Abstract
Perceptual learning not only improves sensitivity, but it also changes our subjective experience. However, the question of how these two learning effects relate is largely unexplored. Here we investigate how subjects learn to see initially indiscriminable metacontrast-masked shapes. We find that sensitivity and subjective awareness increase with training. However, sensitivity and subjective awareness dissociate in space: Learning effects on performance are lost when the task is performed at an untrained location in another quadrant, whereas learning effects on subjective awareness are maintained. This finding indicates that improvements in shape sensitivity involve visual areas up to V4, whereas changes in subjective awareness involve other brain regions. Furthermore, subjective awareness dissociates from sensitivity in time: In an early phase of perceptual learning, subjects perform above chance on trials that they rate as subjectively invisible. Later, this phenomenon disappears. Subjective awareness is thus neither necessary nor sufficient for achieving above-chance objective performance.
Keywords: consciousness, psychophysics, Signal Detection Theory
Our perceptual apparatus is constantly shaped by experience. This fact has been shown, for example, in experiments investigating perceptual learning, where practice on a sensory task leads to increases in perceptual sensitivity (1). Although perceptual learning is a well-studied phenomenon, the question of how it changes subjective awareness has rarely been addressed. Do we actually “see more” after training? Apart from anecdotal reports pointing in this direction, a quantitative analysis of the effects of learning on subjective awareness is largely missing (2) because studies in perceptual learning have almost exclusively focused on objective task performance [i.e., sensitivity in terms of signal detection theory (3)]. However, studies from a different line of research, namely those investigating conscious perception, have found that objective performance in a task and awareness of the stimuli on which the task is performed can dissociate. Such is the case in blindsight (4) but also is found in normal observers (refs. 5–7 but see ref. 8). These data demonstrate that these two aspects of perception cannot be treated as being equivalent. We have recently shown that subjects can be trained to perform on and to see stimuli that are initially invisible to them (9). We thus hypothesize that awareness is trainable, a conclusion that is in accordance with recent findings in blindsight patients (10). However, the time course of those learning effects has not been explored, i.e., whether the improvements in sensitivity and subjective awareness depend on each other. In particular, changes in sensitivity could be a prerequisite for changes in subjective awareness. Alternatively, it could be necessary to subjectively see a stimulus in order for changes in sensitivity to occur. Last but not least, training could affect sensitivity and subjective awareness in parallel without any mutual dependence between these two aspects of perception. The latter question is related to another issue that is still a matter of debate, namely whether objective performance and subjective awareness depend on the same circuits in the normal brain.
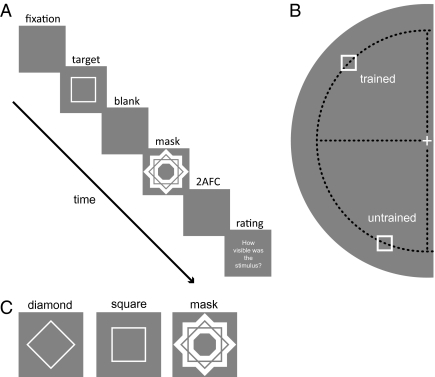
Here, we address both questions by training subjects on a shape-discrimination task under metacontrast-masking conditions (Fig. 1). Stimulus parameters are such that the shapes are objectively indiscriminable before training. Through continuous practice, subjects learn to discriminate a square from a diamond, thus crossing the objective threshold (d′ = 0). Additionally, subjects rate their subjective awareness of the stimuli on a trial-by-trial basis, which allows us to measure the time courses of learning effects on sensitivity and subjective awareness concurrently. To localize the learning effects in the brain by psychophysical techniques, we change the stimulus position after the final training session to a new stimulus location. Based on an estimate of receptive field (RF) size, this manipulation allows us to test the hypothesis that early visual areas up to V4 are the sites of objective learning effects.
Fig. 1.
Stimuli and experimental procedures. (A) Each trial started with a fixation period (1,000–1,500 ms). The target was presented for 10 ms. The mask was presented for 50 ms at SOAs between 20 ms and 150 ms. Subjects then decided between square and diamond in a two-alternative forced-choice (2AFC) task, followed by a rating of subjective visibility of the target stimulus. (B) The training location was in the upper left quadrant at 4° eccentricity. The isoeccentric transfer location was in the lower left quadrant, 6.6° from the trained location. (C) A diamond and a square served as target stimuli, and the mask was star-shaped and did not overlap with the target contours.
