Abstract
Hypoxia is an important regulator of normal and cancer stem cell (CSC) differentiation. Colorectal CSCs from SW1222, LS180, and CCK81 colorectal cancer-derived cell lines are able to differentiate into complex 3D lumen-containing structures in normoxia, whereas in hypoxia, they form undifferentiated dense colonies that have reduced expression of the enterocyte differentiation marker CDX1, lack goblet cell formation, and have increased expression of BMI1 and activated Notch1. Hypoxia increases the clonogenicity of CSCs, which is cumulative as each round of hypoxia enriches for more CSCs. The hypoxic phenotype is reversible, because cells from hypoxic-dense colonies are able to reform differentiated structures when regrown in normoxia. We show that CDX1 is able to stimulate the generation of lumens even in hypoxia and has a negative feedback on BMI1 expression. Knockdown of CDX1 reduces lumen formation but does not affect goblet cell formation, suggesting that enterocytes and goblet cells form from different progenitor cells. Notch inhibition by dibenzazepine (DBZ) allowed CSCs to form goblet cells in both normoxia and hypoxia. Finally, we show that Hif1α, but not CA9, is an important mediator of the effects of hypoxia on the clonogenicity and differentiation of CSCs. In summary, hypoxia maintains the stem-like phenotype of colorectal cell line-derived CSCs and prevents differentiation of enterocytes and goblet cells by regulating CDX1 and Notch1, suggesting that this regulation is an important component of how hypoxia controls the switch between stemness and differentiation in CSCs.
Hypoxia is an important regulator of normal embryonic development (1) and stem cell differentiation (2). Hypoxic cells can be identified in the murine embryonic gut (1), and aberrations in oxygen control during development can lead to gastrointestinal abnormalities (3). Hypoxia is often present in cancers, because the demand for oxygen outstrips the supply of blood when cancers progress and become larger. Low oxygen levels stabilize Hif1α and -2α proteins, basic helix–loop–helix transcription factors that mediate the downstream effects of hypoxia on angiogenesis, tumor progression, and metastasis (4). Hypoxia in tumors is important clinically, because expression of Hif1α (5, 6) and Hif2α (7) proteins is correlated with poorer prognosis. Hif1α expression is also associated with resistance to fluorouracil (5-FU) (8) and oxaliplatin (9).
There is increasing evidence that the cancer stem cell (CSC) population is capable of self-renewal, driving tumor growth and differentiating to form all of the lineages found within a cancer (10, 11). Hypoxia seems to maintain the stem-like phenotype in neuroblastomas (12) and activates signaling pathways that are associated with the undifferentiated phenotype of normal stem cells, including Oct4 (13) and Notch (14). The explanation for hypoxia's association with more aggressive disease and poorer clinical outcome may, in part, be because hypoxia leads to an increase in the proportion of CSCs in a tumor.
In this paper, we refer to CSCs as the subpopulation of cells that drive the growth of a cell line. We have previously shown that CSCs can be isolated from colorectal cancer cell lines and that these CSCs can initiate tumors in vivo and differentiate into all of the lineages found in the original cancer (15), thus fulfilling the widely accepted functional definition of CSCs. The aim of the current study was to investigate the effects of 1% hypoxia on the clonogenicity of CSCs and their ability to differentiate into multiple lineages, with a view to contributing to an understanding of the molecular pathways underpinning the hypoxic response. We show that hypoxia maintains the undifferentiated phenotype of CSCs, increasing their clonogenicity and inhibiting their differentiation into multiple lineages. Our results also suggest that CDX1 and Notch ligands, respectively and separately, control the differentiation of CSCs into columnar and goblet cells and that feedback inhibition of CDX1 by BMI1 may play a role in the maintenance of the CSC state.
Results
Hypoxia Prevents Differentiation of Colorectal Cancer Cells and Maintains a Stem-Like Phenotype.
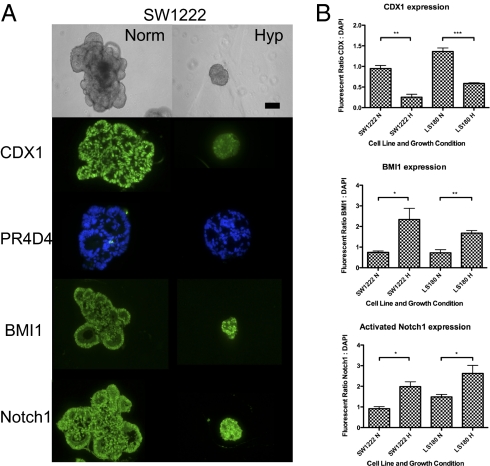
We have previously shown that the large differentiated colonies from the SW1222 cell line grown in 3D Matrigel contain the CSCs, whereas the small colonies are made up of terminally differentiated cells with limited growth potential (15). Single cells from the colorectal cancer (CRC)-derived cell lines LS180 and CCK81 plated in 3D Matrigel in normoxic conditions also form such differentiated colonies with complex internal structures (Fig. 1 and Figs. S1–S3). However, when these three cell lines are grown in hypoxia (1% O2) for a period of 3 (LS180) to 4 wk (SW1222 and CCK81), there is a profound effect on the colony morphology. The colonies become smaller, rounder, and far less well-differentiated, with no obvious features of differentiation (Fig. 1 and Figs. S1–S3). These dense colonies are similar to the undifferentiated colonies that HCT116 forms when grown in normoxia, which reflects the high proportion of CSCs in that cell line (15).
