Abstract
Understanding infertility and sterility requires knowledge of the molecular mechanisms underlying sexual reproduction. We have found that male mice deficient for the gene encoding the protease inhibitor protease nexin-1 (PN-1) show a marked impairment in fertility from the onset of sexual maturity. Absence of PN-1 results in altered semen protein composition, which leads to inadequate semen coagulation and deficient vaginal plug formation upon copulation. Progressive morphological changes of the seminal vesicles also are observed. Consistent with these findings, abnormal PN-1 expression was found in the semen of men displaying seminal dysfunction. The data demonstrate that the level of extracellular proteolytic activity is a critical element in controlling male fertility.
Increasing fertility problems in men led to major efforts for a better understanding of the cellular and molecular mechanisms underlying fertility (1). The two major determinants of the reproductive potential of an individual are the efficiency of the spermatogenesis and the function of the accessory sex glands.
Mice bearing single-gene mutations recently have provided exciting information about proteins required for a normal reproduction capability (2). In males, the genes identified to date are needed either for spermatogenesis or the generation of competent sperm. For example, male infertility was observed in mice lacking the HR6B gene, a homologue of a yeast gene encoding an ubiquitin-conjugating enzyme (3). Furthermore, the absence of an oligodendrocyte-specific protein/claudin-11 gene leads to reproductive deficits due to an abnormal generation of paracellular physical barrier of tight junctions necessary for spermatogenesis (4). Degeneration of spermatogonia was identified as the cause of male infertility in the few mice lacking apoptotic protease-activating factor-1 (Apaf-1) that survive to adulthood (5). Finally, fertilin β-deficient mice were shown to be deficient in sperm-egg membrane adhesion, sperm-egg fusion, and migration from the uterus into the oviduct (6).
However, only restricted information is available about proteins that are crucial for a normal function of the accessory sex glands. The seminal vesicle is crucial for normal reproduction, as shown by vesiculectomy experiments performed in mice (7). Several seminal vesicle functions have been proposed: stimulating sperm motility, serving as an energy source, providing immunosuppressive factors, participating in embryonic development (8–11), and in rodents, forming the copulatory plug (12).
Several serine proteases have been identified in the reproductive tract of both humans and rodents (13, 14). Proteases also are secreted in the lumen of the male genital tract (15). Urokinase and tissue type-plasminogen activators (uPA and tPA) have been described as the major proteases in semen from rodents (16). Their precise function in reproduction is still unknown, but uPA is secreted at the time of ejaculation and binds to the head of spermatozoa (16). Knowledge of how the activity of these proteases is regulated by their inhibitors is important in understanding their role in reproduction. Protein C inhibitor, a serine protease inhibitor secreted in the human seminal plasma, has been linked to cases of infertility (17). Protease nexin-1 (PN-1), a serine protease inhibitor belonging to the serpin superfamily, can modulate the proteolytic activity of thrombin, plasminogen activators, trypsin, and plasmin (18–21). Mouse PN-1 is expressed in a wide variety of tissues (22) but in the adult the highest levels are found to be under androgen control in the seminal vesicle (23). By inhibiting uPA and possibly other serine proteases, PN-1 could regulate the level of proteolytic activity in the seminal fluid.
Here, we report that mice homozygous for a disrupted PN-1 gene (PN-1−/− mice) show male infertility. This infertility is not due to altered spermatogenesis or sperm function but arises from a deficiency in the seminal fluid composition. Because of an enhanced proteolytic profile the seminal fluid is deficient in semenoclotin. Consequently, the copulatory plugs are malformed, lowering the probability of spermatozoa reaching the uterus. Furthermore, we find that infertile men with seminal vesicle dysfunction show abnormal PN-1 expression, thus indicating that controlled extracellular proteolytic activity is important for fertility in humans as well.
Materials and Methods
Animals.
