Abstract
Binge drinking (blood-alcohol levels ≥ 0.08 g% in a 2-h period), is a significant public health burden in need of improved treatment. Gene therapy may offer beneficial alternatives to current psychosocial and pharmacotherapeutic interventions, but identification of the target genes is a clinical challenge. We report that a GABAA α2 siRNA vector (pHSVsiLA2) infused into the central nucleus of the amygdala (CeA) of alcohol-preferring (P) rats caused profound and selective reduction of binge drinking associated with inhibition of α2 expression, decreased GABAA receptor density, and inhibition of Toll-like receptor 4 (TLR4). CeA infusion of a TLR4 siRNA vector (pHSVsiLTLR4a) also inhibited binge drinking, but neither vector functioned when infused into the ventral pallidum. Binge drinking was inhibited by a GABAA α1 siRNA vector (pHSVsiLA1) infused into the ventral pallidum, unrelated to TLR4. The vectors did not alter sucrose intake and a scrambled siRNA vector was negative. The data indicate that GABAA α2-regulated TLR4 expression in the CeA contributes to binge drinking and may be a key early neuroadaptation in excessive drinking.
Keywords: alcoholism, HSV vector, reinforcing effects, ethanol, innate immunity
Approximately 30% of the current drinkers in the United States drink alcohol excessively, a condition that kills ~75,000 people annually (1). Binge drinking (blood-alcohol level ≥ 0.08 g% in a 2-h period) is one form of excessive drinking (2) that is related to impulsivity and anxiety (3, 4), and represents a particularly problematic and hazardous form of excessive alcohol intake (5, 6). Gene therapy may offer a beneficial alternative to psychosocial and pharmacotherapeutic interventions, but a clinical challenge is the identification of the relevant target gene or genes. The GABAA receptors are an established molecular target for excessive drinking (7). Microinfusion of α1-preferring ligands into the ventral pallidum (VP) (8), a locus containing the highest concentrations of α1 subunits in the reward circuitry (9), selectively regulated excessive drinking. Indeed, the VP is an important forebrain substrate receiving GABAergic projections from neurons of the extended amygdala (10), and it has been implicated in effort-related decision making pertaining to drug abuse (11). Marked reductions in alcohol drinking were also seen in α1 knock-out mice (12). However, adaptive changes in gene expression preclude final conclusions (13), and evidence of an association between the α1 subunit and alcohol drinking is relatively scant in humans (14). In contrast, human clinical linkage studies support a role for the α2 subunit in alcohol dependence, potentially reflecting the role of α2 in anxiety, impulsivity, and cognition (4, 15, 16). Unfortunately, compelling preclinical data to support the involvement of the α2 subunit in the control of excessive drinking are not currently available (13, 17).
The CeA is a major component of the extended amygdala that has been implicated in early neuroadaptations associated with excessive drinking (18). Like the VP, the central nucleus of the amygdala (CeA) comprises GABAA receptors that, when activated by ligand binding, suppress excessive drinking (19, 20). However, in contrast to the VP, the CeA comprises primarily α2 subunits (10) hypothesized to regulate states of emotionality [like fear and anxiety (16, 10)], which may be salient in the initiation of alcohol drinking (21). In so far as the anxiogenic response to alcohol withdrawal is a powerful motivator of excessive drinking (18), the α2 subunits may also be salient in regulating the negative affective states of alcohol dependence. However, the direct role of the α1 and α2 subunits in regulating binge drinking and their downstream molecular pathways are unknown.
Our studies follow on recent findings that: (i) proinflammatory cytokines/chemokines are involved in alcohol-related neurodegeneration (22), (ii) increased cytokine activity causes an adaptive change that supports sensitization of ethanol withdrawal-induced anxiety linked to GABAA-receptors (23), and (iii) the innate immunity receptor Toll-like receptor 4 (TLR4) regulates proinflammatory cytokine responses (24) and has a pivotal role in alcohol-induced neuroinflammation and brain damage (25, 26). We report that the vulnerability to initiate/sustain binge alcohol drinking is associated with a GABAA α2-TLR4 pathway in the CeA and GABAA α1 in the VP. To the extent of our knowledge, this article is unique in reporting that an innate immunity receptor functions downstream of GABAA receptors in the brain and is associated with the vulnerability to engage in binge alcohol drinking, providing a paradigm shift in the alcohol field.
Results
Infusion of the pHSVsiLA2 Vector into the CeA Inhibits Binge Drinking.
