Abstract
Recent findings suggest that extrasynaptic δ-subunit–containing GABAA receptors are sensitive to low-to-moderate concentrations of alcohol, raising the possibility that these receptors mediate the reinforcing effects of alcohol after consumption of one or a few drinks. We used the technique of viral-mediated RNAi to reduce expression of the GABAA receptor δ-subunit in adult rats in localized regions of the nucleus accumbens (NAc) to test the hypothesis that δ-subunit–containing GABAA receptors in the NAc are necessary for oral alcohol consumption. We found that knockdown of the δ-subunit in the medial shell region of the NAc, but not in the ventral or lateral shell or in the core, reduced alcohol intake. In contrast, δ-subunit knockdown in the medial shell did not affect intake of a 2% sucrose solution, suggesting that the effects of GABAA receptor δ-subunit reduction are specific to alcohol. These results provide strong evidence that extrasynaptic δ-subunit–containing GABAA receptors in the medial shell of the NAc are critical for the reinforcing effects of oral ethanol.
Keywords: addiction, reward, self-administration, tonic inhibition
GABAA receptors (GABAARs) have long been considered a primary target for alcohol actions, including the voluntary consumption of alcohol (1–5). For example, treatment with the GABAA agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) enhances the acquisition of alcohol consumption in laboratory rats (6), whereas the opposite effect, a reduction of alcohol self-administration, is observed after administration of the GABAAR antagonists Ro15-4513 or picrotoxin (7–9).
GABAARs are pentamers assembled from a variety of subunits to form multiple isoforms that are likely to differ in their alcohol sensitivity (10). Although many GABAAR subunit combinations can be activated by high (anesthetic) alcohol concentrations (11), only specific GABAAR subunit combinations, specifically those containing a δ-subunit, appear to be sensitive to alcohol concentrations in the lower range of blood alcohol levels associated with mild to moderate intoxication in humans (12–15). For example, recombinant α4βδ and α6βδ GABAARs are reported to be sensitive to 3–30 mM concentrations of alcohol (12, 14, 16). Interestingly, δ-subunit–containing GABAARs are exclusively located at extrasynaptic locations (17–19), where they give rise to a tonic form of inhibition that potently suppresses neuronal excitability. A number of studies have shown that alcohol at low concentrations acts through extrasynaptic δ-containing GABAARs to increase tonic inhibition (20–24).
The above findings suggest that actions at extrasynaptic δ-containing GABAARs could be critical for a variety of cognitive and behavioral effects of consumed alcohol, including anxiolysis, behavioral tolerance, and rewarding and reinforcing effects. However, δ-subunit knockout mice, and the related α4-subunit knockout mice, demonstrate relatively few alterations in the effects of alcohol in vivo. For example, the effects of acute alcohol administration on measures of anxiety, hypothermia, and sedation are not altered in these mice (25, 26). In contrast, δ-subunit knockout mice drink less than their wild-type counterparts (25). Thus, δ-containing receptors may be involved in rewarding and reinforcing effects of alcohol.
The δ-subunit is expressed in subregions of the thalamus, cortex, and hippocampus as well as in the dorsal and ventral striata, specifically in the nucleus accumbens (NAc) (27, 28). The NAc is a critical component of the circuitry that underlies the rewarding and reinforcing effects of alcohol and other drugs (29–31). Recently, we found that selective reductions in the expression of the GABAAR α4-subunit in the NAc shell, but not the core, reduces alcohol self-administration (32). This finding supports a role for the extrasynaptic α4βδ GABAAR in the reinforcing effects of alcohol.
Although GABAAR α4-subunits normally partner with δ-subunits to form extrasynaptic α4βδ receptors, recent findings indicate that alcohol can alter the distribution of the α4-subunit, with a shift to synaptic sites after high doses or chronic administration (33, 34). Therefore, it is of interest to determine the role of the δ-subunit, which is exclusively extra- or perisynaptic, in alcohol intake. Here we used viral-mediated RNAi (35–37) to achieve reductions in the expression of the δ-subunit mRNA in specific regions of the NAc in the adult rat. The NAc can be subdivided into the shell and the core. The shell can be further subdivided into at least three subregions—a medial, ventral, and lateral shell (38)—based on findings from immunohistochemical and tract-tracing studies. Therefore, we determined whether reductions in δ-subunit expression in the NAc shell produced subregion-specific effects. Our results indicate that δ-subunit–containing GABAARs contribute to the reinforcing effects of alcohol, and that this effect is localized to the medial subregion of the NAc shell.