PN-1 knockout mice were generated by homologous recombination in embryonic stem cells (24). Mice were backcrossed into the C57BL/6 mouse line. Sexually mature mice from 2 to 10 months were used in all experiments. Each male was placed with four females until copulation occurred as pretraining.
Mating and Counting Spermatozoa in Females.
Ten PN-1+/+ and 11 PN-1−/− samples from 3- to 5-month-old males were analyzed. In all cases, two females were mated with each male. Thirty minutes after coitus, the females were killed, the pubic bones were cut, and the vagina was opened ventrally. The vaginal plug was carefully removed, fixed in 70% ethanol, dried, and weighed. For five PN-1+/+ and five PN-1−/− animals, the uterine contents were retrieved by flushing and aspirating with 100 μl of PBS. Spermatozoa were counted by using a hematocytometer.
Seminal Vesicles.
Seminal vesicles were recovered from 4- and 10-month-old PN-1+/+ and PN-1−/− mice killed by cervical dislocation. After examination of the global morphology of the glands, they were frozen and stored at −80°C. Unstained 25-μm cryostat sections were used to study the internal appearance of the gland and other sections were stained with hematoxylin-eosin following Sigma Diagnostics protocol in the study of epithelial structure.
Collection of Seminal Vesicles and Coagulating Glands Fluid.
Seminal vesicles and coagulating glands were recovered from 2- and 10-month-old adult animals killed by cervical dislocation. Their respective fluids were collected by gently squeezing the glands against the wall of plastic tubes. Seminal vesicle fluid was centrifuged (3,000 × g for 10 min). The precipitate and the supernatant were collected. The liquid phase was diluted in 1/9 volumes of 10 mM Tris⋅HCl (pH 6.8), 1% SDS, 4% glycerol, and the pellet was resuspended in 180 μl of 10 mM Hepes, containing 0.32 M sucrose, and then mixed with 20 μl of 10 mM Tris⋅HCl (pH 6.8), 1% SDS, 4% glycerol. Samples were boiled for 5 min at 95°C. Protein (2.5 μg) was loaded onto SDS/PAGE gels and subsequently stained with Coomassie brilliant blue, as described (25).
Northern Analysis and in Situ Hybridization.
Northern blot analysis and in situ hybridization were performed with digoxigenin-labeled RNA probes for PN-1 prepared as described (26, 27).
Immunoblot Analysis and Immunocytochemistry.
Immunoblot analysis and immunocytochemistry were performed as described (22).
Enzymatic Assays.
The amount of thrombin-like protease and PN-1-like inhibitor present in the seminal vesicle and the coagulating gland fluids was evaluated by using a highly sensitive serine protease assay as described (28). The measured activity was compared with a standard dose–response curve with either thrombin or recombinant PN-1 protein and amount expressed in fmol/mg protein. To measure thrombin inhibition in plug homogenates, entire plugs of PN-1−/− and PN-1+/+ males (n = 3, in both cases) were mechanically homogenized in thrombin dilution buffer. The homogenates (0.8 μg of protein in 80 μl) were mixed with 10 μl human α-thrombin (1 nM in 67 mM Tris, pH 8.0, 133 mM NaCl, 0.13% PEG-6000) per well in a 96-well microtiter plate and incubated for 30 min at 37°C. After preincubation, 10 μl S-2288 substrate (H-D-Ile-Pro-Arg-para-nitroanilide, Chromogenix, Molndal, Sweden; 1.25 mg/ml in H2O) was added, and the remaining amidolytic activity was determined by measuring the rate of hydrolysis at 405 nm over 30 min by using a THERMOmax microplate reader (Molecular Devices) as described (29).
Human Semen Samples.
Human seminal plasma was obtained from semen analysis performed during the work-up of couples attending the sterility clinic. Seminal plasma was separated by centrifugation (2,000 × g for 10 min) and stored in aliquots at −20°C. Protein concentration was determined by using the TCA assay. Semen was analyzed according to the guidelines of the World Health Organization (38). The biochemical markers for accessory gland functions were fructose, zinc, and carnitine for the seminal vesicles, the prostate, and the epididymis, respectively (30).