Fig. 1.
(A) Light microscopy and immunofluorescence of SW1222 grown for 4 wk in 3D Matrigel under normoxia and hypoxia (1% oxygen). (Magnification: 20× objective; scale bar: 200 μm.) (B) Quantitation of immunofluorescence of CDX1, BMI1, and activated Notch1. SI Materials and Methods has methods for obtaining the ratio of fluorescence to DAPI staining. In figures, ***P < 0.001, **P < 0.01, and *P < 0.05.
The effect of hypoxia on differentiation of colonies derived from single cells of the lines SW1222, LS180, and CCK81 grown in Matrigel under hypoxia for 4 wk is shown by quantified immunofluorescence staining with markers of differentiation (Fig. 1 and Figs. S1–S3). As shown before (15), although colonies grown in normoxia expressed high levels of the enterocyte differentiation marker CDX-1, especially in their periphery, and also contained mucin-producing goblet cells (PR4D4), the hypoxic colonies expressed much lower levels of CDX-1 and did not express the goblet cell mucin marker PR4D4 at all. In contrast, staining for activated Notch1 and Bmi1, markers that have been associated with an increased stem-like phenotype, showed increased expression under hypoxia compared with normoxia. This is consistent with the hypothesis that activated Notch1 and Bmi1 play a role in the maintenance of a stem-like phenotype of CSCs by hypoxia.
Hypoxia Increases Clonogenicity of Colorectal CSCs.
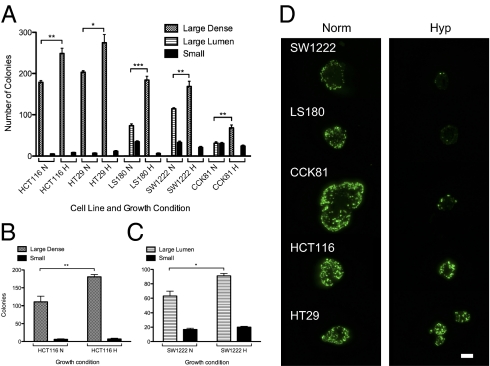
Growth of all five cell lines, HCT116, HT29, LS180, SW1222, and CCK81, in hypoxic conditions for 2–4 wk increased the clonogenicity of large colonies compared with normoxia (Fig. 2A). For the normally differentiating cell lines (LS180, SW1222, and CCK81), this applied to large colonies that did not show any significant evidence of differentiation. For these, the proportion of large vs. small colonies increased in hypoxia compared with normoxia. Fig. 2 B and C (HCT116 and SW1222, respectively) shows that pretreatment of these cell lines for 72 h in hypoxia followed by replating single cells in Matrigel and growing them in normoxia for 2–4 wk increased clonogenicity. This indicates that even the 72-h hypoxic pretreatment is enough to trigger events that affect clonogenicity after up to 4 wk in normoxia.
Fig. 2.
Hypoxia increases clonogenicity. (A) Colony count of five cell lines grown in Matrigel in normoxia (N) or hypoxia (H) for 2–4 wk. Colony count of (B) HCT116 and (C) SW1222 cells that were first exposed to 72 h of hypoxia and then replated in Matrigel in normoxia for 2–4 wk; unpaired t test; 500 cells/well. (D) Hypoxia reduces the proportion of Ki67+ve cells in cell lines that can differentiate (SW1222, LS180, and CCK81) much more than in those that are mostly undifferentiated (HCT116 and HT29). (Magnification: 20× objective; scale bar: 200 μm.)