We studied alcohol-preferring (P) rats, an established model of human alcohol abuse, recently shown to engage in National Institute on Alcohol Abuse and Alcoholism-defined effort-related (operant-lever) “binge” drinking (27, 28) (SI Discussion). Compared with nonalcohol-preferring (NP) rats, P rats have elevated levels of GABAA subunits that are associated with excessive drinking in humans, notably α1 in the VP and both α1 and α2 in the CeA (Fig. S1). To examine whether these neuroanatomical site-specific elevations are associated with binge drinking, we used the siRNA technology that specifically inhibits one gene at one site (29). The siRNAs and the construction and specificity of the HSV-based vectors (amplicons) used to deliver them are described in SI Materials and Methods (Figs. S1 and S2, and Table S1). The vectors specifically inhibit their cognate targets and are not toxic. They do not cause loss of body weight or alter general activity levels in the P rats (Fig. S4), and do not induce cell death/apoptosis in intrastriatally infused mice (Fig. S5). The vectors transduce neurons at the injection sites and do not traffic to distant brain areas as determined by EGFP visualization at 72 h after infusion. This result is shown in Fig. 1 A and B for one of the nine CeA sites given pHSVsiLA2. Most EGFP+ cells likely contain GABAA receptors, because the α2 and α1 subunits are highly expressed at these sites (30). Similar results were obtained for all of the vectors and in all examined neuroanatomical sites.
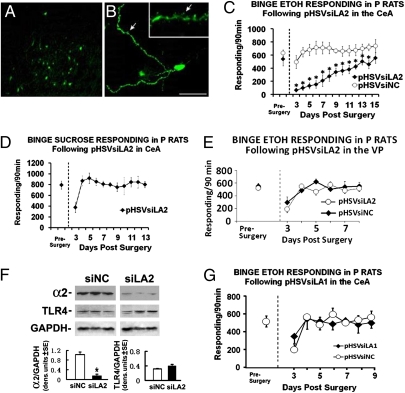
Fig. 1.
CeA-delivered pHSVsiLA2, but not pHSVsiLA1, inhibits binge alcohol drinking. (A and B) A group of infected cells located near one of the pHSVsiLA2 injection sites (A) and an individual EGFP+ neuron (B). (Inset) Arrows show presence in processes and within individual spines. (Scale bar represents 250 μm in A and 25 μm in B, respectively.) (C) Binge alcohol (10% vol/vol) responding in P rats on an FR-4 schedule during presurgery (5 d), and after pHSVsiLA2 infusion into the CeA. The asterisks represent significance for pHSVsiLA2 (n = 8) compared with pHSVsiNC (n = 5) and presurgery controls using Tukey and Dunnett's test, respectively, after significant group × session ANOVA [F(7,56) = 3.34, P < 0.034]. (D) Binge sucrose (0.1% wt/vol) responding in P rats on an FR-4 schedule during the presurgery (5 d) and after pHSVsiLA2 (n = 4) infusion in the CeA. Except for the initial postsurgery day, pHSVsiLA2 did not alter binge sucrose responding (P > 0.05). (E) Binge alcohol responding in P rats on an FR-4 schedule during the presurgery (5 d), and after pHSVsiLA2 (n = 5) or pHSVsiNC (n = 9) infused into the VP. Except for the initial postsurgery day, pHSVsiLA2 did not alter binge alcohol responding (P > 0.05). (F) Extracts of VP micropunches, collected 72 h after pHSVsiLA2 or pHSVsiNC infusion, were immunoblotted with antibodies to α2, TLR4, or GAPDH used as loading control. The blots were stripped between antibodies and results are expressed as densitometric units ± SEM. The levels of α2, but not TLR4, were significantly lower in pHSVsiLA2 than pHSVsiNC rats (*P < 0.001 by ANOVA). (G) Binge alcohol responding in P rats given pHSVsiLA1 (n = 5) or pHSVsiNC (n = 9) into the CeA. Except for the initial postsurgery day, pHSVsiLA1 did not alter alcohol responding (P > 0.05). (See SI Materials and Methods for blood alcohol levels and additional statistical details for C–G.)
To initiate binge drinking, we used the operant drinking-in-the-dark-multiple-scheduled-access (DIDMSA) protocol developed by the Integrative Neuroscience Initiative on Alcoholism (INIA-West) (27, 28). Using this protocol, blood-alcohol levels were ≥ 110 mg/dL. Rats trained to binge on sucrose (0.1% wt/vol) on a similar schedule were studied in parallel. Cohorts of P rats (n = 5–8 per group) trained to self-administer alcohol were randomly given by cohort, pHSVsiLA2 (α2-specific) or pHSVsiNC (scrambled control) into the CeA by bilateral stereotaxic infusion. After 3 d, during which the animals were allowed to recover from the stress of surgery, they were given the opportunity to engage in alcohol or sucrose drinking and examined daily for 15 d (Fig. 1C). On days 3 to 6 after surgery, pHSVsiLA2-infused rats had virtually no alcohol intake. Thereafter, drinking increased with time, returning to the original presurgery levels on day 13 after infusion. This finding is in direct contrast to rats given pHSVsiNC who evidenced minimally reduced alcohol drinking only during the first 3 d after surgery. Throughout the next 14 d of follow-up, the pHSVsiNC-treated rats displayed baseline (presurgery) levels of alcohol drinking (Fig. 1C) and pHSVsiLA2 did not decrease sucrose (Fig. 1D) or water (Fig. S6A) consumption. Significantly, pHSVsiLA2 failed to alter alcohol responding when given into the VP (Fig. 1E) or nucleus accumbens (NAcc) (Fig. S6B), both of which are neuroanatomical control loci. The data indicate that pHSVsiLA2 has both reinforcer and neuroanatomical specificity when infused into the CeA, suggesting that α2 overexpression at this site promotes alcohol drinking vulnerability, at least in P rats.