We find that changes in sensitivity and changes in subjective awareness indeed dissociate: Improved sensitivity does not transfer into another quadrant, whereas subjective awareness does, indicating that subjective awareness and objective performance depend on different brain regions. Furthermore, in an early phase of perceptual learning, subjects perform above chance on trials that they rate as subjectively invisible. This effect disappears with practice. Together, these results support the notion that thresholds of awareness are not fixed, that subjective awareness is neither necessary nor sufficient for changes in sensitivity, and that the cortical loci of learning effects in subjective awareness and in sensitivity are not identical.
Results
Subjective and Objective Learning Effects Dissociate in Space: Thresholds and Transfer.
Thresholds as a function of stimulus onset asynchrony (SOA) were first assessed before training. For each subject, the SOA yielding zero sensitivity (d′ = 0) in this first threshold measurement was subsequently used for training (see below). To evaluate whether improvements in performance were confined to the trained SOA or generalized to untrained SOAs, we also evaluated the full threshold function after training. In addition, we changed the location of the stimulus to a position 6.6° away from the trained location to another quadrant at isoeccentricity after the training and the final threshold assessment to probe which brain regions are involved in the learning effects. In particular, we did this to test whether area V4, an important intermediate stage in the analysis of shape (11), or any higher area in the ventral stream was the locus of perceptual learning in our study. Finally, to address the potential role of feedback in perceptual learning, half of the subjects received blockwise percentage correct feedback during training.
To evaluate whether subjects’ sensitivity, response bias, and subjective awareness changed as a function of training, we first entered their d′, c, and mean Perceptual Awareness Scale (PAS; ref. 12) rating from the threshold assessments separately into repeated-measures ANOVA (rmANOVA) with the factors session (before training, after training, transfer) and SOA (for d′ and c: 20, 30, 40, 50, 70, 90, 110, 130, and 150 ms; for PAS: 20–50 ms see below). For the PAS data, we included the factor accuracy (correct, incorrect). We also tested whether the presence of blockwise feedback affected any of the measures (between-subjects factor feedback). On the PAS, 1 refers to “No experience,” 2 to “Brief glimpse (a feeling that something has been shown),” 3 to “Almost clear experience (ambiguous experience of the stimulus),” and 4 to “Clear experience of the stimulus,” whereby PAS ≥ 2 indexes “subjective detection” and PAS = 4 indexes “subjective discrimination” (see below and SI Materials and Methods).
Objective learning effects.
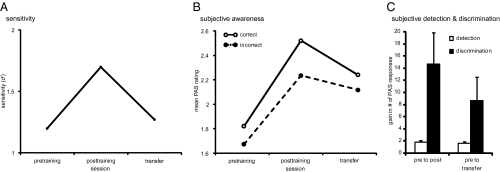
Sensitivity (d′) increased linearly with SOA in all sessions [effect of SOA: F(1.381, 27.618) = 44.794, P < 0.01, η2 = 0.691], a signature of type A metacontrast masking (Fig. S1). Learning clearly affected sensitivity (effect of session: F(1.603, 32.065) = 9.744, P < 0.01, η2 = 0.328]: Mean d′ was significantly higher after the training than before (mean difference = 0.498, SE = 0.141, P < 0.01) and compared with the transfer position (Fig. 2A; mean difference = 0.425, SE = 0.087, P < 0.01). Thus, training on a discrimination task resulted in above-chance performance even if subjects initially showed zero sensitivity. However, there was no significant difference between sensitivity before training and after training at the transfer position (mean difference = −0.073, SE = 0.131, P > 0.58, uncorrected). Thus, subjects’ sensitivity increased at the trained position, but this improvement did not transfer to another quadrant at isoeccentricity, which indicates that changes in sensitivity involve visual areas up to area V4 (Discussion). Separate analyses restricted to the trained SOA and on the subsample of SOAs that we used for the analysis of the PAS data confirmed these results. Blockwise feedback did not affect performance on this task (no significant main effect of feedback or interactions with feedback, all P > 0.41).