The results of staining large colonies after 2–4 wk in hypoxia or normoxia (Fig. 2A) with Ki67 (which identifies dividing cells) are shown in Fig. 2D and Fig. S4. Hypoxia markedly reduced the proportion of cells that were Ki67 positive in SW1222, LS180, and CCK81 colonies, which all differentiate under normoxic conditions. Hypoxia did not, however, seem to affect the proportion of Ki67-positive cells in HCT116 and HT29, which normally have limited capacity to differentiate and a higher proportion of CSCs. However, the size of these colonies under hypoxia was smaller than after a similar period under normoxia. This suggests that, although hypoxia blocks the differentiation of CSCs in the cell lines SW1222, LS180, and CCK81 and therefore leads to a larger number of colonies derived from CSCs, by 4 wk in hypoxia, most of the cells in these colonies are no longer actively dividing. In contrast, a significant proportion of the cells in CSC-derived colonies from HCT116 and HT29, which cannot in any case differentiate, continues active division, although perhaps at a somewhat slower rate. This suggests that the effect of hypoxia on the normally differentiating cell lines has wider effects than only blocking differentiation.
Effects of Hypoxia Are Cumulative and Reversible.
Single cells of SW1222 and LS180 were grown in Matrigel for 4 wk in either normoxia or hypoxia. The resulting large colonies were retrieved, disaggregated into single cells, replated back into Matrigel, and then, regrown in either normoxia or hypoxia for another 4 wk. Fig. 3 A and B shows the effects of these four different growth conditions, respectively, (normoxia to normoxia, hypoxia to normoxia, normoxia to hypoxia, and hypoxia to hypoxia) on clonogenicity. For both cell lines, 8 wk in hypoxia produced the highest clonogenicity. Four weeks in hypoxia, whether this came first or second, had an intermediate effect. As illustrated in Fig. 3C and Fig. S5, the fact that cells grown for 4 wk in hypoxia and then replated in normoxia can again produce differentiating colonies shows that the effects of hypoxia are still reversible after 4 wk.
Fig. 3.
Hypoxic effects are cumulative and reversible. (A) SW1222 and (B) LS180 cell lines were grown in Matrigel in normoxia or hypoxia for 4 wk, and then, they were replated and grown in normoxia or hypoxia for another 4 wk (1,000 cells/well; unpaired t test). (C) SW1222 colonies were grown in Matrigel in either normoxia or hypoxia for 4 wk, and then, they were retrieved and replated as single cells in Matrigel for another 4 wk in either normoxia or hypoxia. (Magnification: 20× objective; scale bar: 200 μm.)
CDX1 Is Sufficient and Necessary for the Formation of Lumens but Not for Goblet Cells.
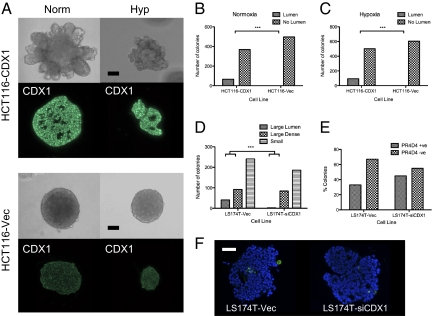
As previously shown (15), the HCT116 cell line does not express CDX1, and its colonies do not form lumen-like structures; however, when stably transfected with a vector expressing CDX-1, a proportion of HCT116-CDX1 colonies are able to form lumen structures in normoxia (Fig. 4 A and B). We now show that this ability is maintained even in hypoxia (Fig. 4 A and C). Staining for PR4D4 (goblet cell mucin) in HCT116-CDX1 colonies was uniformly negative, suggesting that enterocytes and goblet cells arise from different progenitor lineages within the crypt. CDX1 is, as expected, expressed in the nucleus in HCT116-CDX colonies and absent from the nucleus of HCT116 vector control colonies (Fig. 4A). These results suggest that the hypoxic block on differentiation acts upstream of CDX-1, and therefore, forced CDX1 expression allows lumen formation, a marker of columnar cell differentiation, even in hypoxia.
Fig. 4.
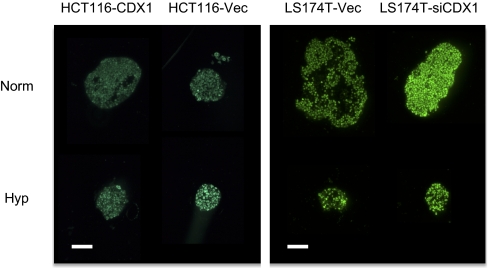
CDX1 is sufficient and required for lumen formation even in hypoxia but not for goblet cell differentiation. (A–C) Unlike the HCT116 vector control (HCT116-vec), HCT116-CDX1 forms lumens in both normoxia and hypoxia. Knockdown of CDX1 in LS174T (LS174T-siCDX1) reduces the proportion of large colonies that can form lumens compared with the vector control (LS174T-vec; D) but has no significant effect on the proportion of mucin expressing goblet cells. (E and F) Goblet cell mucin (PR4D4) is green. Nuclei are stained blue with DAPI. (Magnification: 20× objective.) Fisher's exact tests were significant in B, C, and D, but not E. (Scale bar: 200 μm.)
