Abstract
Individuals with 22q11.2 microdeletions have cognitive and behavioral impairments and the highest known genetic risk for developing schizophrenia. One gene disrupted by the 22q11.2 microdeletion is DGCR8, a component of the “microprocessor” complex that is essential for microRNA production, resulting in abnormal processing of specific brain miRNAs and working memory deficits. Here, we determine the effect of Dgcr8 deficiency on the structure and function of cortical circuits by assessing their laminar organization, as well as the neuronal morphology, and intrinsic and synaptic properties of layer 5 pyramidal neurons in the prefrontal cortex of Dgcr8+/− mutant mice. We found that heterozygous Dgcr8 mutant mice have slightly fewer cortical layer 2/4 neurons and that the basal dendrites of layer 5 pyramidal neurons have slightly smaller spines. In addition to the modest structural changes, field potential and whole-cell electrophysiological recordings performed in layer 5 of the prefrontal cortex revealed greater short-term synaptic depression during brief stimulation trains applied at 50 Hz to superficial cortical layers. This finding was accompanied by a decrease in the initial phase of synaptic potentiation. Our results identify altered short-term plasticity as a neural substrate underlying the cognitive dysfunction and the increased risk for schizophrenia associated with the 22q11.2 microdeletions.
Keywords: 22q11.2 deletion syndrome, synaptic plasticity, field EPSP, patch clamp, neurogenesis
Occuring predominantly de novo, 22q11.2 microdeletions are among the most common chromosomal abnormalities (1). Microdeletion carriers exhibit a spectrum of cognitive deficits (1) and ~30% of them develop schizophrenia (SCZ) in adolescence or early adulthood (1). Recurrent 22q11.2 microdeletions account for up to 1% to 2% of sporadic SCZ cases (2). The majority of cases have a 3-Mb deletion, and 7% have a nested 1.5-Mb deletion that removes 27 known genes (1). Although one or a few genes may have a greater phenotypic impact, it is the imbalance of several deleted genes that determines the overall phenotype (1). Animal model studies seem to support this scenario and have been instrumental in identifying genes responsible for specific deficits (1).
Using chromosomal engineering, we generated a mouse model carrying a 1.3-Mb chromosomal deficiency (Df(16)A) on mouse chromosome 16, which is syntenic to the 22q11.2 1.5-Mb microdeletion [Df(16)A+/− mice] (3). The Df(16)A+/− mouse strain provided compelling evidence that microRNA (miRNA) dysregulation emerges as a result of the 22q11.2 microdeletion (3). The deleted region of human chromosome 22 and mouse chromosome 16 contains Dgcr8, a key component of the “microprocessor” complex essential for miRNA production (4). The Dgcr8 gene is haploinsufficient and an approximate halving of its expression results in the down-regulation (by ~20–80%) of a specific subset of mature brain miRNAs that may account for a portion of the transcript misregulation observed in the brain of Df(16)A+/− mice. To determine if Dgcr8 deficiency and the consequent alterations in miRNA biogenesis contribute to the neuronal abnormalities observed in Df(16)A+/− mice, we also generated heterozygous Dgcr8-deficient mice (homozygous deficiency results in embryonic lethality) (3).
Working memory refers to the short-term retention of information aiming at planning and organizing a forthcoming action that can be delayed. Working memory deficits have become increasingly recognized as a key deficit in SCZ (5, 6), and deficits in spatial working memory tasks have been found in children and adolescents with the 22q11.2 microdeletion (1, 7, 8). Such deficits may reflect a more general disruption of the dynamics of neural networks that subserve sensory perception and cognition. Df(16)A+/− mice show a deficit in acquiring a working memory-dependent task (the T-maze delayed nonmatch to place task) whose successful execution depends on the frontal regions of the mouse neocortex and their interaction with the hippocampus (9, 10). Interestingly, it was determined that this deficit arises, in part, as a result of deficiency of Dcgr8 and miRNA biogenesis, because heterozygous deficiency of Dgcr8 alone affects acquisition of the task without affecting associative memory (3). These findings highlight the contribution of this gene and of miRNAs to the cognitive phenotypes associated with the 22q11.2 microdeletions, and offer a unique opportunity to identify the neural substrates underlying these phenotypes.
Working memory theories typically invoke persistent neuronal activity and invariably propose a central and unique contribution of the prefrontal cortex (PFC). These models include nonmutually exclusive accounts of excitatory cortico–thalamo–cortical loops, reentrent cortico–cortical networks, local cortical circuit dynamics, and intrinsic cellular properties, and most rely on recurrent excitation that persists, even in the absence of continued sensory stimulation (11, 12). Therefore, mutations that affect working memory may affect cortical networks structurally or functionally by impacting upon the establishment of neuronal connections, as well as synaptic transmission or plasticity. Dgcr8 is expressed in the developing and mature cortex but it remains unknown if reduction in Dgcr8 levels alters cortical function and, if so, whether it acutely impairs intrinsic and synaptic properties of cortical neurons or affects cortical circuitry or both.