Fig. 2.
Threshold sessions. (A and B) Average sensitivity (d′) (A) and subjective awareness (mean PAS rating) (B) for correct and incorrect trials on the four short SOAs in the threshold sessions before and after training and at the untrained location (transfer). (C) Average learning-induced gain in subjective detection [(no. of trials with PAS ≥ 2 posttraining)/(no. of trials with PAS ≥ 2 pretraining)] and subjective discrimination [(no. of trials with PAS = 4 posttraining)/(no. of trials with PAS = 4 pretraining)] on the four short SOAs at the trained and transfer location. Error bars represent the SEM.
As for response bias (c), we found that different SOAs were not associated with different response biases, and, importantly, response bias did not change as a result of training or with the transfer (no significant main effects or interactions, all P > 0.16).
Subjective learning effects.
For the PAS data, only the first four SOAs (20–50 ms) were entered into the analyses because 17 of 22 subjects had fewer than 10 wrong answers in at least one of the longer SOAs (70–150 ms), which would have rendered the factor accuracy unreliable (see Fig. S2 for all SOAs). Ratings of subjective awareness increased as a function of SOA [F(1.412, 28.240) = 10.150, P < 0.01, η2 = 0.337] and session [F(1.410, 28.203) = 29.917, P < 0.01, η2 = 0.599], and this increase was especially pronounced on correct trials [Fig. 2B; session × accuracy interaction, F(1.764, 35.275) = 6.654, P < 0.01, η2 = 0.250]. Importantly, the mean PAS rating for correct responses was higher than for incorrect responses in each session (all P < 0.01). Although ratings for both correct and incorrect responses increased after training, this increase was substantially bigger for correct responses (mean difference between PAS rating for correct and incorrect responses: pretraining = 0.37, SE = 0.043, P < 0.01; posttraining = 0.459, SE = 0.082, P < 0.01). Comparable results were obtained when we plotted the rate of incorrect trials with high PAS ratings against the rate of correct trials with high PAS ratings for three levels of visibility to obtain receiver operating characteristics (ROC) and calculated the area under the curve (AUC), an index of how well PAS ratings predict accuracy (SI Materials and Methods). The AUC was above chance (0.5) before and after the training both for the trained SOA [Fig. S3; pretraining: mean difference = 0.0656, T(21) = 5.896, P < 0.01; posttraining: mean difference = 0.1260, T(21) = 6.907, P < 0.01, all one-sided] as well as for the average over the four short SOAs [pretraining: mean difference = 0.0866, T(21) = 7.579, P < 0.01; posttraining: mean difference = 0.1329, T(21) = 7.441, P < 0.01, as above]. This pattern of results rules out that increases in PAS ratings were caused by a bias to indiscriminately respond with higher ratings as learning progresses because such behavior would erase differences between correct and incorrect trials. We found no effect of or interaction with feedback (all P > 0.41).
A dissociation between sensitivity and subjective awareness was observed for the transfer task. Although moving the stimulus to another quadrant resulted in a significant drop of sensitivity, PAS ratings for correct responses remained above pretraining levels (Fig. 2B). Crucially, the difference between correct and incorrect responses remained significant (mean difference transfer = 0.34, SE = 0.059, P < 0.01), and PAS ratings still predicted accuracy when tested against chance [Fig. S3; AUC trained SOA: mean difference = 0.1031, T(21) = 4.976, P < 0.01; AUC four short SOAs: mean difference = 0.0911, T(21) = 6.723, P < 0.01, all one-sided], confirming that subjects continued using the PAS ratings in a meaningful way. Thus, the RFs underlying objective task performance appear to be smaller than the RFs supporting subjective awareness, indicating that training affects different brain regions.
It is unlikely that the high PAS ratings at the untrained location are explainable by a carry-over effect from the posttraining threshold measurement to the transfer location. If a carry-over effect were in place, then the results at the untrained location should resemble in some way the pattern of results obtained at the source of the carry-over effect, i.e., the posttraining session, which was not the case. Neither the absolute PAS ratings for correct and incorrect trials nor the difference between them [trained SOA: mean difference = 0.11, SE = 0.04, T(21) = 2.2924, P < 0.05; average of the four short SOAs: mean difference = 0.16, SE = 0.04, T(21) = 3.4907, P < 0.01] were identical in the posttraining and transfer session (Fig. 2B).