As a complement to the studies with HCT116-CDX1 and HCT116-vec, we have done similar studies on the pair of lines LS174T-siCDX1 (in which the normal expression of CDX1 is stably knocked down) and its control, LS174T-vec (16). As shown in Fig. 4D, after growth in Matrigel for over 3 wk, the proportion of LS174T-siCDX1 colonies that formed lumens was significantly less than the vector control (Fisher exact test, P < 0.0001). These results confirm the role of CDX1 in controlling the proportion of lumen-forming colonies and therefore, its probable role in columnar cell differentiation. A comparison of the staining of LS174T-siCDX1 with LS174T-vec by PR4D4+ for goblet cell mucus shows no significant effect of the CDX1 knockdown. This again shows that CDX1 is not involved in goblet cell differentiation, supporting the hypothesis that enterocytes and goblet cells arise from different progenitor lineages (Fig. 4 E and F).
CDX1 Has a Negative Feedback Control on BMI1.
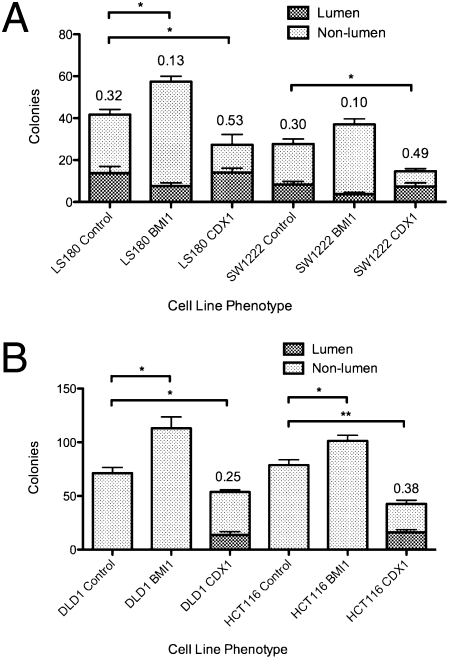
Because of the role of BMI1 in stem cell control, we labeled the two pairs of cell lines, HCT116-CDX1, HCT116-vec, LS174T-siCDX1, and LS174T-vec, with a fluorescent anti-BMI1 antibody under both normoxic and hypoxic conditions and quantified the fluorescence images. The results, shown in Fig. 5 and Fig. S6, suggest a remarkable association between the levels of BMI1 and CDX1 expression. For HCT116-vec, the expression of BMI1 significantly increases in hypoxia, and for LS174T-vec, there is an increase, although it is not significant. However, when CDX1 is expressed in HCT116-CDX1, there is no BMI1 increase in hypoxia, whereas when CDX1 is knocked down in LS174T-siCDX1, BMI1 is expressed both in hypoxic and normoxic conditions. The combined results suggest that CDX1 may have some sort of feedback control over the expression of BMI1 that may be part of a mechanism for controlling the irreversibility of stem cell differentiation. To investigate this relationship further, we examined the effects of overexpression of BMI1 and CDX1 on colony morphology and clonogenicity in the cell lines LS180, SW1222, DLD1, and HCT116 (Fig. 6). Among the lumen-forming lines (LS180 and SW1222), transfection of BMI1 enhances clonogenicity and dampens lumen formation, whereas transfection of CDX1 dampens clonogenicity and enhances lumen formation. Among the dense lines (DLD1 and HCT116), transfection of BMI1 enhances clonogenicity, whereas transfection of CDX1 dampens clonogenicity and stimulates the formation of nascent lumen-containing colonies where none existed before. These data further support the role of CDX1 in controlling differentiation into lumen-forming colonies and the role of BMI1 in maintaining the CSC state.
Fig. 5.
Negative regulation of BMI1 by CDX1. Immunofluorescence staining for BMI1 for the two pairs of cell lines, HCT116-CDX1, HCT116-vec, LS174T-vec, and LS174T-siCDX1, in normoxia and hypoxia. Cell lines were grown for 2 (the HCT116 pair) or 3 wk (the LS174T pair) in Matrigel. (Magnification: 20× objective; scale bar: 200 μm.) For quantitation of immunofluorescence images, see Fig. S6.
Fig. 6.
Overexpression of BMI1 and CDX1 increases and decreases clonogenicity of colorectal CSCs, respectively, in (A) lumen-forming and (B) dense-forming cell lines. Successfully transfected cells expressing BMI1 or CDX1 were selected on the basis of ZsGreen1 reporter expression and grown in Matrigel for 2 wk (1,000 cells/well) (Figs. S7–S11). The mean proportions of lumens were compared with the bulk population by Student t tests; all were significant at P < 0.01. The mean numbers of colonies were compared with the bulk population by t tests.