Results
GABAAR δ-Subunit mRNA and Protein Are Reduced After Ad-shδ Microinfusion into the NAc.
To test the role of the δ-subunit in alcohol intake, the technique of viral-mediated RNAi was applied (32, 35–37). We designed a 21-nt siRNA sequence against the rat δ-subunit that was effective in suppressing δ mRNA expression in HEK293 cells (Fig. S1A). We packaged the siRNA sequence into an adenovirus (termed Ad-shδ) to allow for expression of the shRNA against the δ-subunit in neurons in vitro and in vivo. We confirmed that the Ad-shδ virus reduced expression of δ mRNA in striatal cultures (Fig. S1B).
We next established that infusion of the Ad-shδ virus into the NAc resulted in neuronal infection and down-regulation of the δ-subunit mRNA and protein. For these studies, we waited until 18 d after virus infusion to take brain samples. We expected that δ mRNA and protein would be decreased at this time because our previous studies indicated that effects induced by this adenovirus on other genes [α4-subunit (32); BDNF (37)] are transient, with an effective time window of <2 wk, beginning around day 10 for both mRNA and behavioral changes. Notably, in our prior studies, peak behavioral changes have occurred ~17–18 d after virus infusion (32, 37).
Fig. 1A shows representative examples of the localization of virus into the medial, ventral, or lateral zones of the NAc shell as indicated by GFP fluorescence in brain slices taken 18 d after infusion. As shown in Fig. 1B, virus infection is detected in NAc shell neurons, whereas a relatively low level of infection is seen in glia. In addition, we found decreases in GABAAR δ-subunit mRNA (P < 0.01; Fig. 1C) and protein (P < 0.05; Fig. 1D) levels in the NAc shell 18 d after Ad-shδ infusion compared with infusion of a control virus expressing a nonspecific sequence (termed Ad-NSS) and predicted to have no effect.
Fig. 1.
Confirmation of infection and δ-subunit knockdown after infusion of the Ad-shδ virus into the NAc. Rats were infused with viruses expressing shRNA to target the GABAAR δ-subunit (Ad-shδ) or a control virus expressing a nonspecific sequence (Ad-NSS) into the NAc shell. At 18 d after infusion, brains were removed and prepared for histology (A and B), subunit expression quantification with TaqMan quantitative PCR (C), or protein quantification by Western blotting (D). (A) Presence of GFP after virus infusion into the medial, ventral, or lateral subregions of the NAc shell. (B) Sections of NAc shell after microinfusion stained with anti-GFP (green) antibodies and anti-NeuN (red; Upper) or anti-GFAP (red; Lower). (Upper) Overlap of NeuN and GFP staining (yellow) indicates infected neurons. Arrows point toward examples of Ad-shδ–infected neurons. (Lower) Little overlap of GFAP and GFP staining (yellow) indicates low level of glial infection. (C) Ad-shδ infusion reduces δ-subunit mRNA in NAc. Histogram depicts the mean ratio of δ to GAPDH ± SEM; n = 3 per group. (D) Ad-shδ infusion reduces δ-subunit protein levels. Histogram depicts the mean ratio of δ to actin ± SEM; n = 4 per group. *P < 0.05 compared with Ad-NSS.
Viral-Mediated GABAAR δ-Subunit Knockdown in NAc Shell Decreases Alcohol Intake in an Intermittent-Access Paradigm.
If an extrasynaptic δ-containing GABAAR, such as the α4βδ GABAAR, is required for the reinforcing effects of orally consumed alcohol, then reduction of the expression of the δ-subunit should reduce alcohol intake. We tested the effects of knockdown of the δ GABAAR subunit on alcohol intake by infusing Ad-shδ or Ad-NSS into the medial NAc shell of alcohol-experienced rats. We targeted the medial NAc shell because we previously found that α4 reductions in this region reduced alcohol intake (32). At 5 d after viral infusion, rats were allowed access to alcohol [20% (vol/vol) ethanol (20E)] on the home cage under an intermittent-access schedule in which a bottle of 20E and a second bottle, containing tap water, were available for 24 h on Mondays, Wednesdays, and Fridays only. This schedule leads to alcohol intake sufficient to result in measurable plasma levels (39, 40). Examination of the time course (Fig. 2) of Ad-shδ effects on alcohol intake revealed a main effect of day [F(12, 264) = 5.368, P < 0.001] but not treatment [F(1, 22) = 0.86, P = 0.37] and a significant treatment × day interaction [F(12, 264) = 5.925, P < 0.001]. Intake by Ad-shδ–treated rats began to decrease at day 12 but was not yet significant (P = 0.07); however, intake was significantly decreased at 14 d (P < 0.005), 16 d (P < 0.01), and 19 d (P < 0.02) after infusion compared with control rats. There were no differences between groups by day 21 (P = 0.69). Hence, consumption is reduced at the time points that encompass those at which we found mRNA and protein decreases (Fig. 1 C and D).
