Abstract
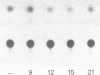
Several classes of oligonucleotide antisense compounds of sequence complementary to the start of the mRNA coding sequence for chloramphenicol acetyl transferase (CAT), including methylphosphonate, alkyltriester, and phosphorothioate analogues of DNA, have been compared to "normal" phosphodiester oligonucleotides for their ability to inhibit expression of plasmid-directed CAT gene activity in CV-1 cells. CAT gene expression was inhibited when transfection with plasmid DNA containing the gene for CAT coupled to simian virus 40 regulatory sequences (pSV2CAT) or the human immunodeficiency virus enhancer (pHIVCAT) was carried out in the presence of 30 microM concentrations of analogue. For the oligo-methylphosphonate analogue, inhibition was dependent on both oligomer concentration and chain length. Analogues with phosphodiester linkages that alternated with either methylphosphonate, ethyl phosphotriester, or isopropyl phosphotriester linkages were less effective inhibitors, in that order. The phosphorothioate analogue was about two-times more potent than the oligo-methylphosphonate, which was in turn approximately twice as potent as the normal oligonucleotide.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Miller P. S., Ts'o P. O. Inhibitory effect of complex formation with oligodeoxyribonucleotide ethyl phosphotriesters on transfer ribonucleic acid aminoacylation. Biochemistry. 1974 Nov 19;13(24):4897–4906. doi: 10.1021/bi00721a004. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Spitz S. A., Glave S. A., Reddy M. P., Ts'o P. O., Miller P. S. Hybridization arrest of globin synthesis in rabbit reticulocyte lysates and cells by oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1985 Oct 22;24(22):6139–6145. doi: 10.1021/bi00343a016. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H. Gene synthesis machines: DNA chemistry and its uses. Science. 1985 Oct 18;230(4723):281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Stoltzfus C. M. Inhibition of Rous sarcoma virus replication by antisense RNA. J Virol. 1987 Mar;61(3):921–924. doi: 10.1128/jvi.61.3.921-924.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Hirashima A., Inokuchi Y., Green P. J., Inouye M. A novel immune system against bacteriophage infection using complementary RNA (micRNA). Nature. 1985 Jun 13;315(6020):601–603. doi: 10.1038/315601a0. [DOI] [PubMed] [Google Scholar]
- Cowling G. J., Jones A. S., Walker R. T. Polynucleotide analogue inhibition of messenger-stimulated transfer RNA binding to ribosomes. Biochim Biophys Acta. 1971 Dec 30;254(3):452–456. doi: 10.1016/0005-2787(71)90879-3. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Pytel B. A., Baglioni C. Loss of (2'-5')oligoadenylate synthetase activity by production of antisense RNA results in lack of protection by interferon from viral infections. Proc Natl Acad Sci U S A. 1987 Feb;84(3):658–662. doi: 10.1073/pnas.84.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge M. D., Hodgson A., Jones A. S., Walker R. T. Synthetic analogues of polynucleotides. 8. Analogues of oligonucleotides containing carboxymethylthymidine. J Chem Soc Perkin 1. 1972;16:1991–1996. doi: 10.1039/p19720001991. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Schryver B. B., Marsh Y. V. Stereoconfiguration markedly affects the biochemical and biological properties of phosphorothioate analogs of 2-5A core, (A2'p5')2A. J Biol Chem. 1986 May 5;261(13):5999–6003. [PubMed] [Google Scholar]
- Gait M. J., Jones A. S., Walker R. T. Synthetic-analogues of polynucleotides XII. Synthesis of thymidine derivatives containing an oxyacetamido- or an oxyformamido-linkage instead of a phosphodiester group. J Chem Soc Perkin 1. 1974;0(14):1684–1686. [PubMed] [Google Scholar]