Notch Pathway Inhibition Stimulates the Development of Goblet Cells in Normoxia and Hypoxia.
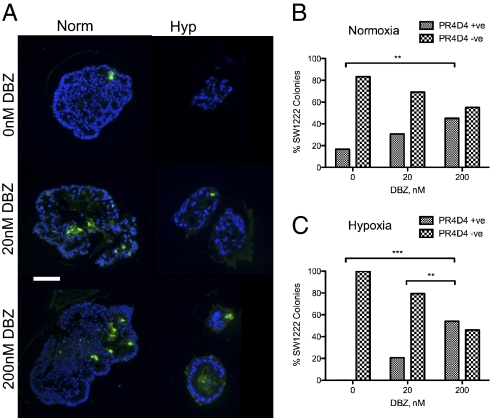
Dibenzazepine (DBZ), an inhibitor of γ-secretase and therefore, of the Notch pathway, has been shown to increase the proportion of goblet cells in the mouse intestine (17). Fig. 7A shows staining of SW1222 colonies with PR4D4 after growth in Matrigel with different concentrations of DBZ and in normoxia and hypoxia. It seems clear that DBZ increases the proportion of PR4D4 staining cells in both normoxia and hypoxia.
Fig. 7.
Notch inhibition of SW1222 induces goblet cell formation in normoxia and hypoxia. (A) PR4D4 (mucin; colored green) staining of colonies after 4 wk of growth with increasing doses of DBZ in normoxia and hypoxia. (Magnification: 20× objective; scale bar: 200 μm.) (B and C) Percent of PR4D4+ve and −ve colonies containing at least one (+) or no (−) stained cells in (B) normoxia and (C) hypoxia. Up to 50 colonies were counted for each growth condition. Fisher's test for proportion of +ve vs. −ve colonies was performed.
Fig. 7 B and C shows the quantitation of the proportions of colonies with at least one stained cell compared with those with none at various DBZ concentrations and in normoxia (Fig. 7B) and hypoxia (Fig. 7C). These data, which show a dose dependence of the number of stained cells with increasing concentrations of DBZ, strongly support the evidence that the Notch signaling pathway controls goblet cell mucin production, most probably through the control of goblet cell differentiation from CSCs. The fact that this effect persists in hypoxia suggests that, as in the case of CDX1 and columnar cell differentiation, the control of goblet cell differentiation by Notch is downstream of the effects of hypoxia on blocking CSC differentiation. DBZ, however, did not induce goblet cell differentiation in cell lines such as HCT116, which could not differentiate in the first place.
Hif1α Knockdown in Hypoxia Reduces Clonogenicity and Increases Differentiation of Colonies and Expression of CDX1.
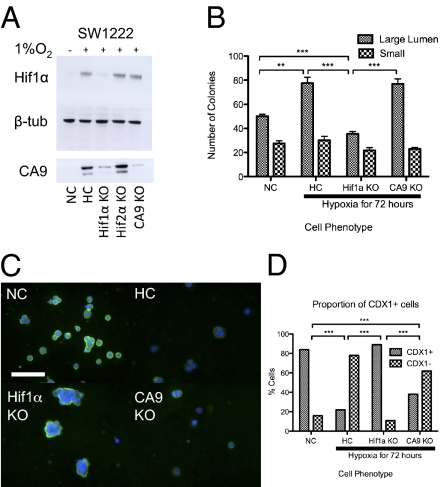
Hif1α is generally considered to be the main molecular mediator of hypoxic effects, whereas CA9 is one of the main targets of Hif1α (18). We, therefore, examined the effects of Hif1α and CA9 knockdown on differentiation of SW1222 in hypoxia. Fig. 8A shows that the transient transfection of SW1222 with siRNA for Hif1α and CA9 successfully reduced their respective expression after 72 h in hypoxia. The effects of the SW1222 knockdowns followed by 72 h in hypoxia on colony numbers after 4 wk in Matrigel in normoxia are shown in Fig. 8B. Only Hif1α knockdown substantially reduced the number of large lumen-forming colonies, whereas the CA9 knockdown gave the same results as the hypoxic control. There was, as before, some increase in overall clonogenicity of the SW1222 hypoxic control compared with normoxia. The results indicate that Hif1α is the main mediator of the increased clonogenicity of SW1222 CSCs in hypoxia and that CA9 does not play a role. The effect of Hif1α knockdown on differentiation is supported by the observation that the SW1222 cells transiently transfected with siRNA to Hif1α and when grown in hypoxia for 72 h, still give rise to cells that express CDX1 as in the normoxic control, whereas the CA9 knockdown and hypoxic control cells show reduced expression of CDX1 (Fig. 8 C and D).