Fig. 2.
Viral-mediated GABAAR δ-subunit knockdown in NAc shell decreases oral alcohol intake. Rats were infused with Ad-shδ (n = 12) to reduce expression of the δ-subunit or the control Ad-NSS (n = 12) into the NAc shell, and drinking within an intermittent-access procedure was assessed. Time course of ethanol intake expressed as grams per kilogram of body weight before (B) and after (days 5–30) virus infusion. (In Figs. 2–4, “B” refers to baseline, which is defined as the average of the last 3 d before virus infusion). *P < 0.02,**P < 0.01, and ***P < 0.005 compared with Ad-NSS. Values depict mean ± SEM.
Similar changes were noted in alcohol preference, expressed as the volume of alcohol consumed relative to the total volume, with a main effect of day [F(12, 264) = 7.940, P < 0.001] and treatment [F(1, 22) = 26.127, P < 0.001] as well as a significant treatment × day interaction [F(12, 264) = 9.951, P < 0.001]. Interestingly, alcohol preference by Ad-shδ–treated rats begin to decrease at day 9 (P < 0.05), although intake was not significantly changed yet; preferences were significantly decreased at day ~12–21 (all P < 0.001) after infusion, compared with control rats. Water intake was increased transiently at the onset and offset of the decrease in alcohol consumption (days 9, 12, and 28; P < 0.05), likely contributing to the changes in preference observed on those days.
Reduction of Alcohol Intake by Viral-Mediated GABAAR δ Knockdown Is Localized to the Medial Zone, but Not the Ventral or Lateral Zones, of the NAc Shell.
To determine whether the effects of knockdown of the δ-subunit were limited to a specific subregion of the NAc shell, the volume of virus microinfused was decreased from 1.0 μL to 0.7 μL, and infusions were made into the medial, ventral, or lateral zones of the NAc shell. To simplify consideration of temporal factors, access to alcohol under the intermittent-access procedure was changed such that a bottle of 20E was available for 24 h every other day rather than for 3 d per week. Brains were removed after the final drinking session, and the location of the viral infusion site was confirmed by visualization of GFP expression in coronal slices as in Fig. 1A (Fig. S2A); rats without bilateral localization of virus infusion within the targeted region were not included in the analyses.
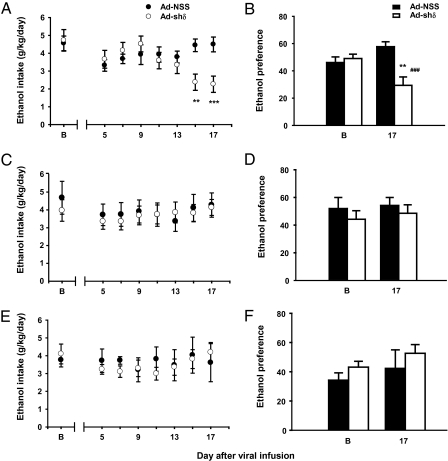
Ad-shδ virus infused into the medial shell reduced alcohol intake (Fig. 3A) and preference (Fig. 3B). Analysis of alcohol intake by rats treated with Ad-shδ and Ad-NSS revealed a main effect of day [F(7, 105) = 3.069, P < 0.01] and a significant treatment × day interaction [F(7, 105) = 4.870, P < 0.001] with no main effect of treatment [F(1, 15) = 1.09, P = 0.314]. Alcohol intake (in grams per kilogram of body weight) was reduced 15 d (P < 0.005) and 17 d (P < 0.001) after infusion in Ad-shδ–treated rats compared with Ad-NSS–treated rats. Preference was also decreased on the final day of measurement, as depicted in Fig. 3B, revealed by a main effect of treatment [F(1, 15) = 8.124, P < 0.05] and a treatment × day interaction [F(1, 15) = 17.726, P < 0.001; preference on day 17, control vs. Ad-shδ, P < 0.001)] but no main effect of day [F(1, 15) = 1.187, P = 0.293]. Water intake was not altered after Ad-shδ infusion in this experiment (all P > 0.05).