Results and Discussion
Infertility in PN-1−/− Male Mice.
To study the impact of protease inhibition on reproduction, reciprocal matings were performed with mice deficient in the serpin PN-1 (Table 1). The formation of vaginal plugs, an indicator of successful copulation, confirmed normal copulatory behavior in all genotypes. There were no indications of impaired fertility in heterozygous or homozygous females. However, all different matings with mutant homozygous males (PN-1−/−) invariably resulted in fewer pregnancies than with wild-type (PN-1+/+) or heterozygous males (PN-1+/−) (18 of 68 vs. 41 of 45 matings). From the matings with homozygous PN-1−/− males, only a few litters were obtained (five of 18 vs. 41 of 41 pregnancies in matings with PN-1+/− and PN-1+/+ males, respectively). These few litters generated by PN-1−/− males had a reduced average size (2.8 pups, n =7 vs. 7.3 pups, n =41). From 24 PN-1−/− males analyzed only four impregnated females, thus indicating a reduced penetrance of the phenotype.
Table 1.
Reproduction data
| Crossing
|
||||||
|---|---|---|---|---|---|---|
| Male | −/− | −/− | +/+ | +/− | +/+ | |
| Female | −/− | +/+ | −/− | +/− | +/+ | |
| Number of males mated | 15 | 24 | 11 | 20 | 5 | |
| Number of females mated | 30 | 38 | 11 | 24 | 10 | |
| Males producing plugs, % | 93 | 64 | 100 | 100 | 100 | |
| Pregnant females, % | 21 | 32 | 81 | 95 | 90 | |
| Delivering females, % | 7 | 9 | 81 | 95 | 90 | |
| Average litter size | 2.5 | 3.1 | 7.3 | 7.4 | 7.4 | |
Sperm Analysis.
Plasminogen activators are serine proteases that can be inhibited by PN-1. Because they play a role in the binding of the head of spermatozoa to the zona pellucida (16), we investigated whether the infertility of PN-1−/− males was caused by sperm dysfunction. Histological examination of the testes of adult PN-1−/− males revealed neither abnormalities nor loss of cells and showed normal spermatogenesis (data not shown). Sperm cells from the epididymis of PN-1+/+ and PN-1−/− males were indistinguishable in motility parameters and in in vitro fertilization assays (data not shown). Thus, PN-1−/− males produce functional spermatozoa in sufficient amounts.
Seminal Vesicle Phenotype.
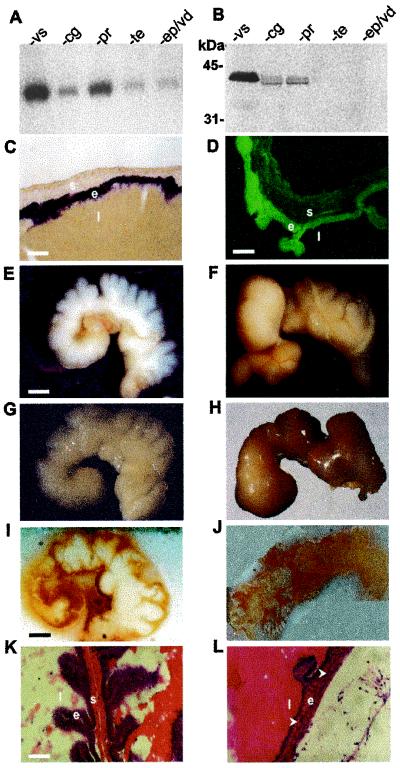
To gain insight into the mechanism leading to impaired fertility, we performed analyses to determine compartments of the male reproductive system in which PN-1 is present. As described earlier (23), the highest levels of PN-1 mRNA and protein were detected in seminal vesicles, especially in the secretory epithelium (Fig. 1 A–D). In line with this, the seminal vesicles of PN-1−/− mice showed progressive abnormalities starting at 4 months (Fig. 1 E–H). The most drastic alterations were the bloody and up to 3-fold enlarged glands found in four of nine PN-1−/− males (data not shown). Unstained frozen sections showed striking differences in the appearance of the vesicle lumens (Fig. 1 I and J). Morphology was normal at 2 months, the onset of sexual maturity, when infertility is already observed. Aberrant organization of the vesicle epithelium and the stroma was obvious at 10 months (Fig. 1 K and L).