Fig. 8.
Hif1α mediates the effects of hypoxia on colorectal CSCs. (A) Western blot of SW1222 cells transiently transfected with siRNA against Hif1α, CA9, and Hif2α (control) and grown under hypoxic conditions for 72 h shows successful knockdown of Hif1α and CA9. NC, normoxic scrambled control; HC, hypoxic scrambled control; KO, knockdown, namely siRNA transient transfection. (B) The effect of siRNA knockdown of Hif1α and CA9 on the production of large lumen-forming colonies by SW1222 cells under 72 h of hypoxia followed by replating and 4 wk in Matrigel in normoxia (unpaired t test; 500 cells/well). Average counts for four wells. (C) SW1222 cells knocked down by siRNA for Hif1α and CA9 and then grown in Matrigel for 72 h in hypoxia are shown. Cells were then recovered from Matrigel and stained for CDX1 (green). Nuclei were stained blue with DAPI. (Magnification: 20× objective; scale bar: 200 μm.) (D) Quantitation of immunofluorescence in C and Fisher's exact test comparing the proportion of CDX1+ vs. CDX1− cells.
Discussion
We provided strong evidence that hypoxia, defined by 1% oxygen, blocks CSC differentiation. Thus, using colony morphology and antibodies to CDX1 and PR4D4, we showed that hypoxia prevents lumen formation in the cell lines that typically differentiate and suppresses expression of CDX1 and PR4D4 while increasing clonogenicity. These changes are accompanied by increased expression of BMI1 and activated Notch-1, which are known presumed markers of CRC stem cells (19, 20), suggesting a key role for BMI1 in maintaining the cancer stem cell state.
The overall results suggest that, in hypoxia, CSCs are maintained in cell lines that normally have the capacity to differentiate because their ability to differentiate is blocked. It has recently been shown that colorectal stem cell division in the mouse is largely symmetrical rather than asymmetrical (21). The most obvious explanation for the effects of hypoxia that we observed is, therefore, that the proportion of CSC divisions is skewed against differentiated cells. Identifying the Hif1a target (s) that causes this switch should then help identify the primary molecular basis for the control of CSC differentiation. These effects of hypoxia on the CRC cell lines parallel many previous studies that, for example, suggest that hypoxia supports self-renewal of embryonic stem cells (22), stimulates proliferation of neural precursor (23) and neural crest stem cells (24), inhibits differentiation of adipocytes (25), maintains an undifferentiated phenotype in neuroblastomas (12), and increases the proportion of glioma CSCs (26).
The fact that the effects of only 72 h in hypoxia are still seen in increased clonogenicity after a further 4 wk of growth in normoxia suggests that hypoxia relatively rapidly induces increased clonogenicity, which then persists after return to normoxia. However, even after 4 wk in hypoxia, cells can still reform differentiated colonies when returned to normoxia. Thus, the effects of hypoxia, although they can persist for some time in normoxia, are still reversible after 4 wk in hypoxia.
Hypoxia also increases clonogenicity in the nondifferentiating cell lines. Ki67 staining, however, shows that cell division in the nondifferentiating cell lines persists in hypoxia, whereas the differentiating cell lines have few dividing cells in their large colonies after 4 wk in hypoxia, which may seem paradoxical. However, the early effects of hypoxia in blocking differentiation may lead to an increase in the number of colonies, which then do not continue to grow. The nondifferentiating cell lines are presumably better adapted to growth in hypoxic conditions, and this is correlated with their high CSC content, their lack of differentiation, and their generally “aggressive” growth, which fits in well with the observation that the more hypoxic-resistant tumors tend to be more aggressive and have a poorer prognosis. (5, 6).
There is good evidence that CDX1 plays an important role in normal intestinal epithelial differentiation (15, 16, 27, 28) and that can then explain downregulation of CDX1 in CRCs through providing a selective advantage by preventing differentiation (29). We now further confirmed the role of CDX1 as a key regulator of columnar cell differentiation by showing that siRNA knockdown of CDX1 in the differentiating cell line LS174T abrogates its ability to form differentiated colonies with lumens. It does not, however, prevent the formation of mucin producing goblet cells as detected by the antibody PR4D4, which implies that the differentiation pathways from the CSCs to columnar cells and to goblet cells are separately controlled. The fact that hypoxia does not inhibit lumen formation when CDX1 expression is forced in HCT116 indicates that the hypoxic block on CSC differentiation acts upstream of the control of columnar cell differentiation by CDX1.