The previous analysis revealed a gradual increase in subjective awareness with practice. However, it is unclear whether the observed increments reflect heightened subjective awareness of discriminant features (i.e., a clearer impression of shape) or heightened subjective detection ability (i.e., seeing something as opposed to seeing nothing) (13) because, by design, the PAS encompasses both. A dissociation between subjective awareness and objective performance could then correspond to differences in task, namely detection versus discrimination. To compare the subjective and objective task when both rely on shape information, we divided the PAS ratings into two categories: (i) trials where subjects reported a clear impression of the shape of the stimulus (PAS = 4) and (ii) trials where subjects at least reported seeing something (PAS ≥ 2). For brevity, we refer to these trials as “subjectively discriminated” and “subjectively detected,” respectively. The proportion of subjectively detected stimuli increased from pretraining to posttraining (trained SOA mean difference = −0.284, SE = 0.052, P < 0.01; four short SOAs: mean difference = −0.252, SE = 0.047, P < 0.01) and was above pretraining levels at the untrained location (trained SOA mean difference = −0.196, SE = 0.057, P < 0.01; four short SOAs: mean difference = −0.185, SE = 0.054, P < 0.01). The proportion of subjectively discriminated trials also increased from pretraining to posttraining (trained SOA mean difference = −0.152, SE = 0.048, P < 0.01; four short SOAs: mean difference = −0.159, SE = 0.047, P < 0.01, all one-sided, Bonferroni corrected for the number of sessions). Most importantly, it remained above pretraining levels at the untrained transfer location (trained SOA: mean difference = −0.072, SE = 0.030, P < 0.05; four short SOAs: mean difference = −0.083, SE = 0.031, P < 0.05, as above), although not fully reaching posttraining levels (trained SOA: mean difference = 0.080, SE = 0.022, P < 0.01; four short SOAs: mean difference = 0.076, SE = 0.021, P < 0.01, as above). This result shows that the dissociation between sensitivity and subjective awareness holds even when objective discrimination and subjective discrimination are directly compared. To assess whether learning effects in subjective discrimination exceeded learning effects in subjective detection, we calculated the respective gain for the trained and transfer locations. Indeed, the gain for discrimination was substantially larger than for detection at both locations [Fig. 2C; measure × session interaction, trained SOA: F(1, 21) = 5.117, P < 0.05, η2 = 0.196; four short SOAs: F(1, 21) = 8.376, P < 0.01, η2 = 0.285]. Thus, subjective discrimination increased with training and transferred to the untrained location, and this learning effect exceeded the learning effect in subjective detection.
Subjective and Objective Learning Effects Dissociate in Time: Training Sessions.
Objective learning effects.
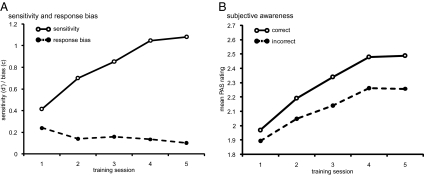
Subjects were trained for five consecutive sessions at an SOA that was initially at the objective threshold, as confirmed by a d′ not significantly different from 0 before training either in the feedback group [mean difference = 0.15, T(10) = 2.129, P > 0.05, two-sided, uncorrected] or in the no-feedback group [mean difference = 0.12, T(10) = 1.873, P > 0.05, two-sided, uncorrected]. We have previously shown that practice on this task leads to increases in sensitivity without changes in response bias (9). Here, we confirmed these results in an rmANOVA with the within-subjects factor training sessions (sessions 1–5) and the between-subjects factor feedback. We found that sensitivity increased with session [F(2.573, 51.454) = 15.830, P < 0.01, η2 = 0.442], whereas response bias did not change significantly [F(2.179, 43.576) = 0.737, P > 0.49, η2 = 0.036]. Sensitivity increased linearly [F(1, 20) = 29.752, P < 0.01, η2 = 0.598], on average by a d′ of 0.66 (SD = 0.56) from the first to the fifth session (Fig. 3A). Blockwise feedback did not affect sensitivity or response bias in this task (all P > 0.11).