- Gallo K. A., Shao K. L., Phillips L. R., Regan J. B., Koziolkiewicz M., Uznanski B., Stec W. J., Zon G. Alkyl phosphotriester modified oligodeoxyribonucleotides. V. Synthesis and absolute configuration of Rp and Sp diastereomers of an ethyl phosphotriester (Et) modified EcoRI recognition sequence, d[GGAA(Et)TTCC]. A synthetic approach to regio- and stereospecific ethylation-interference studies. Nucleic Acids Res. 1986 Sep 25;14(18):7405–7420. doi: 10.1093/nar/14.18.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Arya S. K., Popovic M., Gallo R. C., Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984 Nov 8;312(5990):166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Halford M. H., Jones A. S. Synthetic analogues of polynucleotides. Nature. 1968 Feb 17;217(5129):638–640. doi: 10.1038/217638a0. [DOI] [PubMed] [Google Scholar]
- Harland R., Weintraub H. Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA. J Cell Biol. 1985 Sep;101(3):1094–1099. doi: 10.1083/jcb.101.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M. tcRNA as a naturally occurring antisense RNA in eukaryotes. Nucleic Acids Res. 1986 Aug 26;14(16):6771–6772. doi: 10.1093/nar/14.16.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Sawaki S., Inokuchi Y., Inouye M. Engineering of the mRNA-interfering complementary RNA immune system against viral infection. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7726–7730. doi: 10.1073/pnas.83.20.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe C. A., Lee Y. C. The binding and processing of mannose-bovine serum albumin derivatives by rabbit alveolar macrophages. Effect of the sugar density. J Biol Chem. 1983 Dec 10;258(23):14193–14199. [PubMed] [Google Scholar]
- Hélène C., Montenay-Garestier T., Saison T., Takasugi M., Toulmé J. J., Asseline U., Lancelot G., Maurizot J. C., Toulmé F., Thuong N. T. Oligodeoxynucleotides covalently linked to intercalating agents: a new class of gene regulatory substances. Biochimie. 1985 Jul-Aug;67(7-8):777–783. doi: 10.1016/s0300-9084(85)80167-x. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Jayaraman K., McParland K., Miller P., Ts'o P. O. Selective inhibition of Escherichia coli protein synthesis and growth by nonionic oligonucleotides complementary to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1537–1541. doi: 10.1073/pnas.78.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. S., MacCoss M., Walker R. T. Synthetic analogues of polynucleotides. X. The synthesis of poly-(3'-O-carboxymethyl-2'-deoxyadenosine) and its interaction with polynucleotides. Biochim Biophys Acta. 1973 Feb 4;294(1):365–377. [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- LaPlanche L. A., James T. L., Powell C., Wilson W. D., Uznanski B., Stec W. J., Summers M. F., Zon G. Phosphorothioate-modified oligodeoxyribonucleotides. III. NMR and UV spectroscopic studies of the Rp-Rp, Sp-Sp, and Rp-Sp duplexes, [d(GGSAATTCC)]2, derived from diastereomeric O-ethyl phosphorothioates. Nucleic Acids Res. 1986 Nov 25;14(22):9081–9093. doi: 10.1093/nar/14.22.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre M., Bayard B., Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci U S A. 1987 Feb;84(3):648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Specific hybridization arrest of dihydrofolate reductase mRNA in vitro using anti-sense RNA or anti-sense oligonucleotides. Arch Biochem Biophys. 1987 Feb 15;253(1):214–220. doi: 10.1016/0003-9861(87)90654-0. [DOI] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. Inhibition of heat shock protein synthesis by heat-inducible antisense RNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):399–403. doi: 10.1073/pnas.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Aurelian L., Blake K. R., Murakami A., Reddy M. P., Spitz S. A., Ts'o P. O. Control of ribonucleic acid function by oligonucleoside methylphosphonates. Biochimie. 1985 Jul-Aug;67(7-8):769–776. doi: 10.1016/s0300-9084(85)80166-8. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Blandin M., Murakami A., Reddy P. M., Spitz S. A., Ts'o P. O. Use of methylphosphonic dichloride for the synthesis of oligonucleoside methylphosphonates. Nucleic Acids Res. 1983 Aug 11;11(15):5189–5204. doi: 10.1093/nar/11.15.