The observation that the γ-secretase inhibitor, DBZ, significantly increases the proportion of goblet cells in SW1222 parallels similar effects seen in the mouse (17). The persistence of this effect in hypoxia suggests, as for forced CDX1 expression, that hypoxic inhibition of CSC differentiation acts upstream of the effects of Notch inhibition on goblet cell differentiation, which is consistent with a contribution of activated Notch-1 to maintaining the stem cell phenotype.
Assuming that the basic mechanisms of control of stemness and CSC differentiation in normal tissue will be reflected to a fair extent in differentiating tumors, our results suggest that, at some stage in the development of the crypt from normal stem cells, the pathways of differentiation to columnar and goblet cells begin to diverge and involve different mediators, namely CDX1 and Notch inactivation, respectively. The probability is that this happens during the amplification of the CSC-derived progenitor cells. This would be consistent with models of the colorectal cancer process (30, 31), which suggest that the cell of origin for CSCs may mostly be a progenitor cell, before the commitment has been made to a particular differentiation pathway, rather than, as is often assumed, the normal crypt stem cell (32), and that the proportion of CSCs in a CRC can be highly variable (33).
The patterns of expression of BMI1 and CDX1 in the paired cell lines HCT116-CDX1; HCT116-vec and LS174T-siCDX1; LS174T-vec suggest a remarkable negative correlation, namely that CDX1 seems to repress the expression of BMI1. This is consistent with the differentiation marker CDX1 having a feedback control over the stem cell maintenance marker, BMI1, possibly as a mechanism to inhibit reversion from the differentiated state to an uncommitted progenitor cell or even a CSC.
The observations that transient Hif1α knockdown countered the effect of hypoxia in enhancing clonogenicity and also reversed the inhibition of CDX1 expression suggest that key regulators of the process of CSC differentiation are Hif1α targets. CA9 knockdown had no such effects, indicating that this is not one of the relevant Hif1α targets. Similar results have been published for glioblastomas, where it as been shown that Hif1α knockdown in glioma cells impairs their ability to form tumor spheroids and therefore presumably CSCs (34). It has also been shown that the expression of the cancer stem cell marker CD133 is markedly increased by hypoxia in glioma cells (35). Hif1α may have a direct inhibitory effect on CDX1 transcription as two putative hypoxia response element (HRE)-like sequences have been identified in the 5’ flanking promoter region of human CDX1 (36). Our observation that hypoxia appears to be associated with activated Notch1 is consistent with studies that have shown Hif1α to interact with activated Notch1 and be recruited to Notch responsive promoters upon Notch activation under hypoxia (14). As Hif2a has also been reported to play a role in tumor angiogenesis, it would also be important to examine its downstream targets (37).
Our results show how careful analysis of the relationship between colony morphology and the expression of markers of differentiation and stemness in normoxia and hypoxia, using a panel of CRC-derived cell lines, can provide valuable insights to the controls of CSC function and differentiation. The fact that our in vitro work on the roles of CDX1, BMI1, Notch, and the effects of hypoxia support previous findings obtained from primary mouse tissue, and also that Notch is important for the survival of CSCs (38) and confers a hypoxic resistance phenotype (39) in primary human tissue models, strongly suggests, although does not yet prove, that CSCs in cell lines are equivalent to CSCs in fresh tumors (15). Our approach can serve as a first step to better characterization of these processes at the molecular level in vitro, which can then be extended to the in vivo situation by further work on primary tissue.
Materials and Methods
Detailed materials and methods can be found in SI Materials and Methods.
Cell Lines.
CRC cell lines HCT116, HT29, SW1222, LS180, LS174T, and CCK81 were grown under standard conditions. The stably transfected HCT116-CDX1 and LS174T-siCDX1 cell lines, together with their respective vector controls, were previously generated in our laboratory (16).
Immunofluorescence.
Immunofluorescence was performed as described previously (15). Dilutions of antibodies used are given: anti-CDX1 mAb (1:100; in house), PR4D4 mAb (1:10; in house), anti-Ki-67 mAb (1:100; DAKO), activated Notch1 pAb (1:200; Abcam), Bmi1 pAb (1:200; Cell Signaling), and CA9 mAb (1:100; in house).
Transient Transfection.
Transient transfection was performed according to standard protocols using lipofectamine RNAi max (Invitrogen). siRNA used included negative scrambled control (Invitrogen), Hif1α (Eurogen), Hif2α (Eurogen), and CA9 (Eurogen).
Statistical Analysis.
All data are shown as mean ± SEM. For 2 × 2 contingency tables, Fisher's exact tests were used.