Figure 1.
PN-1 expression in the wild-type male reproductive tract and seminal gland morphological phenotypes in PN-1−/− males. (A) PN-1 transcript and (B) PN-1 protein contents of the seminal vesicle (vs), the coagulating gland (cg), the prostate (pr), the testis (te), and the epididymis-vas deferens (ep/vd) of adult wild-type mice. (C) PN-1 in situ hybridization and (D) PN-1 immunocytochemistry in the adult seminal vesicle. PN-1 mRNA is expressed in the secretory epithelial cells (C); PN-1 protein is predominantly found at the apical side of these cells (D) as well as secreted into the lumen. e, epithelium; l, lumen; s, stroma. (E–H) Morphology of seminal vesicles from 4- and 10-month-old PN-1+/+ mice and PN-1−/− mice. At 4 months, the seminal vesicles of the mutant show a reduction in the number and the depth of the folds of the stromal sheath, obstruction, and/or dilatation of the distal part of the gland and a yellowish tint in the secretory fluid (F compared with E). At 10 months, the phenotype was more severe: these organs showed a massive dilatation and the secretory fluid showed a strong yellow-brownish coloration (H compared with G). (I and J) Compared with the homogenous white appearance of the wild-type seminal vesicle (I) a micro granular-like-structure with a brownish color was detected in the mutant at 6 months (J). (K and L) Histology of seminal vesicle epithelium from 2-month-old (K) and 10-month-old (L) PN-1−/− mice. Frozen sections were stained with hematoxylin and eosin. Note the decreased organization of the epithelium layer and the apparent loss of the stroma in 10-month-old mutant mice (arrows). [Scale bars: (C and K) 50 μm; (D) 35 μm; (E) 1.5 mm; (I) 1.2 mm.]
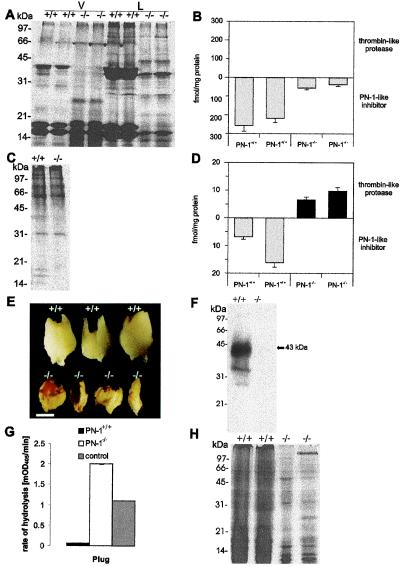
To assess whether the morphological findings were associated with changes in protein composition, we analyzed the protein profile in seminal vesicle fluid from 2-month-old PN-1−/− and wild-type males (Fig. 2A). The absence of many proteins, especially of semenoclotin (31) corresponding to the 38-kDa band, and the appearance of a new prominent band migrating at 28 kDa indicated increased proteolytic activity in the absence of PN-1. Consistent with these findings, a 6.8-fold decrease in thrombin inhibitory activity was detected in the fluid of mutant seminal vesicles (35 vs. 240 fmol PN-1 equivalent per mg protein) (Fig. 2B). On the other hand, in the coagulating glands fluid, the protein profile was normal (Fig. 2C), although loss of inhibitory activity in the mutant males resulted in a slight excess of thrombin-like proteolytic activity (Fig. 2D). Thus, proteolytic activity in seminal vesicle secretions appears to be very tightly controlled. A massive reduction in inhibitory activity could, therefore, have pronounced consequences. The histological and biochemical data strongly suggest that the seminal vesicle is the organ in the reproductive system most affected by PN-1 deletion.