Fig. 3.
Reduction of alcohol intake by viral-mediated GABAA R δ knockdown is localized to the medial zone of the NAc shell. Rats were infused with Ad-shδ or Ad-NSS into the medial (A and B), ventral (C and D), or lateral (E and F) NAc shell, and drinking within an intermittent-access procedure was assessed. (A) Time course of intake expressed as grams per kilogram of body weight before (B) and after (days 5–17) virus infusion into the medial NAc. Ad-shδ, n = 9; Ad-NSS, n = 8. **P < 0.005, ***P < 0.001 compared with Ad-NSS. (B) Alcohol preference [(gram of ethanol consumed/ gram of ethanol + gram of water) × 100] for the same Ad-shδ–treated rats as in A. **P < 0.005 compared with baseline. ###P < 0.001 compared with Ad-NSS within day 17. (C) Time course of alcohol intake before (B) and after (days 5–17) virus infusion into the ventral NAc. Ad-shδ, n = 13; Ad-NSS, n = 5. (D) Alcohol preference for same Ad-shδ–treated rats as in C. (E) Time course of alcohol intake before (B) and after (days 5–17) virus infusion into the lateral NAc. Ad-shδ, n = 9; Ad-NSS, n = 5. (F) Alcohol preference for the same Ad-shδ–treated rats as in E. Values depict mean ± SEM.
In contrast, there was no effect of the Ad-shδ virus on alcohol intake and preference after infusion into the ventral (Fig. 3 C and D) or the lateral (Fig. 3 E and F) shell. Analysis of alcohol intake across each day after infusion in the ventral shell found no effect of treatment [F(1, 16) = 0.068, P = 0.80] or day [F(7, 112) = 1.17, P = 0.33] nor a treatment × day interaction [F(7, 112) = 0.41, P = 0.90; Fig. 3C] as well as no effect of preference for alcohol on treatment [F(1, 16) = 0.456, P = 0.509] or day [F(1, 16) = 0.533, P = 0.48] nor a treatment × day interaction [F(1, 16) = 0.06, P = 0.82; Fig. 3D]. Likewise, analysis of alcohol intake across each day after infusion in the lateral shell found no effect of treatment [F(1, 12) = 0.18, P = 0.68] or day [F(7, 84) = 1.50, P = 0.18] nor a treatment × day interaction [F(7, 84) = 2.10, P = 0.052; Fig. 3E] as well as no effect of preference for alcohol on treatment [F(1, 12) = 1.452, P = 0.251] or day [F(1, 12) = 2.352, P = 0.15] nor a treatment × day interaction [F(1, 12) = 0.016, P = 0.90; Fig. 3F].
Viral-Mediated GABAAR δ-Subunit Knockdown in NAc Core Does Not Alter Alcohol Intake in an Intermittent-Access Paradigm.
Previously, we found that reductions in GABAAR α4-subunit expression in the NAc core had no effect on alcohol intake (32). We likewise found that Ad-shδ microinfusion into the core did not alter alcohol intake [F(7, 126) = 1.055, P > 0.05; Fig. 4A] or preference [F(1, 18) = 0.459, P > 0.05; Fig. 4B] (Fig. S2B).
Fig. 4.
GABAAR δ knockdown in the NAc core does not affect alcohol intake and knockdown in the medial shell does not affect sucrose intake. (A) Rats were infused with Ad-shδ (n = 9) or Ad-NSS (n = 11) into the NAc core, and drinking within an intermittent-access procedure was assessed. Time course of alcohol intake before (B) and after (days 5–17) virus infusion into the NAc core. (B) Alcohol preference for same Ad-shδ–treated rats as in A. (C) Rats were infused with Ad-shδ (n = 9) or Ad-NSS (n = 7) into the NAc medial shell, and sucrose intake within an intermittent-access procedure was assessed. Time course of sucrose intake before (B) and after (days 5–17) virus infusion into the NAc medial shell. (D) Sucrose preference for same Ad-shδ–treated rats as in C. Values depict mean ± SEM.