Infusion of pHSVsiLA1 into the CeA Does Not Inhibit Binge Drinking.
Because α1 expression is also elevated in the CeA from P rats (Fig. S1), we wanted to know if infusion of the α1-specific vector pHSVsiLA1 can similarly reduce binge drinking. Cohorts of P rats (n = 5–9 per group) trained to self-administer alcohol under the “binge operant-lever press model” (27, 28) were randomly given by cohort pHSVsiLA1 or pHSVsiNC into the CeA and examined for alcohol consumption. Except for the first 3 d after surgery, when animals were still recovering from surgery, alcohol drinking was similar to that seen before surgery for both the pHSVsiLA1- and pHSVsiNC-infused rats (Fig. 1G). The data indicate that pHSVsiLA1 infusion into the CeA does not inhibit binge drinking, suggesting that α1 overexpression in the CeA does not contribute to the alcohol-drinking vulnerability of P rats.
Association of pHSVsiLA2-Mediated Decreased Drinking with α2 Inhibition in the CeA.
To examine whether pHSVsiLA2-mediated inhibition of binge drinking is a result of α2 protein inhibition, CeA tissues were collected at 3 and 15 d after infusion, when alcohol drinking was respectively ablated or restored to presurgery levels, and protein extracts were immunoblotted with α2-specific antibody. The blots were stripped and reprobed with α1-specific antibody to control for potential compensatory effects. CeA tissues from animals given pHSVsiNC or PBS, whose drinking was not reduced, were studied in parallel and served as controls. A dramatic decrease in the levels of α2 was caused by pHSVsiLA2 72 h after infusion, when alcohol drinking was ablated, but expression was restored at 15 d after infusion (Fig. 2A), when alcohol drinking was back to presurgery levels. The duration of the inhibitory effect likely reflects that of siRNA integrity/availability and the resulting posttranscriptional gene silencing, as shown for pHSVsiLA1 in Fig. S7. This finding is consistent with previous reports for other unrelated siRNAs (31); α2 was not inhibited in animals given pHSVsiNC (Fig. 2A), and there were no compensatory effects on α1 expression, as evidenced by similar levels of α1 in animals given PBS, pHSVsiLA2, or pHSVsiNC (Fig. 2B). Significantly, pHSVsiLA2 inhibited α2 expression also when given into the VP (Fig. 1F), suggesting that its failure to inhibit binge drinking when given at this site (Fig. 1E) reflects the absence or lack of function of a downstream factor that is operative in the CeA.
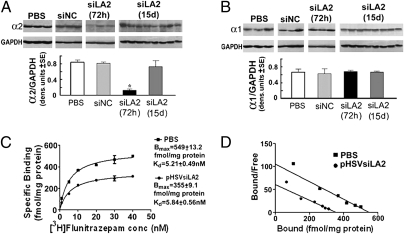
Fig. 2.
Inhibition of α2 expression and receptor density in the CeA by pHSVsiLA2. Cohorts of P rats trained to binge on alcohol as in Fig. 1 were microinfused with PBS, pHSVsiNC, or pHSVsiLA2 into the CeA and micropunches were collected at 72 h or 15 d. Protein extracts were immunoblotted with antibodies to α2 (A), α1 (B), or GAPDH control. The blots were stripped between antibodies and results expressed as densitometric units ± SEM. pHSVsiLA2 inhibited α2, but not α1, at 72 h (*P < 0.001 by ANOVA). Inhibition was not seen for pHSVsiNC and it was lost by day 15 after infusion of pHSVsiLA2 (P > 0.05). (C and D) [3H]flunitrazepam binding in P rats microinfused in the CeA with PBS (n = 10) or pHSVsiLA2 (n = 10) 72 h postinfusion. Significant differences (P < 0.0001) were seen by scatchard analysis (C) and saturation isotherm (D). (See SI Materials and Methods for additional statistical details for A–D.).
Inhibition of Receptor Density in the CeA by pHSVsiLA2.