Figure 2.
Biochemical phenotypes in the accessory sex glands and plug formed by PN-1+/+ and PN-1−/− males. (A and C) PAGE of seminal vesicle-secreted proteins and coagulating gland-secreted proteins from adult PN-1+/+ and PN-1−/− mice. The collected seminal vesicle fluid was separated by centrifugation into a liquid (L) and a viscous (V) fraction. Wild-type seminal liquid phase revealed three major bands, with estimated molecular masses of 38, 17, and 16 kDa (A). In the corresponding PN-1−/− samples, the 38-kDa component was strongly decreased and a component of around 28 kDa was observed. The same general pattern was found in the viscous fraction, but in this case other bands of intermediate size were seen in the mutant samples (A). There was no difference in the coagulating gland protein patterns of PN-1+/+ and PN-1−/− mice (C). (B and D) Amount of thrombin-like protease and PN-1-like inhibitor in the seminal vesicle and the coagulating gland of 14-day-old PN-1+/+ and PN-1−/− mice. In the mutant mice, seminal fluid showed reduced thrombin inhibitory activity (B), whereas coagulating gland fluid showed an excess of unidentified thrombin-like protease activity (D). Notice the different scales used in B and D. (E) A decrease in plug size as well as a modified appearance is clearly seen in PN-1−/− mice compared with the plug generated by wild type. Plugs are oriented with their proximal ends at the top. (Scale bar, 0.75 mm.) (F) Immunoblot indicates that PN-1 is secreted during ejaculation and is then present in plugs generated by PN-1+/+ males. (G) Proteolytic activity assay in plugs homogenates in the presence of added thrombin. The surplus proteolytic activity detected in plugs generated by PN-1−/− males compared with the buffer control with 0.1 nM added thrombin (see Materials and Methods) indicates an endogenous PN-1-sensitive proteolytic activity. (H) Coomassie blue staining of PAGE-analyzed proteins contained in plugs generated by PN-1+/+ and PN-1−/− mice. Each lane was loaded with 5 μg protein.
Vaginal Plug Phenotype.
We compared copulatory plugs produced by wild-type and mutant males (Fig. 2E) to evaluate the effect of reduced protease inhibition in the seminal vesicle fluid. Although plugs were formed by mutant and wild-type males (Table 1) there were differences in size and consistency. Those generated by PN-1+/+ males were hard, tightly filled the vagina, abutting the cervix, and weighed 22.500 ± 0.006 mg SD (n =9), whereas those from PN-1−/− males were smaller, soft and fibrous, did not lodge tightly in the cervical opening, and weighed only 6.255 ± 0.004 mg SD (n =14). Because one function of the vaginal plug is to prevent loss of sperm and increase their chances of reaching the uterus (32), the number of spermatozoa was measured in the uterine cornua 15 min after coitus. No sperm could be counted in the uterus after mating with the group of PN-1−/− infertile males (0.0 ± 0.0 PN-1−/− spermatozoa per uterus, n = 5 vs. 58.7 ± 4.8 × 105 PN-1+/+ spermatozoa per uterus, n = 5 ). The presence of PN-1 protein in the plugs generated by PN-1+/+ males (Fig. 2F) indicates that the serpin stored in the seminal vesicle is one of the substances ejaculated into the female genital tract during copulation. Consequently, much higher thrombin-like activity was detected in homogenates of plugs generated by PN-1−/− than by wild-type mice (data not shown). To check for PN-1-like activity in these plug homogenates, thrombin was exogenously added to the samples and the resulting proteolytic activity was measured (Fig. 2G). Addition of exogenous thrombin to plug homogenates from PN-1+/+ males did not increase proteolytic activity, indicating an excess of PN-1 in these samples. This also implies that the residual proteolytic activity detected in these plug homogenates is PN-1 insensitive. When homogenates with equal amounts of protein derived from mutant and wild-type plugs were compared by SDS/PAGE analysis, significantly fewer protein bands were detected in the mutant sample (Fig. 2H). This finding suggests that in the mutant many of the proteins normally forming the plug had been degraded to peptides too small to be retained by the gel. Abnormal plug formation by PN-1−/− males can be explained by the near absence of the 38-kDa protein semenoclotin, which is known to serve as a substrate for prostatic transglutaminases and become a main constituent of the copulatory plug (31). The small, fibrous, malformed plugs are not able to seal the vagina after coitus, thus leading to the reduced number of sperm found in the female partner.