To explore the effect of Dgcr8 deficiency in the structure and activity of cortical circuits, and to further our understanding of the cellular mechanisms underlying the cognitive dysfunction and psychiatric phenotypes associated with 22q11.2 microdeletions, we conducted electrophysiological and morphological analysis of prefrontal pyramidal neurons from Dgcr8+/− mutant mice and their WT littermates. We found that layer 5 (L5) pyramidal neurons from mutant mice show normal intrinsic membrane properties and normal basal synaptic transmission upon activation of superficial layer afferents. In contrast, L5 neurons displayed a greater level of short-term synaptic depression (STD) and less potentiation following physiologically relevant high-frequency stimulation, likely because of a deficit at the presynaptic level. Changes in cortical synaptic plasticity were accompanied by modest changes in the density of layer 2/4 (L2/4) neurons, as well as a modest but significant decrease in the size of spines of basal dendrites of L5 pyramidal neurons. Our results strongly suggest that alterations in synaptic properties within the PFC due to miRNA dysregulation contribute to the cognitive impairments observed in mouse models and in patients with the 22q11.2 microdeletion. Because the same abnormal cortical processes mediating cognitive deficits may also underlie other symptoms, which cannot be readily modeled in mice (such as positive or negative symptoms), our results may offer more general insights into the nature of the neural substrates underlying 22q11.2-associated cognitive and psychiatric phenotypes.
Results
Basic Synaptic Transmission in the PFC Is Normal in Dgcr8+/− Mice.
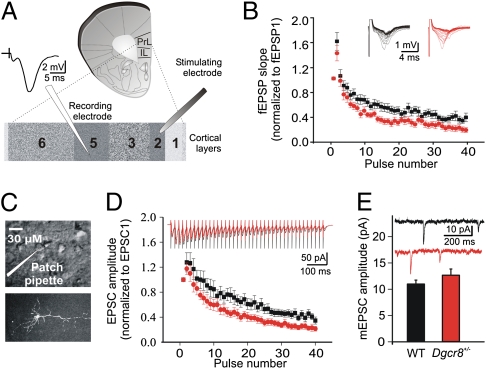
Fibers coursing across long distances in the superficial cortical layers carry top-down information from higher cortical regions (13, 14), as well as feedback information from thalamocortical pathways (15) and feedback pathways through the hippocampus (16). In the PFC, these top-down pathways are thought to contribute to cognitive processes (reviewed in ref. 17) that are impaired in SCZ patients, including working memory. Thus, to assess the top-down information processing in the PFC of Dgcr8+/− mice, we used field recordings and we initially characterized the synaptic properties of neurons in L5 (a major cortical output layer of the PFC network) while stimulating at the border of L1 and L2/3 (Fig. 1A). Stimulation applied at the border of L1 and L2/3 is thought to activate cortico-cortical afferent fibers contacting the apical dendrites of pyramidal neurons (13, 18), as well as L2 pyramidal neurons whose axons collaterize locally in the cortex and provide many synapses onto the dendrites of L5 pyramidal neurons. Such inputs are known to make monosynaptic contacts with pyramidal neurons in L5.
Fig. 1.
The effect of the Dgcr8 deficiency on STD in L5 of mPFC. (A) Schematic representation of the prelimbic (PrL) and infralimbic (IL) areas in the mPFC slice preparation. The stimulating electrode was placed at the border of L1 and L2/3, and the extracellular recording electrode was placed in L5. A sample field fEPSP is shown. (B) Field EPSPs evoked by 40 stimuli at 50 Hz. The upper superimposed sample traces are individual fEPSP responses to each stimulus within the 50-Hz train. STD is significantly greater in Dgcr8+/− mice (N = 7; n = 16) than in the WT controls (N = 8; n = 14) (two-way repeated-measures ANOVA; P < 0.02). Post hoc analysis revealed that the level of depression was significantly greater in the Dgcr8+/− mice compared with WT mice at the seventh pulse (P = 0.048). (C) Voltage clamp recordings were made from visually identified (Upper) and biocytin-filled (Lower) L5 pyramidal neurons of the mPFC. (D) (Upper) Traces show the decrease in EPSC amplitudes in response to 40 pulses applied at 50 Hz and are the averages of the 10 repetitions performed in WT (N = 8, n = 13) and in Dgcr8+/− mice (N = 9, n = 11). Each vertical line represents the stimulation artifact. The short-term depression of the mean EPSC amplitudes is significantly greater in the mutant mice than in WT controls across experiments (two-way repeated-measures ANOVA; P < 0.05). Post hoc analysis revealed that the level of depression was significantly greater in the Dgcr8+/− mice compared with WT mice at the 14th pulse (P = 0.033). (E) The mean amplitude of mEPSCs was not different between genotypes. WT mice (N = 4; n = 8); Dgcr8+/− mice (N = 3; n = 4). Sample mEPSC traces of recordings from WT (black) and Dgcr8+/− (red) mice are shown.
Stimulation of the superficial layers of the medial PFC (mPFC) elicited typical field excitatory postsynaptic potentials (fEPSPs). The small initial nonsynaptic fiber volley, whose amplitude is proportional to the number of recruited afferent fibers, was followed by a prominent synaptic response. This fEPSP was quantified by measuring the initial slope of the linear rising phase (Fig. 1A). The fEPSPs evoked at low stimulation frequency (0.033 Hz) were reproducible with a high signal-to-noise ratio. After a stable baseline, basal synaptic transmission was assessed by gradually increasing the stimulation intensity. Overall, no genotypic differences were observed in the amplitude of the fiber volley, or in the stimulus-response curve (Fig. S1A) (WT: N = 7; n = 17; Dgcr8+/−: N = 7; n = 17; two-way repeated-measures ANOVA; P > 0.05). Thus, when the same number of afferent fibers are recruited, basal transmission is unaffected by the mutation.