Fig. 3.
Training sessions. (A) Sensitivity (d′) and response bias (c) at the trained SOA during the training sessions. (B) Subjective awareness (mean PAS rating) for correct and incorrect trials during the training sessions.
Subjective learning effects.
To examine the time course of subjective awareness as a function of practice, we asked subjects to rate the stimulus visibility on a trial-by-trial basis. Subjective awareness ratings increased over sessions [F(1.437, 28.742) = 12.017, P < 0.01, η2 = 0.375], with a more pronounced increase for correct responses [accuracy × session, F(1.941, 38.828) = 7.672, P < 0.01, η2 = 0.276] but no effect of feedback (all P > 0.26). When considering subjectively detected (PAS ≥ 2) and subjectively discriminated (PAS = 4) trials separately, we found that the proportions of both increased with session [detection: F(1.337, 28.085) = 8.218, P < 0.01, η2 = 0.281; discrimination: F(1.467, 30.816) = 7.384, P < 0.01, η2 = 0.260]. Thus, practice on initially indiscriminable stimuli leads to improvements in objective performance and subjective awareness (Fig. 3). The AUC per session was always above chance (Fig. S4; all P < 0.01, one-sided) and even increased linearly with session [F(1, 20) = 15.680, P < 0.01, η2 = 0.439]. Thus, the improvements in subjective awareness do not simply reflect a change in criterion because a bias to indiscriminately use higher ratings as learning progresses would not lead to a differential increase in the ratings for correct and incorrect responses or to an increase in the AUC.
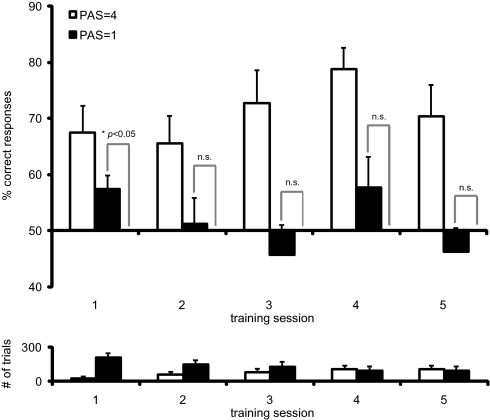
We then turned to the question of whether objective performance depends on subjective awareness, i.e., whether subjects had to consciously perceive the stimuli to respond correctly, and whether this changed over the course of learning. We separately analyzed the percentage of correct responses for trials rated as invisible (PAS = 1) and for trials rated as clearly visible (PAS = 4) per session. We found that subjects performed significantly better than chance for trials that they rated invisible [mean percentage correct = 0.57, T(21) = 2.865, P = 0.0465, two-sided, Bonferroni corrected for the number of sessions] but only during the first session (Fig. 4; all other P > 0.88, as above). [Missing values were replaced by the mean of the respective group (feedback/no feedback), which did not change the overall pattern of results. There were no missing values for PAS = 1 trials in session 1.] This finding suggests that subjective awareness is not necessary for above-chance objective performance at the earliest stage of learning. For trials that were rated as clearly visible, the percentage of correct responses was always higher than chance (all P < 0.02, as above). Because the number of PAS = 1 and PAS = 4 trials develops in opposite directions over sessions (Fig. 4), the above-chance performance for PAS = 4 trials in session 1 rules out the possibility that the lack of above-chance performance for PAS = 1 trials in sessions 2–5 is because of the progressively smaller number of trials. Together, subjects were aware of some trials (correct trials | PAS = 4) even in session 1; concomitantly, they were apparently able to use information that fell below their subjective threshold for the discrimination task (correct trials | PAS = 1). However, although subjects continued to report that some stimuli were invisible in sessions 2–5, they did not continue to perform above chance in these trials. Thus, it appears that, with increasing awareness, information below the subjective threshold is either not available or not used anymore.
Fig. 4.
Objective performance on invisible and clearly visible trials during training. (Upper) Percentage of correct responses on subjectively invisible (PAS = 1) and subjectively clearly visible (PAS = 4) trials per training session. (Lower) Average number of invisible (PAS = 1) and clearly visible (PAS = 4) trials per session. Error bars represent the SEM. n.s., not significant at an α level of 0.05, Bonferroni corrected.
