jan;17(1):85–94. doi: 10.1177/1933719109348025. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura K, Hirsch E. Interleukin-6 is neither necessary nor sufficient for preterm labor in a murine infection model. J Soc Gynecol Investig. 2003 Oct;10(7):423–7. doi: 10.1016/s1071-5576(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 27.Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007 Nov;197(5):518 e1–8. doi: 10.1016/j.ajog.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beutler B. Innate immune responses to microbial poisons: discovery and function of the Toll-like receptors. Annu Rev Pharmacol Toxicol. 2003;43:609–28. doi: 10.1146/annurev.pharmtox.43.100901.135729. [DOI] [PubMed] [Google Scholar]
- 29.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008 Feb;15(2):121–7. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003 Nov;163(5):2103–11. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci. 2007 May;14(4):315–20. doi: 10.1177/1933719107302959. [DOI] [PubMed] [Google Scholar]
- 32.Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, et al. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001 Dec 15;167(12):7069–76. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
- 33.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997 Jul 24;388(6640):394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 34.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998 Dec 11;282(5396):2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999 Feb 15;189(4):615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007 Aug 15;179(4):2501–8. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso G, Midiri A, Biondo C, Beninati C, Gambuzza M, Macri D, et al. Bacteroides fragilis-derived lipopolysaccharide produces cell activation and lethal toxicity via toll-like receptor 4. Infect Immun. 2005 Sep;73(9):5620–7. doi: 10.1128/IAI.73.9.5620-5627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams KM, Lucas J, Kapur RP, Stevens AM. LPS induces translocation of TLR4 in amniotic epithelium. Placenta. 2007 May-Jun;28(5-6):477–81. doi: 10.1016/j.placenta.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, et al. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002 Sep;107(1):145–51. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qureshi N, Takayama K, Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991 Jan;59(1):441–4. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, et al. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4′-phosphatase activities. Cell Microbiol. 2009 Nov;11(11):1587–99. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling RS, Ramamurthy RS, Gibbs RS. A comparison of intrapartum versus immediate postpartum treatment of intra-amniotic infection. Obstet Gynecol. 1987 Dec;70(6):861–5. [PubMed] [Google Scholar]
- 43.Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988 Dec;72(6):823–8. doi: 10.1097/00006250-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol. 1980 Mar 15;136(6):709–13. doi: 10.1016/0002-9378(80)90445-7. [DOI] [PubMed] [Google Scholar]
- 45.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. Jama. 2004 Jul 28;292(4):462–9. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 46.Gravett MG, Thomas A, Schneider KA, Reddy AP, Dasari S, Jacob T, et al. Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J Proteome Res. 2007 Jan;6(1):89–96. doi: 10.1021/pr060149v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hitti J, Lapidus JA, Lu X, Reddy AP, Jacob T, Dasari S, et al. Noninvasive diagnosis of intraamniotic infection: proteomic biomarkers in vaginal fluid. Am J Obstet Gynecol. May 15; doi: 10.1016/j.ajog.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]