Short-Term Synaptic Plasticity Is Impaired in the mPFC of Dgcr8+/− Mice.
Both short- and long-term synaptic plasticity can be induced in PFC networks in acute slices (19–23) and likely play an important role in PFC function. We first determined whether short-term synaptic plasticity was impaired in the mPFC of the Dgcr8+/− mice (WT: N = 7; n = 17; Dgcr8+/−: N = 7; n = 17). The fractional neurotransmitter release, as assessed by a paired-pulse facilitation (PPF) protocol at various interstimulus intervals, was unchanged in the PFC of Dgcr8+/− mice (Fig. S1B) (n = 17 slices per genotype; two-way repeated-measures ANOVA; P > 0.05). During the delay period of working memory tasks, PFC neurons can typically receive trains of inputs from neighboring cells in the 20- to 60-Hz range. Therefore, we determined the consequences of Dgcr8 deficiency on the response to trains of inputs within this physiological frequency range, by applying 40 impulses at 50 Hz. The superimposed sample traces of Fig. 1B show that in both genotypes, the fEPSP could follow a high-frequency stimulation, typically reflecting monosynaptic connections. At this stimulation frequency, the second evoked fEPSP was facilitated but the facilitation was not different between genotypes (Fig. 1B) (WT = 1.60 ± 0.12, N = 8, n = 14; Dgcr8+/− = 1.40 ± 0.12, N = 7, n = 16; t test; P > 0.05). This facilitation was rapidly followed by a strong depression of the fEPSPs, which was greater in the Dgcr8+/− compared with WT mice (two-way repeated-measures ANOVA; P = 0.0154).
To characterize synaptic plasticity at the single-cell level, we made whole-cell recordings and investigated STD under voltage-clamp conditions. Biocytin-filled L5 pyramidal cells were clearly identified having the typical pyramidal cell morphology, with a prominent apical dendrite that extends up to the superficial layers (Fig. 1C). Extracellular field and intracellular voltage-clamp recordings both measure synaptic currents and should corroborate each other. As expected, using the same 50-Hz stimulation paradigm as in the field recordings, intracellular recordings from L5 pyramidal neurons revealed that during the high-frequency stimulation train, the initial facilitation was not different between genotypes but the depression in amplitude of excitatory synaptic currents was greater in mutant mice (Fig. 1D) (WT: N = 8, n = 13; Dgcr8+/−: N = 9, n = 11; two-way repeated-measures ANOVA; P < 0.05). The synaptic depression remained greater in the mutant mice until the end of the train, similar to the field recording results. The greater depression observed in the mutant animals was not simply caused by a change in synaptic glutamatergic receptors, as no effect was observed on the amplitude of the miniature excitatory postsynaptic currents (mEPSCs) (Fig. 1E) (WT: 10.99 ± 0.75 pA, N = 4, n = 8; Dgcr8+/−: 12.66 ± 1.18 pA, N = 3; n = 4). In addition, there was no effect on the spontaneous EPSC (sEPSC) amplitude, area, rise, and decay time (Table S1) (WT: N = 13, n = 21; Dgcr8+/−: N = 13; n = 27; t test; P > 0.05). These results suggest that a change in transmitter-release characteristics may contribute to the enhanced depression of synaptic currents during 50-Hz stimulation.
Dgcr8 Deficiency Alters Postsynaptic EPSP Temporal Summation.
