A 62-year-old man presented with a one-week history of fever and confusion. His family had noted progressive memory impairment and difficulty following verbal instructions. He also had a two-week history of productive cough that had failed to respond to clarithromycin. There was no history of tuberculosis, or recent travel or contact with anyone who was ill. His past medical history included Waldenström macroglobulinemia, which had responded to eight cycles of fludarabine seven years earlier followed by maintenance therapy with chlorambucil. Because of worsening anemia and evidence of increasing bone marrow infiltration, the patient had been given combination chemotherapy with cyclophosphamide, vincristine, prednisone and rituximab six weeks before presentation. He had completed his second cycle of chemotherapy eight days before presentation.
On examination, he appeared unwell. He had a blood pressure of 120/60 mm Hg, a heart rate of 88 beats/min, a respiratory rate of 16 breaths/min and a temperature of 38.3°C. His oxygen saturation was 97% on room air. He was alert but disoriented with respect to place and time. There were no focal neurologic deficits. Chest examination revealed decreased breath sounds and crackles in the right base of the lung. Abdominal examination revealed a palpable spleen tip, but no hepatomegaly. There was no palpable lymphadenopathy. Examination of the skin showed an erythematous papule above the right ear.
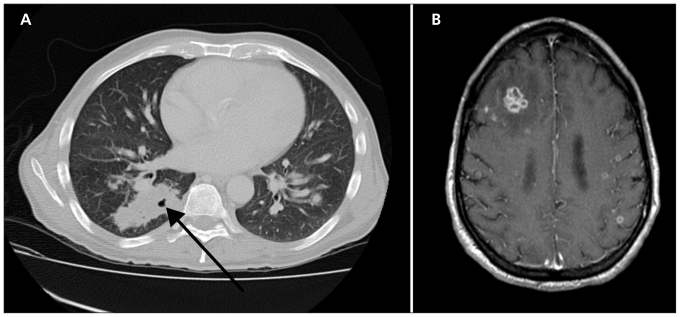
The results of laboratory investigations are summarized in Table 1. Chest radiography showed an opacity in the right lower lobe. A contrast-enhanced computed tomography (CT) scan of the chest revealed multiple, bilateral nodules and a cavity in the right lower lobe of the lung (Figure 1A). A contrast-enhanced CT scan of the brain showed a mass in the right frontal lobe. A subsequent contrast-enhanced magnetic resonance imaging (MRI) scan of the brain showed multiple ring-enhancing lesions (Figure 1B). Despite a one-week course of broad-spectrum antibiotic treatment with piperacillin–tazobactam, the patient’s fever persisted, and consultation by an infectious diseases specialist was requested.
Table 1:
Summary of laboratory investigations in a man with fever, cough and confusion shortly after a course of chemotherapy for Waldenström macroglobulinemia
| Investigation (normal range) | Result |
|---|---|
| Complete blood count | |
| Hemoglobin, g/L (140–180) | 93 |
| Leukocyte count, × 109/L (4.0–11.0) | 0.7 |
| Neutrophil count, × 109/L (2.0–7.5) | 0.2 |
| Lymphocyte count, × 109/L (1.5–4.0) | 0.1 |
| Platelet count, × 109/L (150–400) | 41 |
| Immunologic studies | |
| IgG level, g/L (7–16) | 2.6 |
| IgA level, mg/L (700–4000) | < 250 |
| IgM level, mg/L (400–2300) | 35 300 |
| CD4 count,* × 109/L (0.55–1.65) | 0.18 |
| Cerebrospinal fluid analysis | |
| Leukocyte count, × 106/L (0–5) | 4 |
| Glucose level, mmol/L (2.2–4.4) | 3.9 |
| Protein level, g/L (< 0.45) | 1.5 |
| Bacterial culture | Negative |
| Fungal culture | Negative |
| Cryptococcal antigen | Negative |
| Cytology | Lymphoma cells |
Performed two years before presentation.
Figure 1:
Contrast-enhanced images in a 62-year-old man with fever, cough and confusion eight days after chemotherapy for Waldenström macroglobulinemia. (A) Computed tomography scan of the lungs showing bilateral nodular opacities with a cavity in the right lower lobe of the lung (arrow). (B) Magnetic resonance imaging scan of the brain showing multiple ring-enhancing lesions with surrounding edema.
What is your diagnosis?