Viral-Mediated GABAAR δ-Subunit Knockdown in NAc Shell Does Not Alter Sucrose Intake in an Intermittent-Access Paradigm.
To determine whether the effects of GABAAR δ knockdown in the NAc shell reflect alterations in consumption of preferred substances in general, the effects of virus infusion on oral sucrose intake were tested. Ad-shδ microinfusion into the medial shell of the NAc did not affect intake of a 2% sucrose solution [F(1, 118) = 0.588, P > 0.05; Fig. 4C] or preference [F(1, 14) = 3.062, P > 0.05; Fig. 4D] (Fig. S2B).
Discussion
We found that reduction in the expression of the δ GABAAR subunit in the NAc decreases alcohol intake, and that this effect is localized to the NAc shell, specifically to the medial zone, but not the ventral or lateral zones, of the NAc shell. These results indicate that δ-subunit–containing GABAARs in the medial NAc shell contribute to the voluntary intake of alcohol at the moderate levels experienced after one or a few drinks.
Although alcohol intake was reduced in two separate experiments, there was also a transient effect on water intake in the first experiment. We have not observed effects on water intake in subsequent studies; for example, both alcohol intake (in grams per kilogram of body weight) and preference are reduced 15 and 17 d after δ knockdown in the medial shell (Fig. 3 A and B), but water intake is not changed on those days. Although the reasons for the change in water intake in the first experiment are not clear, the first experiment used a slightly larger infusion volume (1.0 μL) than the second experiment (0.7 μL). Critically, the majority of days with reduced drinking in the first experiment were not accompanied by changes in water intake, supporting the assertion that the δ-subunit effects are selective for alcohol.
GABAergic mechanisms in the medial shell have been implicated in the intake of preferred substances; for example, microinjection of the direct GABA agonist muscimol into the NAc medial shell increases intake of sucrose (41). However, we found no change in sucrose consumption after knocking down the GABAAR δ-subunit, as well as the α4-subunit (32), in the medial shell. Therefore, δ- or α4-containing GABAAR down-regulation is not sufficient to alter sucrose intake, although we have yet to test whether this manipulation will alter the effects of muscimol on sucrose intake. The data thus far support a selective effect of GABAAR δ and α4 reductions for alcohol and not a general change in reward processing or ingestive processes.
Our results indicate that δ-subunit knockdown in the NAc significantly reduced alcohol consumption only when the virus was infused within the medial shell, but not within the ventral or lateral shell, or in the NAc core. The δ-subunit is expressed throughout the NAc (28). Thus, a possible explanation for the localized effect of δ knockdown is the distinct connectivity of the medial shell. Subregions of the shell receive different combinations of afferents from cortical and subcortical sources and project to different pallidal, hypothalamic, and mesencephalic targets (e.g., see ref. 42). Especially relevant to the present study, rats will self-administer alcohol directly into the medial shell, but not the core, of the NAc (43). Other drugs of abuse also are self-administered directly into the medial shell, including amphetamine (44), cocaine (45), and dopamine receptor agonists (30). These findings indicate that the medial shell in particular is involved in the reinforcing effects of self-administered drugs, including alcohol.
GABAAR δ-subunits can partner with α4- and β-subunits to form the α4βδ GABAAR isoform. α4βδ GABAARs in some brain regions are sensitive to low (1–30 mM) concentrations of alcohol (12, 14, 23). Alcohol concentrations in this range are attained after a few drinks in humans, and they impair cognition, judgment, and motor abilities (46). Some studies have failed to detect an effect of alcohol at α4βδ GABAARs (47). The reasons for these discrepancies are not clear; however, issues in expressing functional δ-containing GABAARs (48) and/or requirements for phosphorylation signals (49, 50) may contribute. As part of an effort to address the role of α4βδ in vivo, we previously showed that alcohol intake and preference were decreased by reductions in GABAAR α4-subunit expression in the NAc shell. Thus, our current and previous findings, together with a prior study in which δ knockout mice consumed less alcohol than wild-type mice (25), provide strong evidence that α4βδ GABAARs contribute to mechanisms mediating alcohol intake. Our findings do not, however, address whether alcohol's effects at this receptor are direct or indirect (i.e., via enhanced presynaptic release of GABA, etc.). Interestingly, GABAAR δ- or α4-subunit knockout mice have no alterations in acute behavioral effects of alcohol in tests of anxiety, motor behavior, analgesia, and sedation (25, 26) under normal circumstances, although alcohol's effects on anxiety in models of premenstrual syndrome do depend on α4βδ GABAARs, most likely in the hippocampus (14). Thus, some, but not all, behavioral effects of alcohol appear to be mediated by α4βδ GABAARs. This conclusion suggests the possibility of targeting pharmacological treatments to reduce alcohol's rewarding and/or reinforcing properties.