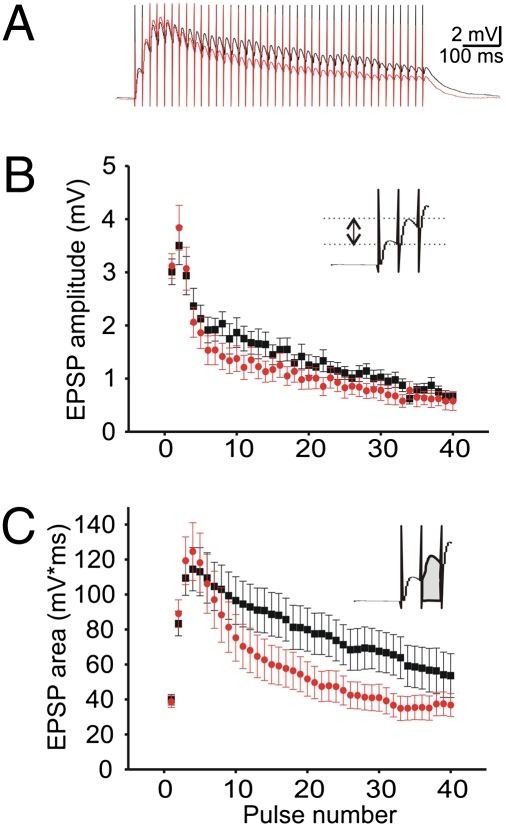
Changes in synaptic depression are likely to affect the temporal summation of synaptic inputs. Therefore, we evaluated the effect of Dgcr8 deficiency under current-clamp conditions (Fig. 2A) (WT: N = 10, n = 12; Dgcr8+/−: N = 10, n = 15). Two approaches were used to measure the depolarizing synaptic responses recorded in L5 pyramidal neurons. First, we measured the amplitude of each individual EPSP from a baseline obtained just after each pulse in the train (Fig. 2B, Inset). Measured in this way, the peak amplitude of the EPSPs ignores the effect of residual depolarization from the preceding EPSP in the train. This measurement revealed no significant genotypic difference (two-way repeated-measures ANOVA; P > 0.05). Second, we measured the area of the depolarizing response for each EPSP, which takes into account the residual depolarization caused by the summation of the EPSPs (Fig. 2C, Inset). This analysis revealed a decrease in the level of the depolarization in mutant mice during the 50-Hz train. Specifically, after the initial 20 pulses, the area values of the mutant mice decreased rapidly toward baseline levels, whereas the values of the WT mice were still 40% above baseline at the end of the stimulation train (Fig. 2C). The interaction between genotype and EPSP area was significantly different, indicating an effect on the shape of EPSP summation (two-way repeated-measures ANOVA; P < 0.0001). This difference in EPSP summation is likely to be a consequence of a decrease in transmitter release in the mutant mice during the stimulation train. Interestingly, the membrane properties of postsynaptic L5 pyramidal neurons tested here, such as the membrane time constant, τ (24), the hyperpolarization-activated, cyclic nucleotide-gated (HCN) cation channel activation (25), and cell excitability do not seem to contribute to the genotypic difference in the amount of EPSP summation during synaptic depression (SI Text, Fig. S2, and Table S2).
Fig. 2.
Synaptic summation of EPSPs in L5 pyramidal neurons of the mPFC of Dgcr8+/− mice. (A) The traces are the averages of the 10 repetitions performed in WT (black; N = 10, n = 12) and Dgcr8+/− mice (red; N = 10, n = 15). Each vertical line is a stimulation artifact. (B) Across experiments, the mean amplitude of each individual EPSP measured from a baseline obtained just after each pulse during the 50-Hz train is unchanged by the mutation (two-way repeated-measures ANOVA; P > 0.05). (C) In contrast, the mean EPSP areas measured from the initial resting level and which includes the summating effect of the previous EPSPs in the train was significantly decreased by the mutation (WT mice; Dgcr8+/− mice; two-way repeated-measures ANOVA; P < 0.0001). The Insets in B and C show the procedures used for measuring the size of the EPSPs.
Initial Phase of Synaptic Potentiation Is Impaired in the mPFC of Dgcr8+/− Mice.
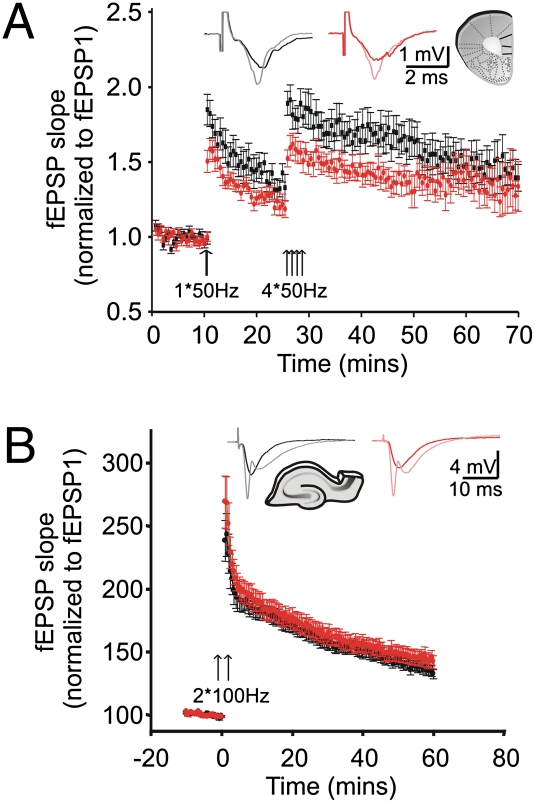
In L5 of the mPFC of rodents, trains of pulses at 50 Hz have previously been used to induce synaptic potentiation (19–21). Thus, we used field recordings to compare the level of potentiation induced by high-frequency stimulation in both mutant and WT mice (WT: N = 8, n = 12; Dgcr8+/−: N = 7, n = 14). A 50-Hz stimulation train applied after a stable 10-min baseline was sufficient to induce synaptic potentiation (Fig. 3A, single arrow; Inset, traces from before and immediately after the first 50-Hz train are shown). However, this potentiation, monitored every 30 s for 15 min, was significantly reduced in the mutant mice (WT = 1.84 ± 0.10; Dgcr8+/− = 1.50 ± 0.07; two-way repeated-measures ANOVA; P = 0.0094). After 15 min, the high-frequency train was applied four additional times, separated by 10 s (Fig. 3A, four arrows) and the fEPSP slopes were monitored for 40 min following the stimulation. This second round of stimulation revealed that potentiation was significantly less in mutant mice, but only during the initial 20-min period, a time approximately corresponding to the time course of short-term potentiation (reviewed in ref. 26). In contrast, the level of long-term potentiation when assayed 40 min after the repeated tetani was not different between genotypes (Fig. 3A) (WT = 1.39 ± 0.09; Dgcr8+/− = 1.33 ± 0.17; t test; P = 0.74).