PN-1 Protein in Semen from Infertile Men.
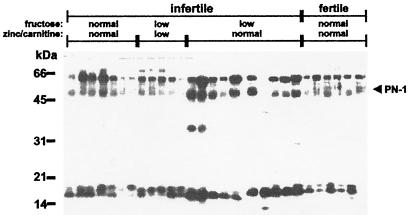
In 10% of cases, male sterility results from various malformations of the seminal vesicle, such as obstruction of the seminal tract and unilateral or bilateral absence of this organ, which lead to characteristic changes in the ejaculate (33). Immediately after ejaculation, human semen forms a coagulum that is liquefied within 5–20 min (34). The function of this phenomenon is not understood but it is reminiscent of plug formation in rodents. As the data in mice revealed the importance of a correct balance between protease and protease inhibitory activity in the semen, we compared PN-1 protein levels in semen samples of men in couples with and without fertility problems (Fig. 3). The seminal plasma of men from infertile couples was classified according to fructose, zinc, and carnitine levels, which are used to evaluate the secretory activity of the seminal vesicle, the prostate, and the epididymis, respectively (30). High PN-1 levels were found in semen of men where seminal dysfunction was characterized by low fructose but normal zinc and carnitine levels (Fig. 3). In contrast, PN-1 protein was only weakly detected in seminal plasma from fertile men, as well as in the two other categories of men belonging to couples with fertility problems. Similar immunoblot profiles were obtained by using a monoclonal antibody raised against rat PN-1 (data not shown).
Figure 3.
High PN-1 levels are typically detected in men with a marker profile indicating altered seminal vesicle secretory activity. PN-1 was detected in 100 μg protein of seminal plasma from fertile men and men from sterile couples using rabbit polyclonal antibody (RAB-1) raised against human PN-1. Samples obtained from infertile men are grouped into three classes depending on the concentrations of fructose, zinc, and carnitine. The range of concentrations were: fructose, normal = 25–104 μmol/ejaculate, low = 6–16 μmol/ejaculate; zinc, normal = 4–14 μmol/ejaculate, low = 1–3 μmol/ejaculate; and carnitine, normal = 0.8–2.9, low = 0.0–0.7 μmol/ejaculate. Seminal plasma from fertile men was analyzed as a control. The bands detected at about 60 kDa and below 20 kDa are considered to be high molecular mass complexes and degradation products, respectively.
Conclusions.
The increase of PN-1 levels found in the semen of a subgroup of men with abnormal seminal vesicle secretory activity is in contrast to the situation in mice, where deletion of the PN-1 gene leads to infertility through the impaired control of proteolytic activity in the seminal fluid. Nevertheless, these results obtained from both mouse and human males highlight the importance of a balance between proteases and their inhibitors in semen. In this context, altered levels of plasminogen activators in semen were found in some cases where abnormal liquefaction or sperm immobility lead to human sterility (35, 36). Inactive protein C inhibitor, another member of the serpin family, also has been detected in cases of human sterility (17). The identification of a protein belonging to the serpin superfamily among new genes specific for the male accessory gland in Drosophila melanogaster (37) suggests that the function of protease inhibitors in reproduction spans many species. Our report demonstrates a link between infertility and disturbed composition of the seminal fluid caused by a single gene deletion. As semen is readily accessible to analysis (e.g., proteomics) and experimental modification in human and other species, our data open up perspectives on the role played by the seminal vesicle fluid. They should stimulate research in the field of human fertility control, as well as in situations where the efficiency of artificial insemination needs to be improved, for example in endangered species.