Studies of subunit expression in thalamic and hippocampal neurons indicate that δ-containing GABAARs are primarily extrasynaptic (51, 52). Extrasynaptic GABA receptors have high affinity for GABA, slowly desensitize, and mediate a tonic inhibitory current that regulates neuronal excitability (17). GABAergic tonic currents in the dorsal striatum have been measured; in the adult mouse, they are much larger in medium spiny neurons of the indirect pathway and are mediated by receptors containing the GABAAR δ-subunit (53). It thus seems likely that striatal neurons within the NAc shell also express a δ-subunit–dependent GABAergic tonic current. Although the alcohol sensitivity of tonic inhibition in the striatum has not been reported, the GABAergic tonic currents mediated by δ-containing GABAARs in the hippocampus (23, 24), thalamus (54), and cerebellum (20) are alcohol-sensitive, although those of the thalamus require higher alcohol concentrations (54). Together, these findings lead us to hypothesize that moderate levels of alcohol produce reinforcing/rewarding effects by enhancing GABAergic tonic inhibition in the medial shell, in agreement with proposals that reductions in the excitability of NAc shell neurons may provide a reinforcement signal (55). Therefore, one interpretation of the present study is that alcohol intake decreased as a result of a reduction in the ability of alcohol to enhance tonic inhibition in the NAc shell.
In conclusion, the current findings indicate that δ-containing GABAARs in the medial NAc shell play an important role in alcohol drinking behavior, strengthening the hypothesis that the α4βδ GABAAR in a restricted region of the NAc shell is a key brain substrate for the reinforcing properties of oral alcohol.
Materials and Methods
Animals.
Male Long Evans rats (200–250 g on arrival; Harlan) were individually housed in a temperature-regulated (21 ± 1 °C) and light-regulated (12 h light/dark cycle; lights on 0700 hours) vivarium, with unrestricted access to rat chow and water, except as noted otherwise. All procedures were approved by the Institutional Animal Care and Use Committee of the Ernest Gallo Clinic and Research Center.
Cloning and Preparation of shRNA Constructs.
A 21-nt siRNA sequence (5′-GGACGUGAGGAACGCCAUUGU-3′) of the rat GABAAR δ-subunit mRNA was designed with the GenScript web-based program; specificity was verified by a BLAST search. A nonrelated 19-nt sequence (5′-AUGAACGUGAAUUGCUCAA-3′) was used as a negative siRNA control (56). For viral delivery of double-stranded siRNA, the adenoviral shuttle vector pRNAT-H1.1 (GenScript) and the adenoviral vector Adeno-X (Clontech) were used. pRNAT-H1.1 is a vector for vector-based siRNA cloning with an H1 promoter to express shRNA and contains GFP under control of the CMV promoter. For each siRNA sequence, two complementary DNA oligonucleotides, containing sense and antisense siRNA sequences with a stem-loop structure, were synthesized, annealed, and ligated into BamHI and HindIII sites of pRNATH1.1 vector following the vector cloning protocols. The pRNAT-shRNA recombinants were confirmed by sequencing before subcloning into the cloning sites of I-CeuI and PI-SceI of Adeno-X vector.
Adenovirus Production.
Preparation of Ad-shδ and Ad-NSS adenoviruses was initiated by transfection of recombinant adenoviral constructs into HEK293 cells with Lipofectamine 2000 (Invitrogen). Recombinant viruses were amplified in HEK293 cells, followed by purification using Adeno-X Virus Purification Kit (Clontech). Viruses were titered based on GFP-visualized infection.
TaqMan Quantitative RT-PCR and Western Blot Analysis.