Fig. 3.
Synaptic plasticity in L5 of the mPFC and in CA1 of Dgcr8+/− mice. (A) Synaptic potentiation in WT mice (N = 8, n = 12) and Dgcr8+/− mice (N = 7, n = 14). There is a significant difference in the degree of potentiation of fEPSPs over time (two-way repeated-measures ANOVA; P < 0.05). Immediately after the first 50-Hz train (first arrow), the level of potentiation is significantly lower in the Dgcr8+/− mice. Similarly, after the four consecutive 50-Hz trains (four arrows), post hoc testing revealed that the difference in potentiation lasts for about 20 min. In contrast, long-term potentiation assayed 40 min after the tetanization is unaffected by the mutation. (Inset) Sample fEPSPs traces before (WT and Dgcr8+/− mice are black and red traces, respectively) and immediately after the first tetanization (WT and Dgcr8+/− are gray and pink traces, respectively). (B) Synaptic potentiation induced in CA1 by two trains (arrows) of 100 Hz (100 pulses, 1 s) applied to Schaffer collaterals is normal in Dgcr8+/− mice (two-way repeated-measures ANOVA; P > 0.05). WT mice (N = 5; n = 20); Dgcr8+/− mice (N = 5; n = 20). (Inset) Sample fEPSPs traces before (WT and Dgcr8+/− mice are black and red traces, respectively) and immediately after the tetanization (WT and Dgcr8+/− are gray and pink traces, respectively).
Synaptic Transmission and Plasticity Are Normal at the CA1/CA3 Synapse of Dgcr8+/− Mice.
In contrast to impaired spatial working memory performance, Dgcr8+/− mice show normal hippocampal-dependent long-term associative memory (3). Using field recordings in acute hippocampus slices, we probed hippocampal synaptic transmission and plasticity by recording in the stratum radiatum of the CA1 region while stimulating Schaffer collaterals of Dgcr8+/− mice (Fig. S3A). At this CA3 to CA1 synapse, the strength of the baseline synaptic transmission was unaltered by Dgcr8 deficiency (Fig. S3B). PPF, reflecting the release probability, was also unchanged (Fig. S3C). Further analysis of synaptic plasticity showed that both STD (Fig. S3D) and the initial and later phases of long-term potentiation (Fig. 3B) were normal in this brain region.
Thus, unlike the robust deficits observed in the PFC and in agreement with the previously shown lack of deficits in hippocampus-dependent cognitive tasks, basic synaptic transmission and plasticity at the CA3/CA1 synapse of Dgcr8+/− mice appear to be normal. This discrepancy may be related to differences in the specific miRNAs affected in the two areas (3) or to some yet poorly understood resilience of CA3/CA1 synapse to miRNA dysregulation. Therefore, the effects of Dgcr8 deficiency on synaptic plasticity are not manifested ubiquitously in the brain.
Laminar Organization in the Cortex of Dgcr8+/− Mice.
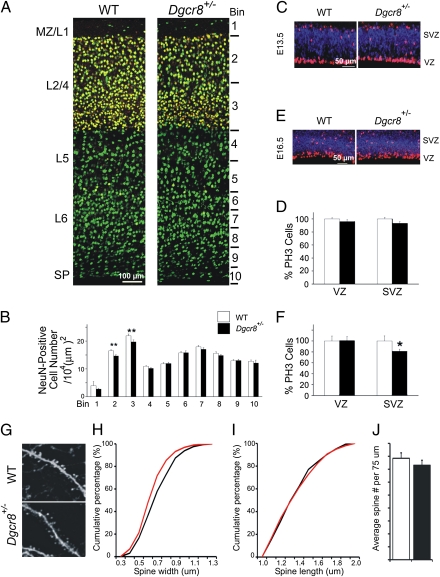
Alterations in the laminar organization of the neocortex may also impact upon the network dynamics underlying working memory and other cognitive processes that are impaired in SCZ patients. We therefore looked for changes in the positional arrangement and neuronal density of the cortical layers in the frontal cortex of 6-wk-old Dgcr8+/− mice, using the pan-neuronal marker, NeuN. There were no overt alterations in laminar organization (Fig. 4A), and with the exception of a modestly diminished NeuN cell frequency in medial, dorsal, and lateral L2/4 (Fig. 4B and Fig. S4A) [Bin 2 (superficial L2/4): 11.1% reduction, P < 0.001; Bin 3 (deeper L2/4): 12% reduction, P < 0.001; n = 18 per genotype], we found no overall significant change in the total frequency of neurons across the cortical layers. We further evaluated the change in neuronal density in L2/4 by staining for Cux1, a L2/4 selective marker (27). Consistent with the NeuN staining, there was a decrease in the frequency of neurons labeled for Cux1 in the medial, dorsal, and lateral frontal cortex (Fig. S4B).
Fig. 4.