Acknowledgments
We thank P. King, M. Meins, U. Müller, P. Matthias, G. Thomas, and J. D. Vassali for critical reading of the manuscript, D. Hantai for the gift of the RAB-1 anti-human PN-1 antibody, and S. Taieb and E. Fries for technical assistance. We are also grateful to the Novartis Research Foundation for financial support.
Abbreviation
- PN-1
protease inhibitor protease nexin-1
References
- 1.Carlsen E, Giwercman A, Keiding N, Skakkebaek N E. Br Med J. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brüning J C, Gautam D, Burks D J, Gillette J, Schubert M, Orban P C, Klein R, Krone W, Müller-Wieland D, Kahn C R. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 3.Baarends W M, Hoogerbrugge J W, Roest H P, Ooms M, Vreeburg J, Hoeijmakers J H, Grootegoed J A. Dev Biol. 1999;207:322–333. doi: 10.1006/dbio.1998.9155. [DOI] [PubMed] [Google Scholar]
- 4.Gow A, Southwood C M, Li J S, Pariali M, Riordan G P, Brodie S E, Danias J, Bronstein J M, Kachar B, Lazzarini R A. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 5.Honarpour N, Du C, Richardson J A, Hammer R E, Wang X, Herz J. Dev Biol. 2000;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- 6.Cho C, O'Dell Bunch D, Faure J E, Goulding E H, Eddy E M, Primakoff P, Myles D G. Science. 1998;281:1857–1960. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- 7.Pang S F, Chow P H, Wong T M. J Reprod Fertil. 1979;56:129–132. doi: 10.1530/jrf.0.0560129. [DOI] [PubMed] [Google Scholar]
- 8.Peitz B. Biol Reprod. 1986;83:169–176. [Google Scholar]
- 9.Mann T, Lutwak-Mann C. Male Reproductive Function and Semen. Berlin: Springer; 1981. [Google Scholar]
- 10.Thaler C J. Am J Reprod Immunol. 1989;21:147–150. doi: 10.1111/j.1600-0897.1989.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 11.O W S, Chen H Q, Chow P H. J Reprod Fertil. 1988;84:341–344. doi: 10.1530/jrf.0.0840341. [DOI] [PubMed] [Google Scholar]
- 12.Fawell S E, Higgins S J. Mol Cell Endocrinol. 1987;53:1949–1952. doi: 10.1016/0303-7207(87)90201-2. [DOI] [PubMed] [Google Scholar]
- 13.Chen L Y, Lin Y H, Lai M L. Biol Reprod. 1998;92:1498–1505. doi: 10.1095/biolreprod59.6.1498. [DOI] [PubMed] [Google Scholar]
- 14.Charlesworth M C, Young C Y, Miller V M, Tindall D J. J Androl. 1999;20:220–229. [PubMed] [Google Scholar]
- 15.Clements J A, Matheson B A, Wines D R, Brady J M, MacDonald R J, Funder J W. J Biol Chem. 1988;263:16132–16137. [PubMed] [Google Scholar]
- 16.Huarte J, Belin D, Bosco D, Sappino A P, Vassali J D. J Cell Biol. 1987;104:1281–1289. doi: 10.1083/jcb.104.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He S, Lin Y L, Liu Y X. Mol Hum Reprod. 1999;5:513–519. doi: 10.1093/molehr/5.6.513. [DOI] [PubMed] [Google Scholar]
- 18.Baker J B, Low D A, Simmer R L, Cunningham D D. Cell. 1980;21:37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- 19.Guenther J, Nick H, Monard D. EMBO J. 1985;4:1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gloor S, Odink K, Guenther J, Nick H, Monard D. Cell. 1986;47:687–693. doi: 10.1016/0092-8674(86)90511-8. [DOI] [PubMed] [Google Scholar]