Discussion
We found that both sensitivity and subjective awareness change in perceptual learning. However, learning does not affect these two aspects of perception in the same way, evidencing that they should not be treated as being equivalent. Subjective awareness is not sufficient for achieving or maintaining objective performance: At an untrained location in another quadrant, sensitivity drops back to pretraining levels, whereas learning effects on subjective awareness are preserved. This differential generalization of objective performance and subjective awareness across the retinotopic map indicates that the cortical loci of learning effects in subjective awareness and sensitivity are not identical. Furthermore, subjective awareness is also not necessary for above-chance objective performance because performance on trials that were rated as subjectively invisible was above chance for the discrimination task. However, this is only the case in the first training session. Thus, the immediate use of subjectively unavailable information vanishes as perceptual learning progresses. Still, changes in sensitivity can occur even when the stimuli used for training do not cross the threshold of subjective awareness. Together, these findings support the notion that perceptual thresholds are not fixed and that, to fully characterize perceptual learning, both objective and subjective measures need to be considered.
Progression of Sensitivity and Subjective Awareness.
Both sensitivity and subjective awareness increased with training. Comparing the pretraining with the posttraining thresholds, we found that the learning effects are not limited to the trained SOA but are spread in time to all other tested SOAs. The training-related improvements can have diverse reasons. Given that subjects could not infer the precise moment of appearance of the target (randomized fixation period, randomized SOAs), learning effects cannot be attributed to an improved focusing of attention in time. Another possibility is that subjects learned to suppress the mask, as has been found for pattern masking (14, 15). This hypothesis cannot be directly tested in metacontrast masking because the mask cannot be changed for a given target. However, mask suppression by a second mask has been shown to lead to target recovery in metacontrast masking (16). Alternatively or additionally, the representation of the target stimuli might have been strengthened through learning (17).
The changes in objective performance were accompanied by changes in subjective awareness. The more pronounced increase in subjective awareness on correct than on incorrect trials and the increase of the AUC with sessions show that the higher PAS ratings were not attributable to an indiscriminate response bias toward higher scores as learning progresses. Notably, the learning curves for sensitivity and subjective awareness dissociate. Only in the first session does information remaining below the subjective threshold lead to above-chance performance. This effect vanishes in the remaining sessions. Previous studies in blindsight patients as well as in normal observers have found similar dissociations within sessions (4–7). Thus, under certain conditions, visual stimuli allow for correct behavior without concomitant subjective experience. Learning studies have also reported dissociations between sensitivity and subjective awareness: For example, normal observers can learn to discriminate motion directions (18), orientations (19), and emotional expressions in masked faces (20) without awareness. However, contrary to our results, practice did not improve subjective awareness in these studies.
The above-chance performance on trials that subjects rated as invisible suggests that information that has entered the visual system can be used even if this information is not accessible to subjective awareness. A change in subjective awareness requires that the initial representation of the stimulus is transformed to make it accessible for subjective report. It has been proposed that this conversion requires the formation of a higher-order representation of the stimulus (21). In this framework, higher-order representations are learned from the underlying signal and noise distributions on which the discrimination task is performed. Learning-induced changes in the underlying distributions (as captured by d′) and/or changes in the signal and noise distributions of the higher-order representations can then lead to increased subjective awareness. It has also been hypothesized that conscious representations are stronger, more stable, and more distinct than unconscious representations (22). A recent functional MRI study lends support to this proposal by showing that activity patterns in the temporal lobe elicited by consciously perceived stimuli are less variable than those evoked by unperceived stimuli (ref. 23 but see ref. 24). Accordingly, once a representation has reached sufficient quality and stability through learning, it becomes accessible to report. Our study expands previous findings by showing that the immediate use of subjectively unavailable information vanishes once a consciously accessible representation has been established.