Structural changes in the cortex of Dgcr8+/− mice. (A) Schematic representation of probe locations for quantifying frequency of neurons labeled with a pan-neuronal marker (NeuN, green) and L2/4 selective marker (Cux1, red) in the cortex of 6-wk-old WT and Dgcr8+/− mice. (B) NeuN-labeled cells in medial frontal cortex of WT and Dgcr8+/− mice. The frequency of NeuN-labeled cells across 10 equal bins from pia to white matter at the medial region of the frontal cortex is shown: frequency of NeuN-labeled cells is represented as number of cells per 104 mm2. (C–F) Progenitor cells were counted throughout the entire cortex at E13.5 (C and D, n = 17 per genotype) and E16.5 (E and F, n = 16 per genotype) and normalized to corresponding WT values. Basal and apical progenitors, both identified by phosphohistone 3 (PH3) labeling (red), are distinguished by their positions in the SVZ versus VZ via immunostaining for Tbr-2 (blue), a SVZ marker. (G) High-magnification representative images of basal dendrites of EGFP-expressing pyramidal neurons in the L5 region of the mPFC of WT Thy1-GFP/M+/− (n = 5) and Dgcr8+/−;Thy1-GFP/M+/− (n = 5) mice. (H and I) Analysis of the width and length of mushroom spines in Dgcr8+/−;Thy1-GFP/M+/− L5 neurons basal dendrites. Reduction in the width (H), but not length (I) was observed. (J) The density of all spines (estimated over 75 μm of dendritic length) was unaffected in Dgcr8+/−;Thy1-GFP/M+/− neurons. Data are shown as mean ± SEM. *P < 0.05, **P < 0.001.
Given the well-established role of miRNA in cellular proliferation (28), we asked whether this cortical abnormality could be a result of changes in cortical neurogenesis. Basal progenitors located at the cortical subventricular zone (SVZ), at E16.5 give rise primarily to L2/4 projection neurons (29). We found reduced frequency of mitotic basal progenitors, identified by phosphohistone 3 immunoreactivity (PH3; a G2/M-phase cell-cycle marker), as well as their SVZ location, at embryonic day E16.5 (n = 16, P < 0.02) (Fig. 4 C–F). In contrast, apical progenitors cells located in the cortical ventricular zone (VZ) appeared to be unchanged. We found no difference in the frequency of PH3-positive progenitors at E13.5, suggesting that Dgcr8 deficiency selectively influences late stages of corticogenesis. To further relate the observed change in L2/4 neuron frequencies to altered basal progenitor proliferation at E16.5, we analyzed neurons birth-dated by BrdU injection on E16.5 in the P5 cortex. As expected, E16.5 birth-dated–labeled neurons were primarily located in superficial L2/4 and their frequency was significantly decreased (n = 18, P < 0.003) (Fig. S5). Notably, the decrease in the density of L2/4 neurons due to Dgcr8 deficiency is consistent with recent reports of similarly altered frequency of L2/4 neurons in the cortex of a mouse model of the 22q11.2 microdeletion (30).
Dendritic Branching and Spine Development in the Cortex of Dgcr8+/− Mice.
We looked for deficits in dendritic branching and dendritic spine development in the basal dendrites of L5 neurons by intercrossing the Dgcr8+/− mice with a reporter strain (Thy1-GFP/M line), where L5 pyramidal neurons are intrinsically labeled in a relatively sparse mosaic manner with GFP throughout the cell body and dendritic tree. Cortical pyramidal neurons in L5 receive inputs from all cortical layers but numerous excitatory cell types target almost exclusively the basal dendritic arborization (18). Morphotypic analysis of mushroom spines showed a slight decrease in the width of spines in the basal dendrites of PFC pyramidal neurons from Dgcr8+/− mice (~8%, Kolmogorov-Smirnov test, P < 0.007), whereas their length was unaffected (Fig. 4 G, H, and I). In addition, the mushroom spine density was normal (t test, P = 0.21) (Fig. 4J). Analysis of gross dendritic development in the PFC of Dgcr8+/−;Thy1-GFP/M+/− mice did not reveal any significant genotypic effect on dendritic complexity at varying distances from the soma (n ≥ 25, P > 0.05) (Fig. S6A). In addition, no significant differences were observed when comparing the number of primary dendrites or branches and total dendritic length (Fig. S6 B–D).
Discussion
Emerging evidence suggests a strong association between miRNA-dependent dysregulation of gene expression and susceptibility to psychiatric disorders, such as SCZ (3, 31, 32). In particular, previous work has shown that impaired miRNA formation (caused by Dgcr8 deficiency) is one of the genetic factors contributing to the behavioral and neuronal phenotypes associated with 22q11.2 deletions, a well-established SCZ predisposing mutation. In that respect, the analysis presented here sheds light on how impaired miRNA formation alters the structure and function of neural circuits thought to underlie susceptibility to SCZ and other psychiatric disorders.