The effect of pHSVsiLA2 in radioligand ([3H]flunitrazepam) receptor binding was assayed, as previously described (32). [3H]flunitrazepam binding was reduced by pHSVsiLA2 relative to the control condition (PBS-treated rats). Saturation isotherm showed significant differences in specific binding between the two groups. The binding (Bmax) was reduced from 549 ± 13.2 fmol/mg protein in the control group to 355 ± 9 fmol/mg protein in the pHSVsiLA2-treated animals with no significant change in affinity [Kd] (control, 5.21 ± 0.49 nM; pSHVsiLA2-treated, 5.84± 0.56 nM) (Fig. 2 C and D). Although flunitrazepam detects α1, α2, α3, and α5 receptor subunits (33), we conclude that the majority of the involved receptors are α2, because: (i) the CeA in P rats contains primarily α2 and α1 receptor subunits and, (ii) α1 expression was not altered by pHSVsiLA2. The data support the interpretation that the ability of pHSVsiLA2 to inhibit binge drinking is a result of receptor downregulation.
Inhibition of TLR4 Expression in the CeA, but Not VP, by pHSVsiLA2.
We considered the possibility that the putative downstream factor that contributes to the α2 effect in the CeA is TLR4, because: (i) cytokines were implicated in alcohol-mediated brain damage potentially linked to GABAA-receptors (22, 23), (ii) TLR4 signaling was associated with neuroinflammation and brain damage (25, 26), and (iii) alcohol up-regulates genes in the Toll-innate immunity pathway in Drosophila (34). Cohorts of naive P rats (n = 3–4 per group) were given pHSVsiLA2 or pHSVsiNC in the CeA, and tissues collected 72 h after infusion were immunoblotted with antibodies to α2 or TLR4. Antibody to another innate immunity receptor, CCR2, served as a control. There was a significant decrease in the levels of both α2 and TLR4 caused by pHSVsiLA2, but expression was not altered by pHSVsiNC (Fig. 3A). The expression of CCR2 did not decrease because of pHSVsiLA2 (Fig. 3A) and TLR4 expression was not reduced by pHSVsiNC. TLR4 inhibition is associated with the ability of pHSVsiLA2 to inhibit binge drinking and is not an off-target effect of pHSVsiLA2, because pHSVsiLA2 did not inhibit TLR4 expression in RAW264.7 cells (Fig. S3B) nor when infused into the VP (Fig. 1F), a site at which it does not decrease binge drinking (Fig. 1E).
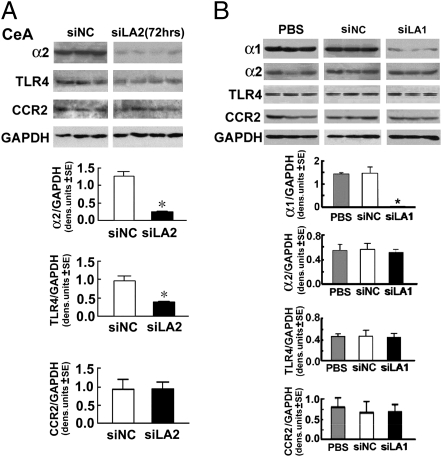
Fig. 3.
CeA-delivered pHSVsiLA2, but not pHSVsiLA1, inhibits TLR4 expression. (A) Micropunches from the CeA of naive P rats microinfused with pHSVsiNC or pHSVsiLA2 collected 72 h after infusion were immunoblotted with antibodies to α2, TLR4, or CCR2 using GAPDH control. Both α2 and TLR4 were significantly lower in pHSVsiLA2 than pHSVsiNC rats (*P < 0.001 by ANOVA). (B) Micropunches of the CeA from naive P rats microinfused with PBS, pHSVsiNC, or pHSVsiLA1 were collected 72 h after infusion and immunoblotted with antibodies to α1, α2, TLR4, or CCR2 using GAPDH control; α1 was significantly lower in pHSVsiLA1 than PBS or pHSVsiNC-treated rats (*P < 0.001 by ANOVA). The levels of α2, TLR4, and CCR2 were similar (P > 0.05). (See SI Materials and Methods for additional statistical details for A and B.)
To further examine the relationship between the α2-regulated TLR4 and binge drinking, a second series of experiments asked whether TLR4 is also inhibited by pHSVsiLA1, which does not affect binge drinking when infused into the CeA. Cohorts of naive P rats (n = 3 per group) were given PBS, pHSVsiLA1, or pHSVsiNC in the CeA and tissues collected 72 h after infusion were immunoblotted with antibodies to α1, α2, TLR4, or CCR2, used as control. A significant reduction in the expression of α1, but not α2, TLR4, or CCR2, was caused by pHSVsiLA1, and pHSVsiNC was negative (Fig. 3B). Because pHSVsiLA1 does not inhibit binge drinking when infused into the CeA, the data implicate TLR4 as a downstream component of an α2 pathway in the CeA that regulates binge drinking.
CeA-Delivered pHSVsiLTLR4a Selectively Inhibits Binge Alcohol Drinking.