Interestingly, objective performance on clearly seen trials was not at ceiling throughout the training sessions, which is consistent with previous masking studies (25–27). This finding can be accounted for by a model in which subjective awareness and objective performance do not depend on each other. However, other factors, e.g., motor errors when reporting the target stimulus in the two-alternative forced-choice task and/or subjective awareness or illusory percepts, could also account for part of the variance. In masking, it is believed that illusory percepts can arise when top-down processes incorrectly complete the highly degraded target information (26). Such illusory percepts should prevail with short SOAs when bottom-up information is scarce and the influence of top-down mechanisms is strong (Fig. S5).
Different Effects of a Change of Stimulus Location.
A further dissociation between sensitivity and subjective awareness was observed for the transfer task. After the training and the final threshold assessment, we changed the location of the stimulus to a position 6.6° away from the trained location to another quadrant at isoeccentricity. At 4° eccentricity, RFs in human area V4 are smaller than 6° (28). Furthermore, RFs in area V4 usually do not cross the horizontal meridian (29, 30), whereas the RFs at later stages in the ventral stream, such as those of the anterior inferotemporal cortex, are on average much larger and not constrained to an individual quadrant (31). The fact that all learning effects in sensitivity were lost when the stimulus location was changed indicates that learning most likely involved area V4 and/or preceding areas. In support of this notion, electrophysiological experiments in monkeys have found suppressive effects of metacontrast masks on neuronal activity of V4 neurons (32).
The change in stimulus location did not have the same effect on subjective awareness. In fact, subjective awareness remained close to posttraining levels at the new location, which was even the case when only considering trials on which subjects could subjectively discriminate the stimuli, showing that the dissociation between objective performance and subjective awareness was not solely because of a transfer of subjective detection ability or a difference in task. Crucially, an unspecific carry-over effect from the posttraining threshold measurement cannot easily explain the results because such an effect should either preserve the absolute PAS ratings and/or the relative difference between PAS ratings for correct and incorrect responses, which was not the case. Thus, sensitivity and subjective awareness generalized differently across the visual field, implying that the respective learning effects involve different brain regions. Similar results have been obtained for visual priming: Subliminal visual primes (affecting sensitivity) are only effective when displayed in the same quadrant as the supraliminal targets, whereas supraliminal primes (affecting sensitivity and subjective awareness) are translation invariant (33). Furthermore, our results indicate that subjective awareness is not only not necessary for correct responses, it is also not sufficient to support generalization of learning effects to the untrained location.
A region suggested to be particularly relevant for subjective awareness is the dorsolateral prefrontal cortex (DLPFC; ref. 7). As in our study, the authors compared matched performance levels for differences in subjective awareness, which was achieved by comparing two SOAs in type B metacontrast masking (7). The DLPFC showed higher blood oxygen level-dependent activity at matched performance levels for SOAs that led to more “seen” responses than another SOA with fewer seen responses. The RFs of visually responsive neurons in the DLPFC are larger than 6.6° at 4° eccentricity (34, 35), which would be compatible with the generalization of subjective awareness across space. Also, learning to discriminate visual objects leads to a sparsening of population activity and a sharpening of the tuning of individual neurons in this area (36). Thus, given its RF characteristics and malleability by practice, the DLPFC is a possible site of learning effects for subjective awareness.
No Measurable Effect of Blockwise Feedback.
The factor feedback remained insignificant in all analyses, i.e., blockwise feedback did not influence the development of sensitivity, response bias, or subjective awareness. Previous evidence on the role of feedback in perceptual learning is inconclusive. Although some studies have found that feedback is necessary (trial-by-trial; ref. 37) or at least beneficial (blockwise; ref. 38), others have found no effects (trial-by-trial; refs. 39 and 40). Our results indicate that repeated performance on the stimuli is sufficient for learning under metacontrast-masking conditions, as has also been found for learning effects in blindsight patients (10). However, it remains possible that trial-by-trial feedback is more effective in driving learning in our task.
Conclusions
Our results show that perceptual thresholds, objective or subjective, are not fixed but can be changed through practice. Awareness is trainable. However, the dissociations between subjective awareness and objective performance show that the respective improvements are not simple byproducts of each other. In particular, their neuronal substrate seems to differ. Thus, if we want to understand how perceptual learning affects not only sensory processing but also higher cognitive functions, and unravel the brain regions involved in such training effects, we will have to go beyond assessing performance in isolation.