- 21.Stone S R, Nick H, Hofsteenge J, Monard D. Arch Biochem Biophys. 1992;252:237–244. doi: 10.1016/0003-9861(87)90028-2. [DOI] [PubMed] [Google Scholar]
- 22.Mansuy I M, van der Putten H, Schmid P, Meins M, Botteri F M, Monard D. Development (Cambridge, UK) 1993;119:1119–1134. doi: 10.1242/dev.119.4.1119. [DOI] [PubMed] [Google Scholar]
- 23.Vassalli J D, Huarte J, Bosco D, Sappino A P, Sappino N, Velardi A, Wohlwend A, Ernø H, Monard D, Belin D. EMBO J. 1993;12:1871–1878. doi: 10.1002/j.1460-2075.1993.tb05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luethi A, van der Putten H, Botteri F M, Mansuy M, Frey U, Sansig G, Portet C, Schmutz M, Schroeder M, Nitsch C, et al. J Neurosci. 1997;17:4688–4699. doi: 10.1523/JNEUROSCI.17-12-04688.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley; 1989. [Google Scholar]
- 26.Schaeren-Wiemers N, Gerfin-Moser A. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- 27.Küry P, Schaeren-Wiemers N, Monard D. Development (Cambridge, UK) 1997;124:1251–1262. doi: 10.1242/dev.124.6.1251. [DOI] [PubMed] [Google Scholar]
- 28.Altrogge L M, Monard D. Anal Biochem. 2000;277:33–45. doi: 10.1006/abio.1999.4356. [DOI] [PubMed] [Google Scholar]
- 29.Hengst U, Albrecht H, Hess D, Monard D. J Biol Chem. 2001;276:535–540. doi: 10.1074/jbc.M002524200. [DOI] [PubMed] [Google Scholar]
- 30.Gonzales G F, Garcia-Hjarles M, Napuri R. Arch Androl. 1988;21:135–142. doi: 10.3109/01485018808986724. [DOI] [PubMed] [Google Scholar]
- 31.Lundwall A, Peter A, Lovgren J, Lilja H, Malm J. FEBS Lett. 1997;249:39–44. doi: 10.1111/j.1432-1033.1997.t01-2-00039.x. [DOI] [PubMed] [Google Scholar]
- 32.Carballada R, Esponda P. J Reprod Fertil. 1992;95:639–648. doi: 10.1530/jrf.0.0950639. [DOI] [PubMed] [Google Scholar]
- 33.Abbit P L, Watson L, Howards S. Am J Roentgenol. 1991;157:337–339. doi: 10.2214/ajr.157.2.1853818. [DOI] [PubMed] [Google Scholar]
- 34.Tauber P F, Propping D, Schumacher G F B, Zaneveld L J D. J Androl. 1980;1:281–288. [Google Scholar]
- 35.Arnaud A, Schved J F, Gris J C, Costa P, Navratil H, Humeau C. Fertil Steril. 1994;61:741–745. doi: 10.1016/s0015-0282(16)56655-2. [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Liu Y X, Du Q, Zhou H M, Lin X, Hu Z Y, Zhang G Y, Zhang G H. Mol Hum Reprod. 1996;2:99–104. doi: 10.1093/molehr/2.2.99. [DOI] [PubMed] [Google Scholar]
- 37.Wolfner M F, Harada H A, Bertram M J, Stelick T J, Kraus K M, Kalb J M, Lung Y O, Neubaum D M, Park M, Tram U. Insect Biochem Mol Biol. 1997;27:825–834. doi: 10.1016/s0965-1748(97)00056-8. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. World Health Organization Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interactions. 4th Ed. Geneva: World Health Organization; 1999. [Google Scholar]