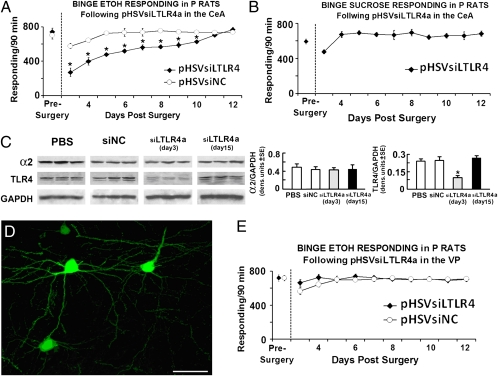
To confirm that TLR4 in the CeA contributes to the vulnerability to engage in binge drinking, cohorts of P rats trained to self-administer alcohol were randomly given, by cohort, pHSVsiLTLR4a (n = 10) or pHSVsiNC (n = 8) into the CeA. After 3 d, during which the animals were allowed to recover from the stress of surgery, alcohol drinking was examined daily for 12 d. We found that pHSVsiLTLR4a inhibited alcohol drinking, with maximal reduction seen on days 3 to 6 postinfusion. Drinking returned to presurgery levels on day 11 after infusion. In contrast, animals given pHSVsiNC evidenced minimally reduced alcohol drinking and only on days 3 to 5 after surgery, likely related to surgical stress (Fig. 4A). Binge sucrose-motivated responding was not altered (Fig. 4B). Reflecting its effect on binge drinking, pHSVsiLTLR4a inhibited TLR4 protein expression on day 3, but not day 15 postinfusion (Fig. 4C). The data indicate that pHSVsiLTLR4a treatment has both neuroanatomical and reinforcer specificity, supporting the interpretation that TLR4 is involved in the CeA effect on binge drinking. We conclude that the TLR4 effect is neuronal, because pHSVsiLTLR4a localized in neurons (Fig. 4D). Significantly, studies of P rats randomly given, by cohort, pHSVsiLTLR4a (n = 7) or pHSVsiNC (n = 8) into the VP indicated that binge drinking was not altered by pHSVsiLTLR4a given at this site (Fig. 4E). This finding is consistent with the failure of pHSVsiLA2 to inhibit binge drinking when given into the VP and suggests that the α2 contribution to binge drinking is through a TLR4-encompassing pathway that is not operative in the VP.
Fig. 4.
CeA-delivered pHSVsiLTLR4a selectively inhibits binge alcohol drinking. (A) Binge alcohol responding in P rats on an FR-4 schedule during the presurgery (5 d), and after pHSVsiLTLR4 infusion into the CeA. The asterisk represents significance for pHSVsiLTLR4 (n = 10) compared with pHSVsiNC (n = 8) and the presurgery control using the Tukey and Dunnett's test, respectively, following significant group [F(1,10) = 15.46, P < 0.003] and session [F(10, 10) = 3.42, P < 0.032] effects. (B) Binge sucrose responding in P rats (n = 5) on an FR-4 schedule during the presurgery (5 d) and after pHSVsiLTLR4 infusion in the CeA. Except for the initial postsurgery day, pHSVsiLTLR4a did not significantly alter binge sucrose responding (P > 0.05). (C) Cohorts of binged P rats were microinfused with PBS, pHSVsiNC, or pHSVsiLTLR4a into the CeA and micropunches collected 72 h or 15 d after infusion. Protein extracts were then immunoblotted with antibodies to α2, TLR4, or GAPDH control and blots stripped between antibodies and expressed as densitometric units ± SEM pHSVsiLTLR4a inhibited TLR4 at day 3, but not day 15 relative to PBS and pHSVsiNC (P > 0.05); pHSVsiLTLR4a also failed to alter the α2 protein (P > 0.05). (D) Group of EGFP+ neurons near one of the pHSVsiLTLR4a injection sites in the CeA. (Scale bar, 25 μm.) (E) Binge alcohol responding in P rats on an FR-4 schedule during the presurgery (5 d), and after pHSVsiLTLR4 (n = 7) and pHSVsiNC (n = 8) infused into the VP; pHSVsiLTLR4a did not alter binge alcohol responding in the VP [F(1, 10) = 2.78, P > 0.05]. (See SI Materials and Methods for blood alcohol levels and additional statistical details for A–C and E.)
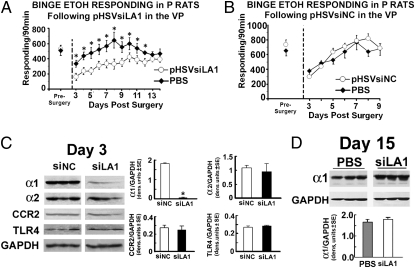
VP-Infused pHSVsiLA1 Inhibits Binge Drinking Unrelated to TLR4 Expression.