Tissue punches from the medial NAc were collected 18 d after infusion with Ad-shδ or Ad-NSS. GABAAR δ mRNA levels were determined by TaqMan quantitative PCR using a primer/probe kit (Applied Biosystems) with GAPDH as an internal control. For Western blot analysis, samples were separated by 4–12% SDS/PAGE (Bis-Tris Gel System; Invitrogen) and transferred onto a nitrocellulose membrane (Millipore). The membrane was blocked in 5% milk in PBS containing 0.05% Tween 20 and incubated with specific primary anti-δ antibodies (1:2,000; Sigma) and then with HRP-conjugated secondary antibodies. Immunoreactivity was detected by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and processed by exposure to Kodak BioMax film. The film was developed with the SRX-10A Medical Film Processor (Konica Minolta). The membrane was stripped in 200 mM NaOH at room temperature for 20 min, washed three times in PBS containing 0.05% Tween 20, and reprobed with anti-actin antibodies (1:2,000, Santa Cruz Biotechnology), which was used as an internal control. The images were scanned, and the immunoreactivity signal of proteins was quantified by densitometry using ImageJ. The intensity of the immunoreactivity of δ-subunit was normalized to actin.
Intermittent Access to Alcohol or Sucrose.
Rats were given 2 d of access to a single bottle of ethanol (20E) as the only fluid available to ensure that all rats had sampled ethanol to reduce possible neophobia. For the next 10 d, rats were allowed home-cage access to 10E and tap water every other day and tap water on the alternative days. The 10E was replaced with 15E for 6 d, followed by 20E. The placement (left or right) of each solution was alternated between each session to control for side preference. The water and ethanol bottles were weighed before and after each 24-h access period. For the first experiment, after 6 wk of 20E exposure, Ad-shδ or Ad-NSS was microinfused into the NAc medial shell. Rats were allowed 5 d of recovery before resuming the intermittent-access procedure for 30 d, with ethanol available only on Monday, Wednesday, and Friday. To examine the role of specific NAc subregions, after 6 wk of 20E exposure, Ad-shδ or Ad-NSS was microinfused into the medial, ventral, or lateral zones of the NAc shell as well as the NAc core. Rats were allowed 5 d of recovery before resuming intermittent access to 20E with ethanol available every other day for 17 d. To determine whether alterations in drinking were alcohol-specific, a two-bottle preference test comparing 2% sucrose (wt/vol) and water was conducted in separate rats. After 6 wk of 2% sucrose exposure, Ad-shδ or Ad-NSS was microinfused into the medial shell. After 5 d of recovery, the intermittent-access two-bottle procedure (2% sucrose and water) was resumed for 17 d.
Surgery and Microinfusion.
Rats anesthetized with isoflurane were stereotaxically infused with Ad-shδ or Ad-NSS bilaterally in the medial (relative to bregma: anteroposterior, +1.6 mm; mediolateral, ±0.78 mm; dorsolateral, −6.8 mm from dura), ventral (anteroposterior, +1.6 mm; mediolateral, ±1.3 mm; dorsolateral, −8.2 mm), or lateral (anteroposterior, +1.2 mm; mediolateral, ±2.7 mm; dorsolateral, −8.0 mm) shell or the core (anteroposterior, +1.2 mm; mediolateral, ±1.9 mm; dorsolateral, −6.8 mm) of the NAc. A stainless-steel infuser (30 gauge) connected via polyvinyl chloride tubing to a 10-μL Hamilton GASTIGHT syringe was used to infuse 0.7–1.0 μL of virus (1 × 1010 to 3 × 1010 transducing units/mL) at a rate of 0.1 μL/min for 7 or 10 min. After an additional 7 min, the infuser was removed, and the scalp was closed with sutures.
Histology and Immunohistochemistry.
Rats were perfused transcardially with fixative (4% paraformaldehyde), then 50-μm coronal slices were cut. The area of virus infection was indicated by expression of the GFP reporter gene. Sections from two rats with virus infusion were subjected to immunohistochemistry as described (37). Primary antibodies used were rabbit anti-GFP polyclonal antibody (Ab290, 1:10,000) with antibodies for the neuronal marker (monoclonal anti-NeuN, 1:100). Secondary antibodies for immunofluorescence were a mixture of Alexa Fluor 594–labeled donkey anti-mouse or donkey anti-mouse Cy3 conjugate (to detect NeuN) and Alexa Fluor 488–labeled donkey anti-rabbit (to detect GFP). Images were acquired by using a Zeiss confocal microscope and visualized with LSM software.
Data Analysis.