We show that L5 neurons of Dgcr8+/− mice display robust alteration in STD upon stimulation, at firing rates within the physiological firing range (20–60 Hz) observed in PFC neurons during the delay period of working memory tasks (33–35). It is widely hypothesized that sustained activity of PFC neurons, during a delay period between the presentation of a brief, informative cue and the generation of a behavioral response, underlies the transient retention of information, in the absence of continued sensory stimulation, in working memory tasks. In this respect, our finding that Dgcr8 mutants display increased synaptic depression upon sustained activation of afferent fibers in the PFC predicts that similar changes would occur during persistent bursts of synaptic inputs in vivo during working memory tasks. Such changes may interfere with the induction and maintenance of sustained activity and ultimately alter circuit dynamics and working memory performance. In addition to the greater level of STD, we show that the activation of superficial layers’ afferent fibers projecting onto L5 neurons leads to a robust decrease in the initial but not late phase of synaptic potentiation in the Dgcr8+/− mutants. This observation provides further support for the notion that Dgcr8 deficiency specifically affects the expression of short-term forms of synaptic plasticity in response to repetitive stimulation. Such aberrant short-term synaptic dynamics within PFC networks may represent a pathological neural substrate underlying dysconnectivity, cognitive dysfunction, and inability to accurately interpret and integrate incoming sensory information present in SCZ and other psychiatric disorders (36, 37). Therefore, our study identifies a candidate synaptic mechanism that can be tested in vivo for its role in behavioral and cognitive performance and can be targeted for pharmacotherapy.
Although the physiological changes highlighted here are clear, the mechanisms underlying short-term plasticity are complex and diverse, and the precise role of Dgcr8 in this process remains to be determined. The early phases of the time courses of facilitation, depression, and potentiation overlap (26, 38), making their mechanisms difficult to dissect in time. However, because STD and short-term potentiation are thought to strongly depend on presynaptic neurotransmitter release, and because, as shown here, both are impaired in the mutant mice, our results are consistent with a deficit at the presynaptic level. In particular, the observed reduction in the size of the EPSCs during the 50-Hz train in both genotypes likely reflects a decrease in probability of presynaptic neurotransmitter release (26). Moreover, because the amplitude of mEPSCs was unaltered, it seems unlikely that the mutation affects postsynaptic sensitivity. Synaptic depression could be caused by a depletion of a readily releasable pool of synaptic vesicles, and that this pool could be smaller in size or show delayed recycling in mutant animals, possibly because of miRNA-dependent aberrant regulation of intraterminal calcium level or its downstream effects. The relevant miRNA targets remain to be identified. Similarly, the mechanisms mediating changes in the initial phase of synaptic potentiation remain equally unclear. However, such deficits may simply emerge as a result of greater synaptic depression and lower neurotransmitter release, with subsequently diminished postsynaptic depolarization at the end of the tetanization.
In contrast to the robust deficits observed at the synaptic level, at the structural level we observed relatively modest and circumscribed changes. Nevertheless, the observed modest and layer-specific structural changes in the Dgcr8-deficient cortex may result in local and long-distance disruptions in cortical circuitry and partly contribute to the cognitive deficits observed in the Dgcr8+/− and Df(16)A+/− mice.
Communication between neurons at synapses must be involved in neuropsychatric disorders (39–41). However, more specific hypotheses are needed to discriminate among key synaptic mechanisms that may contribute to impaired transformation and transmission of information at existing synapses in the brains of individuals afflicted with various psychiatric and neurodevelopmental disorders. Results presented here, from our analysis of a gene and a pathway disrupted by the 22q11.2 microdeletion, suggest that altered STD within prefrontal cortical microcircuits may be a key trigger of altered working memory in SCZ and emphasize a more general role of disturbances of short-term plasticity as a key feature of mutations predisposing to SCZ, and more broadly to mental illness.
Materials and Methods
Animals.
Experiments were performed on 4- to 6-wk-old Dgcr8+/− mutant mice and their WT littermates (3). Generation of Dgcr8+/− mice has been described previously (3). The Dgcr8 mutation was backcrossed into the C57BL/6J background for more than six generations. The animal procedures were carried out in accordance with and approved by the Columbia University Institutional Animal Care and Use Committee.
Analysis of Spine and Dendritic Morphology.
This analysis was performed as described previously (3). See SI Materials and Methods.
Electrophysiological Recordings.
Electrophysiological experiments were performed on coronal PFC slices (300 μm) containing the prelimbic and infralimbic PFC areas (42) (Fig. 1A). The fEPSP responses were evoked using a concentric bipolar stimulating electrode (FHC, Bowdinham, ME) placed at the border of L1 and L2/3 (300 μm from midline). The stimulation site was always aligned ~ 200-μm away from the recording site along the axis perpendicular to the pial surface. Basic synaptic transmission was characterized at 0.033 Hz, with stimulation intensities of 3 to 15, 18, 20, and 25 V (pulse duration = 0.1 ms). The following experiments were performed at a stimulus intensity generating a fEPSP one-third of the maximum obtained at 25 V. Short- and long-term synaptic plasticity were induced using paired-pulses (interstimulus intervals of 20, 50, 100, 200, 400, and 800 ms) and an 800-ms 50-Hz train stimulation (pulse duration = 0.1 ms) protocols, respectively. The frequency, size and kinetics of sEPSCs were obtained from sEPSC recorded for a period of 5 min at −70 mV. Miniature EPSCs were recorded with 0.5 μM TTX present in the bathing solution. To assess STD, EPSCs and EPSPs were evoked by stimulating at the border of layers 1 to 2/3 using an 800-ms 50-Hz train (pulse duration = 0.1 ms). Intrinsic membrane properties were measured by intracellularly injected square current pulses of 500 ms, from −0.150 nA to +0.210 nA (0.015-nA increments). For additional details on electrophysiological recordings, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Megan Sribour and Dr. Kimberly L. Stark for support with the generation and maintenance of the mouse colony; Dr. Papiya Choudhury, Dr. Chi-Kun Tong, Merilee Teylan, and Naoko Haremaki for technical assistance; and Dr.Tomonori Takazawa for insightful discussion. This work was supported by US National Institute of Mental Health Grants MH67068 (to M.K. and J.A.G.) and MH077235 (to J.A.G.), and by a grant from the March of Dimes Foundation and the McKnight Endowment Fund for Neuroscience (to M.K.); J.M., B.X., and L.J.D. have been supported in part by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101219108/-/DCSupplemental.