Having seen that α1 is overexpressed in the VP from P rats (Fig. S1), we wanted to know whether it contributes to the vulnerability to engage in binge drinking. Cohorts of P rats (n = 4–10 per group) trained to self-administer alcohol under the “binge operant-lever press model” (27, 28) were randomly given, by cohort, pHSVsiLA1, pHSVsiNC, or PBS into the VP and examined for alcohol consumption. After 3 d of recovery from the stress of surgery, alcohol drinking was examined daily for 13 d. Alcohol drinking was significantly decreased in animals given pHSVsiLA1, with maximal inhibitory levels seen on days 3 to 5 after infusion and returned to the presurgery levels on day 13 after infusion (Fig. 5A). This finding is in direct contrast to rats given PBS (Fig. 5 A and B) or pHSVsiNC (Fig. 5B), who evidenced reduced alcohol drinking only during the first 3 to 4 d after surgery, likely related to the recovery from the surgical stress. We found that pHSVsiLA1 also failed to alter alcohol responding when given into the NAcc (Fig. S6C). Immunoblotting revealed a strong correlation between the inhibition of binge drinking and α1 expression (Fig. 5C), with α1 levels returning to the original presurgery levels by day 15 (Fig. 5D). The expression of α2 and TLR4 was not altered (Fig. 5C). The data indicate that α1 overexpression in the VP promotes alcohol drinking vulnerability, unrelated to TLR4.
Fig. 5.
VP-delivered pHSVsiLA1 inhibits binge alcohol drinking unrelated to TLR4. (A) Binge alcohol responding in P rats on an FR-4 schedule during the presurgery (5 d), and after pHSVsiLA1 infusion into the VP. The asterisk represents significance for pHSVsiLA1 compared with PBS and the presurgery control using the Tukey and Dunnett's test, respectively, following significant group [F(1, 12) = 50.31, P < 0.0001] and session [F(12, 182) = 3.151, P < 0.0004] effects. (B) Binge alcohol responding in P rats on an FR-4 schedule for presurgery (5 d), and after pHSVsiNC or PBS microinfused into the VP. Both displayed a similar profile of effects (P > 0.05). (C and D) Cohorts of P rats trained to binge on alcohol were microinfused with pHSVsiNC or pHSVsiLA1 into the VP and tissues collected at 72 h (C) or 15 d (D) were immunoblotted with antibodies to α1, α2, CCR2, or TLR4 using GAPDH as loading control. Results are densitometric units ± SEM. pHSVsiLA1 inhibited α1 at day 3 (P < 0.05), but not 15 d postinfusion (P > 0.05). Expression of α2, CCR2, and TLR4 was not inhibited (P > 0.05). (See SI Materials and Methods for blood alcohol levels and additional statistical details for A–D.)
Discussion
The GABAA receptor is a well-recognized contributor to excessive alcohol drinking (4), but the development of effective therapies requires a better understanding of the specific receptor subunits, their brain loci, and the molecular pathways that transmit the signal and contribute to the regulation of the vulnerability to engage in excessive drinking. We report that the vulnerability to engage in binge drinking is differentially related to the GABAA α2 and α1 subunits at distinct brain sites and is regulated by GABAA α1 receptors in the VP and a GABAA α2-regulated pathway that includes TLR4 in the CeA. To the extent of our knowledge, this article is unique in reporting that an innate immunity receptor is under the control of the GABAA receptors in the brain, and is associated with the vulnerability to engage in binge drinking.
To better evaluate the contribution of the differential GABAA expression patterns to binge drinking, we used the siRNA technology that allows for specific inhibition of target genes at distinct brain sites. The specificity of our siRNA vectors is documented both in cultured cells and in the brains from microinfused rats and all experiments were done in parallel with an identical vector for scrambled siRNA (pHSVsiNC). The vectors are not toxic, as documented both at the gross and histologic levels, and they are expressed at the sites of stereotaxic delivery without evidence of trafficking to distant brain areas. Using these vectors, we found that pHSVsiLA2 infusion into the CeA caused a profound reduction in binge drinking that lasted 14 d and was not seen for pHSVsiNC. Inhibition of binge drinking was directly related to the inhibition of α2 expression through siRNA-mediated posttranscriptional gene silencing. Both were maximally reduced on days 3 to 6 postinfusion and increased with time thereafter returning to presurgery levels on day 14 postinfusion. This relatively long-lasting effect is consistent with previous reports for amplicon-delivered siRNAs (31). Loss of α2 expression (on day 3 postinfusion) had a profound and selective negative effect on the density of the GABAA receptor (reduction to 65%), but additional studies are needed to define the quantitative relationship between the levels of α2 and the extent of binge drinking (EtOH responding/90 min) and verify the effect of alcohol-induced changes and their siRNA modulation on receptor function.