Waldenström macroglobulinemia with lung and brain involvement
Nocardiosis
Tuberculosis
Invasive aspergillosis
Pyogenic lung and brain abscesses due to mixed oral flora
The most likely answers are (b) nocardiosis and (d) invasive aspergillosis. Although Waldenström macroglobulinemia could not be excluded at this point, an infectious cause was considered most likely. The first step in determining the likely pathogen included a careful consideration of the patient’s specific immunologic deficits. He had chemotherapy-induced neutropenia, placing him at increased risk of both gram-positive and gram-negative bacterial infections as well as invasive fungal infections caused by Candida and Aspergillus species. The lack of response to empiric therapy with piperacillin–tazobactam suggested that a traditional bacterial infection due to mixed oral flora was unlikely; however, an invasive fungal infection remained possible.
The patient also had evidence of humoral immunodeficiency because his IgG and IgA levels were less than 50% of the lower limits of the normal ranges. This immunodeficiency was likely secondary to the Waldenström macroglobulinemia, which is a B-lymphocyte disorder, as well as to treatment with rituximab, which is an anti-CD20 monoclonal antibody that results in significant B-lymphocyte depletion. A state of humoral immunodeficiency places a patient at increased risk of infection with encapsulated bacteria, such as Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis. These bacteria likely would have responded to the piperacillin–tazobactam treatment.
Prior treatment with fludarabine and current treatment with prednisone also raised the possibility of impaired cell-mediated immunity. Fludarabine is a purine-analogue chemotherapeutic agent that results in prolonged and profound T-lymphocyte depletion. A review of the patient’s records from two years earlier revealed a CD4 count of 0.18 × 109/L, indicating substantial cellular immunodeficiency. This condition would predispose him to infection with opportunistic viral, fungal, mycobacterial and protozoal pathogens, as well as to infection with specific bacteria such as Nocardia species, Salmonella species, Legionella species and Listeria monocytogenes.
After recognizing the patient’s specific immunologic deficits, we considered infectious causes of concurrent lung and brain disease (Box 1).1 Among those listed, nocardiosis or a disseminated fungal infection were thought to be most likely in the context of his impaired cell-mediated immunity, the lack of epidemiologic risk factors for tuberculosis and the recent use of broad-spectrum antibacterial treatment without clinical improvement. We began empiric treatment with trimethoprim–sulfamethoxazole for coverage of (b) Nocardia species and voriconazole for coverage of fungi, including (d) Aspergillus species.
Box 1: Common infectious causes of concurrent lung and brain infections1.
Bacteria
-
Mixed oral flora
- Streptococci and anaerobic organisms
Streptococcus anginosus
Staphylococcus aureus
Klebsiella pneumoniae (hypermucoviscous variant)
Nocardia species
Mycobacteria
Mycobacterium tuberculosis
Fungi
Aspergillus species
Agents of mucormycosis
Cryptococcus neoformans
How would you confirm your diagnosis?
Stop antibiotics and repeat blood cultures in 48 hours
Bronchoscopy with bronchoalveolar lavage
CT-guided lung biopsy
Skin biopsy
Brain biopsy
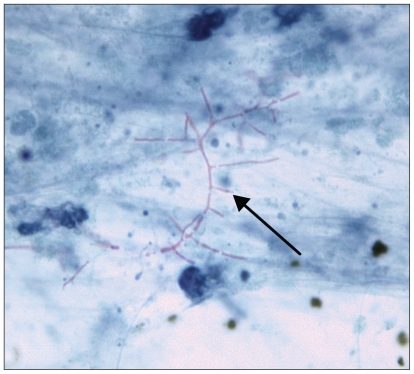
A tissue sample for diagnosis was needed at this point. Both (c) CT-guided lung biopsy and (d) skin biopsy were performed because of their lower procedural risks compared with brain biopsy. Biopsies of the skin nodule and the right lower lobe of the lung revealed filamentous gram-positive bacilli that were partially acid-fast (Figure 2), consistent with nocardiosis. The organism was later confirmed by culture and molecular detection techniques to be Nocardia cyriacigeorgica2 (formerly of the N. asteroides complex), which was susceptible to both trimethoprim–sulfamethoxazole and meropenem.
Figure 2:
Modified acid-fast stain of a biopsy specimen from the right lower lobe of the lung (original magnification × 1200), showing branching, filamentous organisms compatible with Nocardia species (arrow).
Discussion
Nocardia species are branching gram-positive filamentous bacteria that are ubiquitous in soil and water. Human infection most commonly occurs in patients with impaired cell-mediated immunity but may occur in otherwise healthy individuals.3 Common predisposing conditions include HIV infection, malignant lymphoreticular disorders, autoimmune disorders treated with prednisone and cytotoxic chemotherapeutic agents, and solid organ and hematopoetic stem cell transplantation.4 Patients with chronic pulmonary disorders, most notably pulmonary alveolar proteinosis, are also at increased risk of infection.3
Inhalation is the most common route of infection, with secondary dissemination to extrapulmonary sites. Isolated pulmonary infection is most common, occurring in about 70% of those affected, followed by disseminated disease (13.5%) and isolated involvement of the skin (8.1%) and central nervous system (5.4%).4 Imaging of the central nervous system should be performed in all patients with nocardiosis to exclude the possibility of subclinical disease of the central nervous system,3 because of the implications for the duration of treatment.3,4 An exception might be considered for immunocompetent patients who have isolated cutaneous nocardiosis resulting from direct skin inoculation.
