Abstract
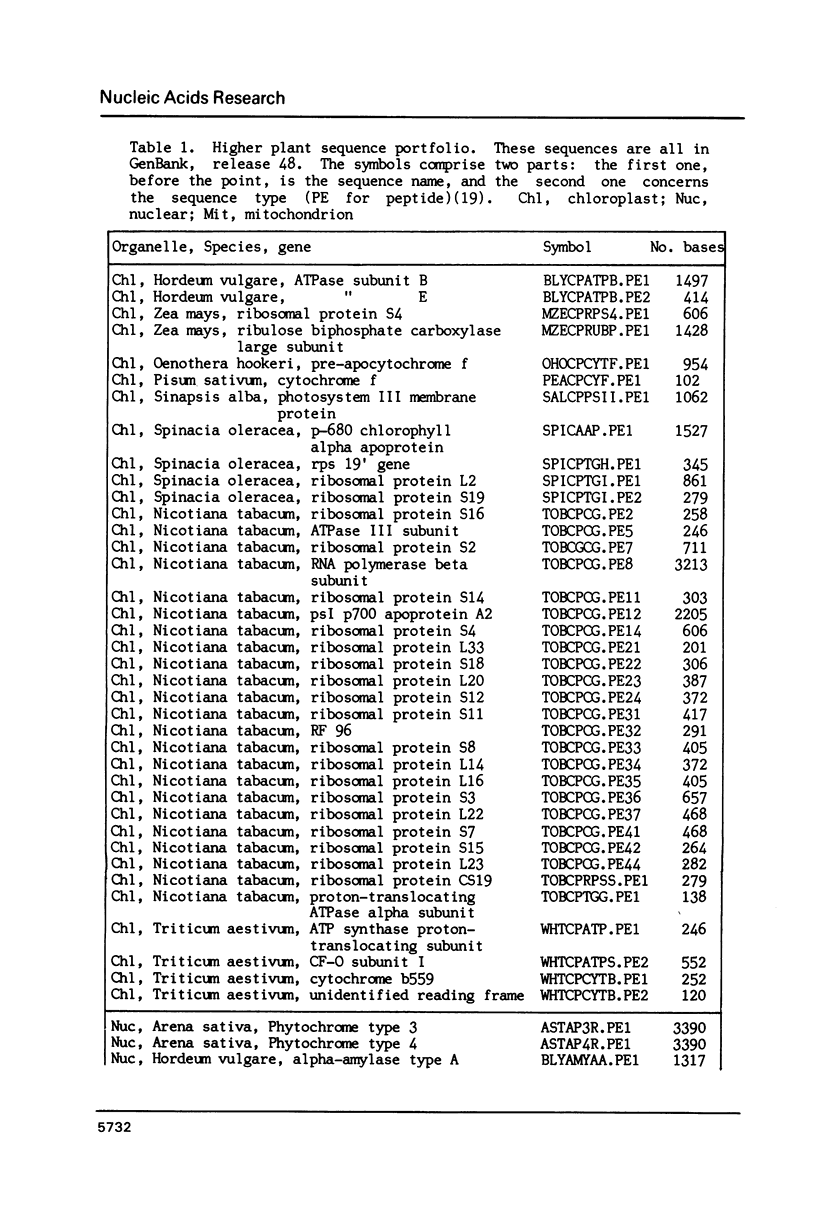
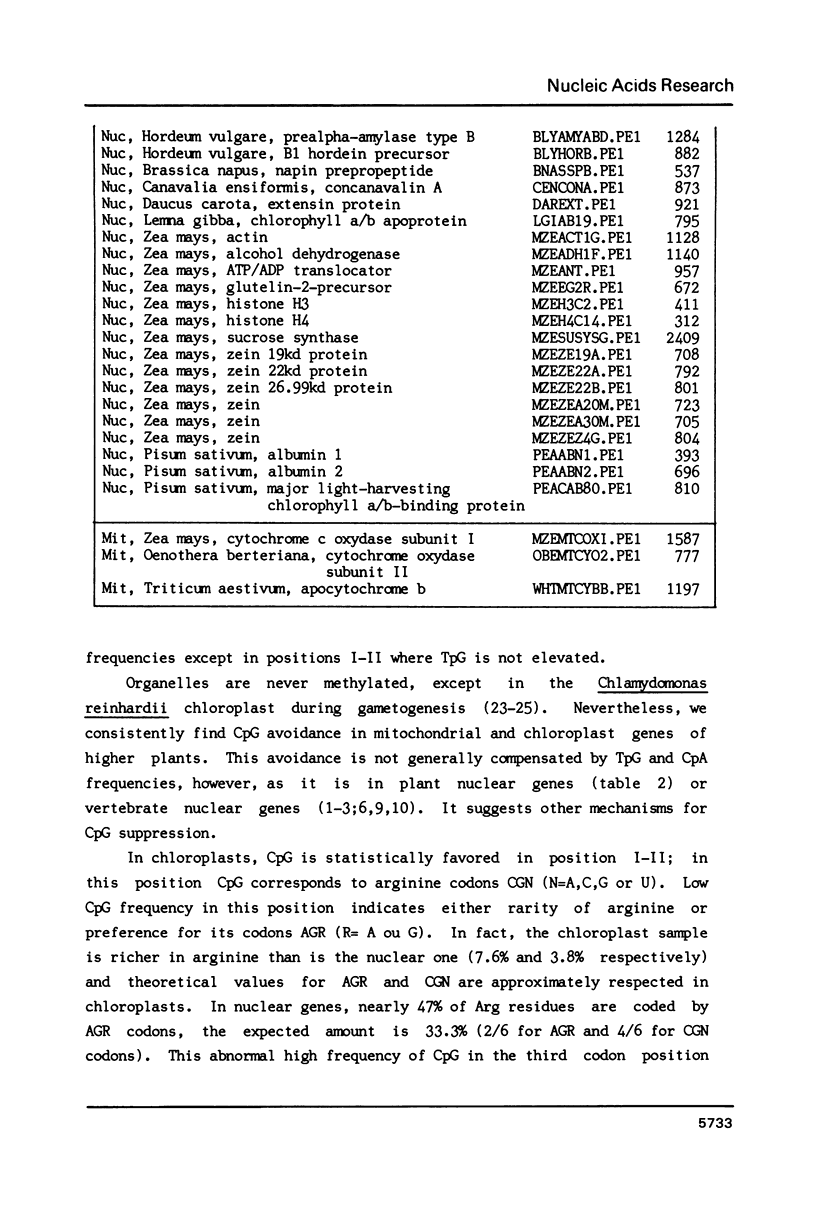
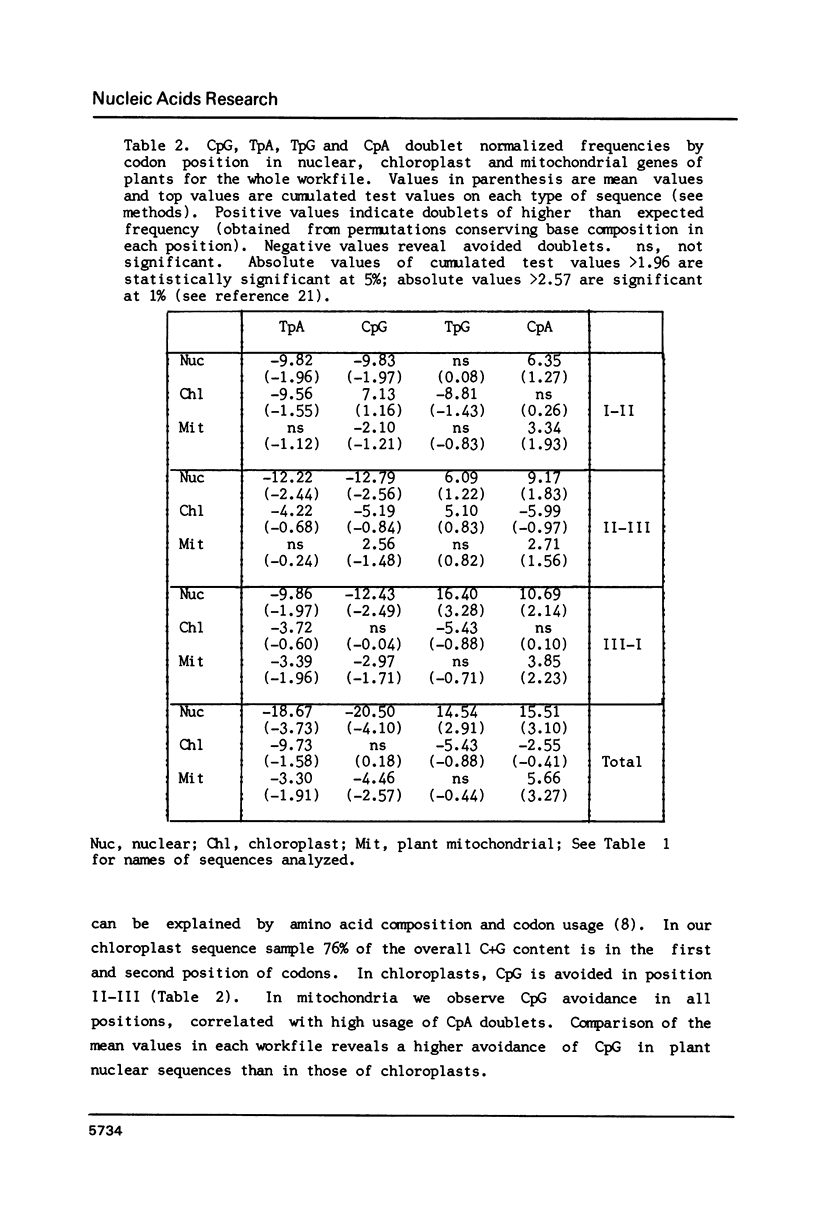
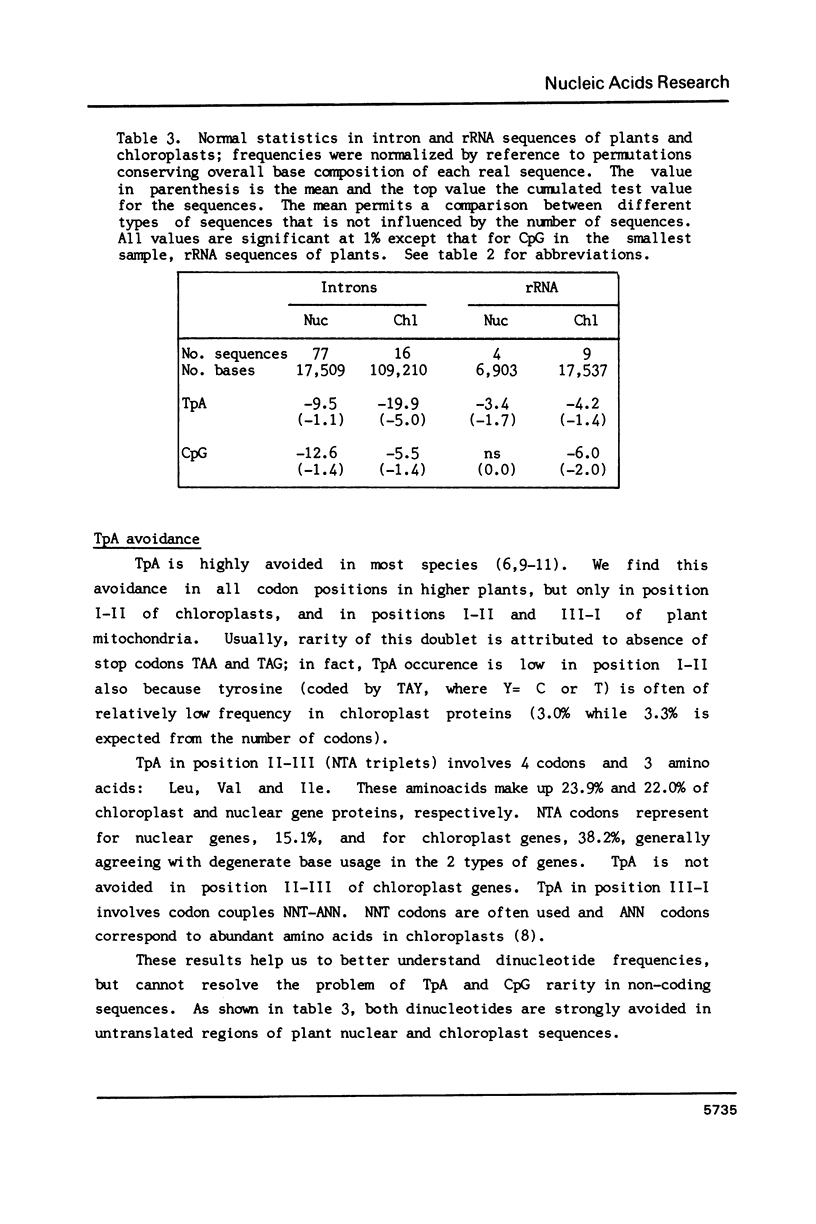
Higher plant nuclear sequences reveal avoidance of CpG and TpA doublets. Chloroplast sequences avoid the TpA doublet in all codon positions. The chloroplast genome is not methylated but codon positions II-III and untranslated regions avoid CpG. The mitochondrial genome, also unmethylated, avoids CpG in all codon positions. We therefore deduce that methylation is not sufficient to explain CpG avoidance in the higher plant systems. Other factors must be taken into account such as amino acid composition, codon choices and perhaps stability of the DNA helix.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G., Olofsson B., Filipski J., Zerial M., Salinas J., Cuny G., Meunier-Rotival M., Rodier F. The mosaic genome of warm-blooded vertebrates. Science. 1985 May 24;228(4702):953–958. doi: 10.1126/science.4001930. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CG doublet difficulties in vertebrate DNA. Nature. 1985 Feb 7;313(6002):437–438. [PubMed] [Google Scholar]