Significantly, although α1 was also overexpressed in the CeA from P rats, its inhibition by infused pHSVsiLA1 did not reduce binge drinking. Conversely, drinking was inhibited by pHSVsiLA1-mediated inhibition of α1 expression in the VP, underscoring the different contributions of distinct GABAA receptors at various brain regions to binge drinking and suggesting that they may function through distinct mechanisms. The NAcc, a well-established GABAA alcohol-reward locus (18), was not associated with GABAA α1- or α2-mediated binge drinking. The ability of pHSVsiLA2 to inhibit binge drinking when infused into the CeA was associated with inhibition of both α2 and TLR4 expression, but only α2 was inhibited by pHSVsiLA2 infusion into the VP. We conclude that in the CeA, TLR4 contributes to binge drinking downstream of α2, because the pHSVsiLTLR4a vector infused at this site also inhibited binge drinking specifically associated with inhibition of TLR4, but not α2 expression. Further supporting the specificity of the α2–TLR relationships, TLR4 was not inhibited by the CeA-infused pHSVsiLA1 vector—which does not inhibit binge drinking when given at this site—nor by pHSVsiLA1 infusion into the VP, where it inhibits binge drinking.
It seems reasonable to suggest that the alcohol-related connection between the α2 and TLR4 receptors is through elevated GABA production (35). GABA could stimulate the TLR4 responses directly (as a ligand) or indirectly via stimulation of chemokine/cytokine networks. This connection could have autocrine or paracrine effects that involve neurons and glial cells, all of which express TLR4. Previous studies have shown that ethanol induces neuronal damage involving TLR4-dependent microglial activation (25). However, the contribution of TLR4 to the initiation or sustaining of binge drinking, its regulation and mechanism, and the cells that are involved, are unknown. The convergence of our findings with existing topographical, neuroanatomical, neuropharmacological, and linkage studies suggests that binge drinking is a result of impaired neuronal inhibition caused by overactive α2-containing GABAA receptors in the CeA and α1-containing GABAA receptors of the VP. TLR4 contributes to binge drinking downstream of α2 in the CeA, but not VP, underscoring the relevance of TLR4 and specific neuroanatomical sites. The neuronal localization of pHSVsiLTLR4a suggests that the α2–TLR4 axis that contributes to binge drinking is neuronal. However, we cannot exclude the contribution of a small number of pHSVsiLTLR4a-targeted glial cells that escaped detection, nor the potential crosstalk between the TLR4+ neurons and glial cells. An important, as yet unanswered question is: why does TLR4 contribute to binge drinking only downstream of α2 and only in the CeA? It is possible that TLR4 contributes to binge drinking at other, as yet unstudied brain sites, potentially through distinct mechanisms implicated in the addiction cycle and involving different cell types. Ongoing studies are designed to address these questions and elucidate the potential contribution to binge drinking of chemokines/cytokines that were implicated in inflammation-related brain damage (22) and are TLR4-regulated (24). The amplicon-delivered siRNA technology provides an important tool to address these questions and may represent a promising therapeutic strategy in attenuating binge drinking behaviors.
Materials and Methods
Antibodies.
The generation and specificity of the rabbit-derived GABAA α1 and α2 antibodies have been previously described (30); they recognize amino acids 1 to 9 and 322 to 357 of the α1 and α2 proteins, respectively.
Small Interfering RNA Vectors.
The siRNAs and the construction and specificity of the EGFP-containing vectors used for their delivery is described in SI Materials and Methods.
Immunoblotting.
Tissue micropunches (300-μm thick) were used in immunoblotting, done as previously described (36) and in further detail in SI Discussion.
Radioligand Binding.
CeA membrane homogenates were prepared from adult male P rats and binding of [3H]flunitrazepam was determined by filtration assay (32). Briefly, homogenates were incubated (1 h, 4 °C) with varying concentrations of [3H]flunitrazepam (0.5–40 nM) in 50 mM Tris-HCl buffer pH 7.4, containing 120 mM NaCl and 5 mM KCl. Nonspecific binding was determined with 100 μM diazepam. Radioactivity was counted by liquid scintillation spectroscopy (Packard; TRI-CARB 2900 TR).
Binge Drinking.
To initiate excessive alcohol drinking, we used the DIDMSA binge operant model developed by INIA-West (27, 28). The 90-min drinking sessions were conducted on a 5-d binge and 2-d withdrawal schedule that emulates human binge-drinking patterns (6). Rats consumed alcohol for 21 d before the presurgery phase.
Stereotaxic Procedures.
Rats were anesthetized by intraperitoneal injection of nembutal (50 mg/kg) and positioned in a stereotaxic apparatus (8). Microinjection was in the CeA and VP (37). Each bregma level of each locus received 200 nL of PBS or amplicon (2.5 × 105 TU) delivered by calibrated glass micropipettes (~20-μm tip) connected to a pneumatic pressure injector (Science Products GmbH). The Institutional Animal Care and Use Committee and Biosafety Committees, University of Maryland approved the procedures. See SI Materials and Methods for additional details.
Statistics.
Data were analyzed by appropriate ANOVAs. Significant ANOVAs were followed by Dunnett and Tukey post hoc tests. Analyses were performed using the Stat Most 5.0 programs (Dataxiom Software Inc.).