Arriving at a diagnosis of nocardiosis may be challenging. A high degree of clinical suspicion is required, and communication with the microbiology laboratory is paramount. Nocardia species grow slowly on standard culture media and may require up to several weeks to reach detectable levels. Given that most microbiology laboratories discard specimens after five days of incubation, laboratory staff must be alerted to the potential diagnosis of nocardiosis so that specimens are kept for prolonged incubation.2 With the wider availability of molecular detection techniques, such as 16S rRNA gene polymerase chain reaction, identification of the species should be performed on all nocardial isolates, because antimicrobial susceptibility differs between species. Standards for testing antimicrobial susceptibility have existed for Nocardia species since 2003 and should be requested routinely.2 However, caution is needed in interpreting the results, because studies correlating clinical outcomes with laboratory susceptibility testing are not as extensive as for other organisms.2
Treatment
Although trimethoprim–sulfamethoxazole is considered the first-line treatment of nocardiosis, not all isolates are susceptible to this agent.2–4 Alternative therapies include imipenem, meropenem, amikacin and linezolid. In seriously ill patients, including those with central nervous system disease, empiric therapy with trimethoprim–sulfamethoxazole in combination with meropenem or amikacin, or both, is recommended until susceptibility data are available.3,5 Based on small observational studies, the recommended duration of treatment is 12 months or longer for immunocompromised patients and those with central nervous system disease.3,4 The clinical outcome for nocardiosis depends on the sites of infection. Mortality may be as high as 50% among patients with involvement of the central nervous system.3
After the start of combination therapy with trimethoprim–sulfamethoxazole and meropenem, our patient’s mental status gradually improved and his skin lesion disappeared. A follow-up MRI scan performed nine weeks later showed substantial resolution of the brain lesions; a chest radiograph appeared normal. The patient’s clinical course, however, was complicated by nosocomial infections as well as transfusion-dependent anemia and thrombocytopenia from progression of his underlying lymphoma. A decision was made to pursue a palliative direction of care, and he died six months following admission.
This case illustrates several important points. First, in immunocompromised patients who present with infectious syndromes, an understanding of their specific immune defects can allow clinicians to predict the causative organism more accurately and initiate appropriate, empiric antimicrobial therapy. Second, concurrent lung and brain infections are caused by a select group of pathogens. Nocardiosis should be considered in patients with impaired cell-mediated immunity. Finally, careful collaboration between the clinicians and the microbiology laboratory is essential when a diagnosis of nocardiosis is suspected, to ensure that specimens are incubated for sufficient time to improve the chance of making a microbiologic diagnosis.
CMAJ invites submissions to “What is your call?” Clinical details (including images) are presented on the first page along with a multiple-choice question about the diagnosis. The answer and a brief discussion of the condition follow on the second page. We specifically invite submissions illustrating common or important radiographic and electrocardiographic diagnoses of appeal to a general audience. We allow up to 1200 words and five references and require authors to obtain consent from the patient for publication of his or her story (form available at www.cmaj.ca/authors/checklist.shtml). Submit manuscripts online at http://mc.manuscriptcentral.com/cmaj.
Acknowledgement
The authors gratefully acknowledge the assistance of Dr. Scott L. Boerner in the preparation of this manuscript and provision of pathology images.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
References
- 1.Tunkel AR. Brain abscess. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. Vol 2, 7th ed Philadelphia (PA): Churchill Livingstone Elsevier; 2010. p. 1265–78 [Google Scholar]
- 2.Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. Based on current molecular taxonomy. Clin Microbiol Rev 2006;19:259–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorrell TC, Mitchell DH, Iredell JR, et al. Nocardia species. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. Vol 2, 7th ed Philadelphia (PA): Churchill Livingstone Elsevier; 2010. p. 3199–207 [Google Scholar]
- 4.Minero MV, Marin M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine 2009;88:250–61 [DOI] [PubMed] [Google Scholar]
- 5.Gombert ME, Aulicino TM. Synergism of imipenem and amikacin in combination with other antibiotics against Nocardia asteroides. Antimicrob Agents Chemother 1983;24:810–1 [DOI] [PMC free article] [PubMed] [Google Scholar]