- Dyer T. A. Methylation of chloroplast DNA in Chlamydomonas. Nature. 1982 Jul 29;298(5873):422–423. doi: 10.1038/298422a0. [DOI] [PubMed] [Google Scholar]
- Gautier C., Gouy M., Louail S. Non-parametric statistics for nucleic acid sequence study. Biochimie. 1985 May;67(5):449–453. doi: 10.1016/s0300-9084(85)80263-7. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C., Attimonelli M., Lanave C., di Paola G. ACNUC--a portable retrieval system for nucleic acid sequence databases: logical and physical designs and usage. Comput Appl Biosci. 1985 Sep;1(3):167–172. doi: 10.1093/bioinformatics/1.3.167. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C., Milleret F. System analysis and nucleic acid sequence banks. Biochimie. 1985 May;67(5):433–436. doi: 10.1016/s0300-9084(85)80260-1. [DOI] [PubMed] [Google Scholar]
- Gouy M., Milleret F., Mugnier C., Jacobzone M., Gautier C. ACNUC: a nucleic acid sequence data base and analysis system. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):121–127. doi: 10.1093/nar/12.1part1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R. Viral, prokaryote and eukaryote genes contrasted by mRNA sequence indexes. FEBS Lett. 1978 Nov 1;95(1):1–11. doi: 10.1016/0014-5793(78)80041-6. [DOI] [PubMed] [Google Scholar]
- Jou W. M., Verhoeyen M., Devos R., Saman E., Fang R., Huylebroeck D., Fiers W., Threlfall G., Barber C., Carey N. Complete structure of the hemagglutinin gene from the human influenza A/Victoria/3/75 (H3N2) strain as determined from cloned DNA. Cell. 1980 Mar;19(3):683–696. doi: 10.1016/s0092-8674(80)80045-6. [DOI] [PubMed] [Google Scholar]
- Knight W. A run-like statistic for ecological transects. Biometrics. 1974 Sep;30(3):553–555. [PubMed] [Google Scholar]
- Lindahl T. DNA methylation and control of gene expression. Nature. 1981 Apr 2;290(5805):363–364. doi: 10.1038/290363b0. [DOI] [PubMed] [Google Scholar]
- Max E. E. New twist to DNA methylation. Nature. 1984 Jul 12;310(5973):100–100. doi: 10.1038/310100a0. [DOI] [PubMed] [Google Scholar]
- Nussinov R. Doublet frequencies in evolutionary distinct groups. Nucleic Acids Res. 1984 Feb 10;12(3):1749–1763. doi: 10.1093/nar/12.3.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Grabowy C., Sager R. Loss of chloroplast DNA methylation during dedifferentiation of Chlamydomonas reinhardi gametes. Mol Cell Biol. 1984 Oct;4(10):2103–2108. doi: 10.1128/mcb.4.10.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S., Sadler J. R. Statistical characterization of nucleic acid sequence functional domains. Nucleic Acids Res. 1983 Apr 11;11(7):2205–2220. doi: 10.1093/nar/11.7.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykocinski M. L., Max E. E. CG dinucleotide clusters in MHC genes and in 5' demethylated genes. Nucleic Acids Res. 1984 May 25;12(10):4385–4396. doi: 10.1093/nar/12.10.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]