Supplementary Material
Acknowledgments
We thank the following from the University of Oxford, United Kingdom: Prof. Adrian Harris, Simon Wigfield, and Ioanna Ledaki for generously providing DBZ, the siRNA constructs for Hif1α and CA9, the CA9 antibody, and for helpful discussion; Jenny Wilding for her comments and advice; Joy Winter for processing histological specimens; and Neil Ashley for his advice on quantitation of fluorescence images. This work was funded by a Royal College of Surgeons of England Research Fellowship (to T.M.Y.), a Cancer Research UK (CRUK) Clinical Research Fellowship (to T.M.Y.), and a CRUK program grant.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014519107/-/DCSupplemental.
References
- 1.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tibboel D, van Nie CJ, Molenaar JC. The effects of temporary general hypoxia and local ischemia on the development of the intestines: An experimental study. J Pediatr Surg. 1980;15:57–62. doi: 10.1016/s0022-3468(80)80404-0. [DOI] [PubMed] [Google Scholar]
- 4.Poon E, Harris AL, Ashcroft M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med. 2009;11:e26. doi: 10.1017/S1462399409001173. [DOI] [PubMed] [Google Scholar]
- 5.Baba Y, et al. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176:2292–2301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao D, et al. Expression of HIF-1alpha and VEGF in colorectal cancer: Association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432. doi: 10.1186/1471-2407-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jubb AM, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer. 2009;101:1749–1757. doi: 10.1038/sj.bjc.6605368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravizza R, et al. Effect of HIF-1 modulation on the response of two- and three-dimensional cultures of human colon cancer cells to 5-fluorouracil. Eur J Cancer. 2009;45:890–898. doi: 10.1016/j.ejca.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DL, et al. Contribution of HIF-1 and drug penetrance to oxaliplatin resistance in hypoxic colorectal cancer cells. Br J Cancer. 2009;101:1290–1297. doi: 10.1038/sj.bjc.6605311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 11.Yeung TM, Mortensen NJ. Colorectal cancer stem cells. Dis Colon Rectum. 2009;52:1788–1796. doi: 10.1007/DCR.0b013e3181a8738c. [DOI] [PubMed] [Google Scholar]
- 12.Jögi A, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covello KL, et al. HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafsson MV, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci USA. 2010;107:3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CW, et al. Gastrointestinal differentiation marker Cytokeratin 20 is regulated by homeobox gene CDX1. Proc Natl Acad Sci USA. 2009;106:1936–1941. doi: 10.1073/pnas.0812904106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 18.Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3:164–167. [PubMed] [Google Scholar]
- 19.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fre S, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 22.Chen HF, et al. Hypoxic culture maintains self-renewal and enhances embryoid body formation of human embryonic stem cells. Tissue Eng Part A. 2010;16:2901–2913. doi: 10.1089/ten.tea.2009.0722. [DOI] [PubMed] [Google Scholar]
- 23.Studer L, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison SJ, et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 26.Soeda A, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 27.Freund JN, Domon-Dell C, Kedinger M, Duluc I. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:957–969. doi: 10.1139/o99-001. [DOI] [PubMed] [Google Scholar]
- 28.Silberg DG, et al. CDX1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium. Gastroenterology. 1997;113:478–486. doi: 10.1053/gast.1997.v113.pm9247467. [DOI] [PubMed] [Google Scholar]
- 29.Wong NA, et al. Loss of CDX1 expression in colorectal carcinoma: Promoter methylation, mutation, and loss of heterozygosity analyses of 37 cell lines. Proc Natl Acad Sci USA. 2004;101:574–579. doi: 10.1073/pnas.0307190101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston MD, Edwards CM, Bodmer WF, Maini PK, Chapman SJ. Examples of mathematical modeling: Tales from the crypt. Cell Cycle. 2007;6:2106–2112. doi: 10.4161/cc.6.17.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston MD, Edwards CM, Bodmer WF, Maini PK, Chapman SJ. Mathematical modeling of cell population dynamics in the colonic crypt and in colorectal cancer. Proc Natl Acad Sci USA. 2007;104:4008–4013. doi: 10.1073/pnas.0611179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 33.Johnston MD, Maini PK, Jonathan Chapman S, Edwards CM, Bodmer WF. On the proportion of cancer stem cells in a tumour. J Theor Biol. 2010;266:708–711. doi: 10.1016/j.jtbi.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Méndez O, et al. Knock down of HIF-1alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol Cancer. 2010;9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griguer CE, et al. CD133 is a marker of bioenergetic stress in human glioma. PLoS ONE. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi YJ, et al. Transcriptional regulation of the Drosophila caudal homeobox gene by bHLH-PAS proteins. Biochim Biophys Acta. 2007;1769:41–48. doi: 10.1016/j.bbaexp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura H, et al. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: Correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 2004;10:8554–8560. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- 38.Pannuti A, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sansone P, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.