References
- 1.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: Linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karayiorgou M, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark KL, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 4.Tomari Y, Zamore PD. MicroRNA biogenesis: Drosha can't cut it without a partner. Curr Biol. 2005;15(2):R61–R64. doi: 10.1016/j.cub.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 5.Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- 6.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 7.Sobin C, et al. Networks of attention in children with the 22q11 deletion syndrome. Dev Neuropsychol. 2004;26:611–626. doi: 10.1207/s15326942dn2602_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kates WR, et al. The neural correlates of non-spatial working memory in velocardiofacial syndrome (22q11.2 deletion syndrome) Neuropsychologia. 2007;45:2863–2873. doi: 10.1016/j.neuropsychologia.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantinidis C, Wang XJ. A neural circuit basis for spatial working memory. Neuroscientist. 2004;10:553–565. doi: 10.1177/1073858404268742. [DOI] [PubMed] [Google Scholar]
- 12.Curtis CE, Lee D. Beyond working memory: The role of persistent activity in decision making. Trends Cogn Sci. 2010;14:216–222. doi: 10.1016/j.tics.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szentágothai J. The neuron network of the cerebral cortex: A functional interpretation. Proc R Soc Lond B Biol Sci. 1978;201:219–248. doi: 10.1098/rspb.1978.0043. [DOI] [PubMed] [Google Scholar]
- 14.Szentágothai J. Local neuron circuits of the neocortex. In: Schmitt FO, Worden FG, editors. The Neurosciences: Fourth Study Program. Cambridge, MA: MIT Press; 1979. pp. 399–415. [Google Scholar]
- 15.Diamond ME. Cerebral Cortex: The Barrel Cortex of Rodents. New York: Plenum Press; 1995. Somatosensory thalamus of the rat; pp. 189–219. [Google Scholar]
- 16.Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb Cortex. 2010;20:393–403. doi: 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 18.Bannister AP. Inter- and intra-laminar connections of pyramidal cells in the neocortex. Neurosci Res. 2005;53(2):95–103. doi: 10.1016/j.neures.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch JC, Crepel F. Use-dependent changes in synaptic efficacy in rat prefrontal neurons in vitro. J Physiol. 1990;427:31–49. doi: 10.1113/jphysiol.1990.sp018159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hempel CM, Hartman KH, Wang XJ, Turrigiano GG, Nelson SB. Multiple forms of short-term plasticity at excitatory synapses in rat medial prefrontal cortex. J Neurophysiol. 2000;83:3031–3041. doi: 10.1152/jn.2000.83.5.3031. [DOI] [PubMed] [Google Scholar]
- 21.Gemperle AY, Enz A, Pozza MF, Lüthi A, Olpe HR. Effects of clozapine, haloperidol and iloperidone on neurotransmission and synaptic plasticity in prefrontal cortex and their accumulation in brain tissue: an in vitro study. Neuroscience. 2003;117:681–695. doi: 10.1016/s0306-4522(02)00769-8. [DOI] [PubMed] [Google Scholar]
- 22.Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci USA. 2004;101:3236–3241. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: Roles in psychiatric disorders. Biol Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Wessel R, Kristan WB, Jr, Kleinfeld D. Supralinear summation of synaptic inputs by an invertebrate neuron: Dendritic gain is mediated by an “inward rectifier” K+ current. J Neurosci. 1999;19:5875–5888. doi: 10.1523/JNEUROSCI.19-14-05875.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, et al. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 27.Nieto M, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J Comp Neurol. 2004;479(2):168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, et al. Embryonic stem cell specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai T, et al. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci USA. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry. 2011;69(2):188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu B, Karayiorgou M, Gogos JA. MicroRNAs in psychiatric and neurodevelopmental disorders. Brain Res. 2010;1338:78–88. doi: 10.1016/j.brainres.2010.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 34.Sawaguchi T, Matsumura M, Kubota K. Catecholaminergic effects on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63:1385–1400. doi: 10.1152/jn.1990.63.6.1385. [DOI] [PubMed] [Google Scholar]
- 35.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fletcher PC, Frith CD. Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10(1):48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 38.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Zoghbi HY. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 40.Owen MJ, O'Donovan MC, Harrison PJ. Schizophrenia: A genetic disorder of the synapse? BMJ. 2005;330(7484):158–159. doi: 10.1136/bmj.330.7484.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.