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Murakami A., Reddy P. M., Spitz S. A., Ts'o P. O. Preparation of oligodeoxyribonucleoside methylphosphonates on a polystyrene support. Nucleic Acids Res. 1983 Sep 24;11(18):6225–6242. doi: 10.1093/nar/11.18.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Annan N. D., McParland K. B., Pulford S. M. Oligothymidylate analogues having stereoregular, alternating methylphosphonate/phosphodiester backbones as primers for DNA polymerase. Biochemistry. 1982 May 11;21(10):2507–2512. doi: 10.1021/bi00539a033. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Barrett J. C., Ts'o P. O. Synthesis of oligodeoxyribonucleotide ethyl phosphotriesters and their specific complex formation with transfer ribonucleic acid. Biochemistry. 1974 Nov 19;13(24):4887–4896. doi: 10.1021/bi00721a003. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Reddy M. P., Murakami A., Blake K. R., Lin S. B., Agris C. H. Solid-phase syntheses of oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Sep 9;25(18):5092–5097. doi: 10.1021/bi00366a017. [DOI] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A., Blake K. R., Miller P. S. Characterization of sequence-specific oligodeoxyribonucleoside methylphosphonates and their interaction with rabbit globin mRNA. Biochemistry. 1985 Jul 16;24(15):4041–4046. doi: 10.1021/bi00336a036. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. L'ARN non sens (nsARN): un outil pour inactiver spécifiquement l'expression d'un gène donné in vivo. C R Acad Sci III. 1984;299(8):271–274. [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Packman L. C., Burleigh B. D., Dell A., Morris H. R., Hartley B. S. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature. 1979 Dec 20;282(5741):870–872. doi: 10.1038/282870a0. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcich B., Ratner L., Josephs S. F., Okamoto T., Gallo R. C., Wong-Staal F. Characterization of long terminal repeat sequences of HTLV-III. Science. 1985 Feb 1;227(4686):538–540. doi: 10.1126/science.2981438. [DOI] [PubMed] [Google Scholar]
- Stec W. J., Zon G., Uznanski B. Reversed-phase high-performance liquid chromatographic separation of diastereomeric phosphorothioate analogues of oligodeoxyribonucleotides and other backbone-modified congeners of DNA. J Chromatogr. 1985 Jun 19;326:263–280. doi: 10.1016/s0021-9673(01)87452-5. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Zamecnik P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stridh S., Oberg B., Chattopadhyaya J., Josephson S. Functional analysis of influenza RNA polymerase activity by the use of caps, oligonucleotides and polynucleotides. Antiviral Res. 1981 Jun;1(2):97–105. doi: 10.1016/0166-3542(81)90036-x. [DOI] [PubMed] [Google Scholar]
- Summers M. F., Powell C., Egan W., Byrd R. A., Wilson W. D., Zon G. Alkyl phosphotriester modified oligodeoxyribonucleotides. VI. NMR and UV spectroscopic studies of ethyl phosphotriester (Et) modified Rp-Rp and Sp-Sp duplexes, (d[GGAA(Et)TTCC])2. Nucleic Acids Res. 1986 Sep 25;14(18):7421–7436. doi: 10.1093/nar/14.18.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerton J. Sequence-specific crosslinking agents for nucleic acids: design and functional group testing. J Theor Biol. 1979 May 7;78(1):61–75. doi: 10.1016/0022-5193(79)90326-6. [DOI] [PubMed] [Google Scholar]
- To R. Y., Booth S. C., Neiman P. E. Inhibition of retroviral replication by anti-sense RNA. Mol Cell Biol. 1986 Dec;6(12):4758–4762. doi: 10.1128/mcb.6.12.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov V. V., Zarytova V. F., Kutiavin I. V., Mamaev S. V., Podyminogin M. A. Complementary addressed modification and cleavage of a single stranded DNA fragment with alkylating oligonucleotide derivatives. Nucleic Acids Res. 1986 May 27;14(10):4065–4076. doi: 10.1093/nar/14.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Wickstrom E., Simonet W. S., Medlock K., Ruiz-Robles I. Complementary oligonucleotide probe of vesicular stomatitus virus matrix protein mRNA translation. Biophys J. 1986 Jan;49(1):15–17. doi: 10.1016/S0006-3495(86)83574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]