Supplementary Material
Acknowledgments
We thank Dr. Cynthia Smith for help with the siRNA studies, Dr. Jennifer Laing for help with the vector toxicity evaluation studies, and Kaitlin Warnock and Mingfei Wang for assisting with the TLR4 behavioral studies. This research was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health Public Health Service Grant NS45169 (to L.A.) and National Institute on Alcohol Abuse and Alcoholism Grants R21AA016933, 1R01AA017963-01A1, and R01AA017963 (to H.L.J.,Sr.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019020108/-/DCSupplemental.
References
- 1.Town M, Naimi TS, Mokdad AH, Brewer RD. Health care access among U.S. adults who drink alcohol excessively: Missed opportunities for prevention. Prev Chronic Dis. 2006;3(2):A53. [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute on Alcohol Abuse and Alcoholism NIAAA Council approves definition of binge drinking. 2004 NIAAA Newsletter 04-5346(3) [Google Scholar]
- 3.Ducci F, et al. Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol. 2007;41(1):3–12. doi: 10.1016/j.alcohol.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edenberg HJ, et al. Variations in GABA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chikritzhs TN, Jonas HA, Stockwell TR, Heale PF, Dietze PM. Mortality and life-years lost due to alcohol: A comparison of acute and chronic causes. Med J Aust. 2001;174:281–284. doi: 10.5694/j.1326-5377.2001.tb143269.x. [DOI] [PubMed] [Google Scholar]
- 6.Naimi TS, et al. Binge drinking among US adults. JAMA. 2003;289(1):70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 7.Harris RA, Trudell JR, Mihic SJ. Ethanol's molecular targets. Sci Signal. 2008;1(28):re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey SC, et al. The GABAA receptor subtype in the ventral pallidum regulates alcohol-seeking behavior. J Neurosci. 2002;22:3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchill L, et al. GABAA receptors containing alpha 1 and beta 2 subunits are mainly localized on neurons in the ventral pallidum. Synapse. 1991;8(2):75–85. doi: 10.1002/syn.890080202. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann WA, Humpel C, Alheid GF, Marksteiner J. Compartmentation of alpha 1 and alpha 2 GABA(A) receptor subunits within rat extended amygdala: Implications for benzodiazepine action. Brain Res. 2003;964(1):91–99. doi: 10.1016/s0006-8993(02)04082-9. [DOI] [PubMed] [Google Scholar]
- 11.Mingote S, et al. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008;28:9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June HL, Sr, et al. Dopamine and benzodiazepine-dependent mechanisms regulate the EtOH enhanced locomotor stimulation in the GABAA alpha1 subunit null mutant mice. Neuropsychopharmacology. 2007;32(1):137–152. doi: 10.1038/sj.npp.1301097. [DOI] [PubMed] [Google Scholar]
- 13.Boehm SL, 2nd, et al. Gamma-aminobutyric acid A receptor subunit mutant mice: New perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Dick DM, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin Exp Res. 2006;30:1101–1110. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 15.Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B, Neuropsychiatr Genet. 2006;141B:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Löw K, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290 doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 17.Blednov YA, et al. Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive α2-containing GABAA receptors. J Pharmacol Exp Ther. 2011;336(1):145–154. doi: 10.1124/jpet.110.171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 19.Foster KL, et al. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 21.Spanagel R, et al. Anxiety: A potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- 22.Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: Key role of NF-kappaB and proinflammatory cytokines. Alcohol Clin Exp Res. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 23.Breese GR, et al. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2008;33:867–876. doi: 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollen KP, et al. Emerging paradigm: Toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- 26.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell RL, Lumeng ZA, Murphy L, Mc JM, Bride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 28.McBride WJ, Li T-K. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 29.Grimm D, Kay MA. RNAi and gene therapy: A mutual attraction. Hematol Am Soc Hematol Educ Program, 2007;2007:473–481. doi: 10.1182/asheducation-2007.1.473. [DOI] [PubMed] [Google Scholar]
- 30.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 31.Saydam O, et al. Herpes simplex virus 1 amplicon vector-mediated siRNA targeting epidermal growth factor receptor inhibits growth of human glioma cells in vivo. Mol Ther. 2005;12:803–812. doi: 10.1016/j.ymthe.2005.07.534. [DOI] [PubMed] [Google Scholar]
- 32.Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABA(A) receptor alpha1 subunit knockout mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- 33.Sieghart W. Structure and pharmacology of gamma-aminobutyric acid-A receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 34.Kong EC, et al. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res. 2010;34:302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wales SQ, Li B, Laing JM, Aurelian L. The herpes simplex virus type 2 gene ICP10PK protects from apoptosis caused by nerve growth factor deprivation through inhibition of caspase-3 activation and XIAP up-regulation. J Neurochem. 2007;103:365–379. doi: 10.1111/j.1471-4159.2007.04745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. San Diego: Academic Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.