Gene expression and protein data were tested with a two-sample t test. Behavioral data were analyzed with two-factor ANOVA (between-subjects factor of treatment and within-subjects factor of day) followed by post hoc tests when indicated by significant (α = 0.05) main effects or interactions.
Supplementary Material
Acknowledgments
We thank Drs. Rachel Jurd and Dao-Yao He for contributions to the design and production of the shRNAs and Dr. Viktor Kharazia for expert histochemical and histological assistance. This work was supported by National Institutes of Health Grant R01 AA016835 and funds from the state of California for medical research on alcohol and substance abuse through the University of California, San Francisco (to P.H.J. and D.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016156108/-/DCSupplemental.
References
- 1.Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- 2.Chester JA, Cunningham CL. GABAA receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Lobo IA, Harris RA. GABAA receptors and alcohol. Pharmacol Biochem Behav. 2008;90:90–94. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris RA, Trudell JR, Mihic SJ. Ethanol's molecular targets. Sci Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith BR, Robidoux J, Amit Z. GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcohol. 1992;27:227–231. [PubMed] [Google Scholar]
- 7.Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer GJ, Michael RP. Interactions between RO 15-4513 and ethanol on brain self-stimulation and locomotor activity in rats. Pharmacol Biochem Behav. 1989;34:785–790. doi: 10.1016/0091-3057(89)90275-x. [DOI] [PubMed] [Google Scholar]
- 9.Samson HH, Tolliver GA, Pfeffer AO, Sadeghi KG, Mills FG. Oral ethanol reinforcement in the rat: Effect of the partial inverse benzodiazepine agonist RO15-4513. Pharmacol Biochem Behav. 1987;27:517–519. doi: 10.1016/0091-3057(87)90357-1. [DOI] [PubMed] [Google Scholar]
- 10.Olsen RW, Sieghart W. GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihic SJ, Whiting PJ, Harris RA. Anaesthetic concentrations of alcohols potentiate GABAA receptor-mediated currents: Lack of subunit specificity. Eur J Pharmacol. 1994;268:209–214. doi: 10.1016/0922-4106(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 12.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on γ-aminobutyric acid type A receptors: Are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Sundstrom-Poromaa I, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: The “one glass of wine” receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 18.Mody I. Aspects of the homeostatic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: Modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang J, et al. Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 22.Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41:211–221. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glykys J, et al. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 25.Mihalek RM, et al. GABAA-receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- 26.Chandra D, et al. Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor α4 subunit knockout mice. Alcohol Clin Exp Res. 2008;32:10–18. doi: 10.1111/j.1530-0277.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzer C, et al. Distribution of the major γ-aminobutyric acidA receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- 29.Koob GF, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 30.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: Intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 31.Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: Recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rewal M, et al. α4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang J, et al. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 35.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 36.Lasek AW, Janak PH, He L, Whistler JL, Heberlein U. Downregulation of mu opioid receptor by RNA interference in the ventral tegmental area reduces ethanol consumption in mice. Genes Brain Behav. 2007;6:728–735. doi: 10.1111/j.1601-183X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 37.Jeanblanc J, et al. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jongen-Rĕlo AL, Groenewegen HJ, Voorn P. Evidence for a multi-compartmental histochemical organization of the nucleus accumbens in the rat. J Comp Neurol. 1993;337:267–276. doi: 10.1002/cne.903370207. [DOI] [PubMed] [Google Scholar]
- 39.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simms JA, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basso AM, Kelley AE. Feeding induced by GABAA receptor stimulation within the nucleus accumbens shell: Regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- 42.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 43.Engleman EA, et al. Ethanol is self-administered into the nucleus accumbens shell, but not the core: Evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: Is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- 46.Eckardt MJ, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 47.Borghese CM, et al. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 48.Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: Tracking δ subunit incorporation into functional receptors. Mol Pharmacol. 2010;78:918–924. doi: 10.1124/mol.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi DS, et al. Protein kinase Cδ regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abramian AM, et al. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia F, et al. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 52.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santhakumar V, Jones RT, Mody I. Developmental regulation and neuroprotective effects of striatal tonic GABAA currents. Neuroscience. 2010;167:644–655. doi: 10.1016/j.neuroscience.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326:475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ptasznik A, Nakata Y, Kalota A, Emerson SG, Gewirtz AM. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drug-resistant, BCR-ABL1(+) leukemia cells. Nat Med. 2004;10:1187–1189. doi: 10.1038/nm1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.