Materials and Methods
Twenty-two subjects participated in this study. The experiment took place on 5 consecutive d. On the first day, we individually determined the SOA at which performance in the discrimination task was at chance and used this SOA for the later training. Training started immediately after the first threshold measurement and lasted 5 d. On the last day, we again assessed the masking threshold, followed by a threshold measurement at the transfer position. Details on the participants, stimuli, procedures, and analyses can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank T. Übelacker and P. Jacob for help with data acquisition and F. Aedo-Jury, J. Aru, T. Bachmann, B. Bridgeman, A. Kohler, M. Schmid, and two anonymous reviewers for their helpful comments on the manuscript. This work was supported by the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009147108/-/DCSupplemental.
References
- 1.Goldstone RL. Perceptual learning. Annu Rev Psychol. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- 2.Fahle M. Perceptual learning. In: Bayne T, Cleeremans A, Wilken P, editors. The Oxford Companion to Consciousness. Oxford: Oxford Univ Press; 2009. pp. 407–409. [Google Scholar]
- 3.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 4.Stoerig P. Blindsight, conscious vision, and the role of primary visual cortex. Prog Brain Res. 2006;155:217–234. doi: 10.1016/S0079-6123(06)55012-5. [DOI] [PubMed] [Google Scholar]
- 5.Meeres SL, Graves RE. Localization of unseen visual stimuli by humans with normal vision. Neuropsychologia. 1990;28:1231–1237. doi: 10.1016/0028-3932(90)90039-q. [DOI] [PubMed] [Google Scholar]
- 6.Schärli H, Brugger P, Regard M, Mohr C, Landis T. Localisation of “unseen” visual stimuli: Blindsight in normal observers? Swiss J Psychol Schweiz Z Psychol Rev Suisse Psychol. 2003;62:159–165. [Google Scholar]
- 7.Lau HC, Passingham RE. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc Natl Acad Sci USA. 2006;103:18763–18768. doi: 10.1073/pnas.0607716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzopardi P, Cowey A. Blindsight and visual awareness. Conscious Cogn. 1998;7:292–311. doi: 10.1006/ccog.1998.0358. [DOI] [PubMed] [Google Scholar]
- 9.Schwiedrzik CM, Singer W, Melloni L. Sensitivity and perceptual awareness increase with practice in metacontrast masking. J Vis. 2009;9:11–18. doi: 10.1167/9.10.18. [DOI] [PubMed] [Google Scholar]
- 10.Sahraie A, et al. Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proc Natl Acad Sci USA. 2006;103:14971–14976. doi: 10.1073/pnas.0607073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasupathy A. Neural basis of shape representation in the primate brain. Prog Brain Res. 2006;154:293–313. doi: 10.1016/S0079-6123(06)54016-6. [DOI] [PubMed] [Google Scholar]
- 12.Ramsøy TZ, Overgaard M. Introspection and subliminal perception. Phenom Cogn Sci. 2004;3:1–23. [Google Scholar]
- 13.Dienes Z, Seth AK. Measuring any conscious content versus measuring the relevant conscious content: Comment on Sandberg et al. Conscious Cogn. 2010;19:1079–1080. doi: 10.1016/j.concog.2010.03.009. discussion 1081–1083. [DOI] [PubMed] [Google Scholar]
- 14.Schubö A, Schlaghecken F, Meinecke C. Learning to ignore the mask in texture segmentation tasks. J Exp Psychol Hum Percept Perform. 2001;27:919–931. doi: 10.1037//0096-1523.27.4.919. [DOI] [PubMed] [Google Scholar]
- 15.Wolford G, Marchak F, Hughes H. Practice effects in backward masking. J Exp Psychol Hum Percept Perform. 1988;14:101–112. doi: 10.1037//0096-1523.14.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Breitmeyer BG, Rudd M, Dunn K. Metacontrast investigations of sustained-transient channel inhibitory interactions. J Exp Psychol Hum Percept Perform. 1981;7:770–779. doi: 10.1037//0096-1523.7.4.770. [DOI] [PubMed] [Google Scholar]
- 17.Gold J, Bennett PJ, Sekuler AB. Signal but not noise changes with perceptual learning. Nature. 1999;402:176–178. doi: 10.1038/46027. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Náñez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- 19.Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61:700–707. doi: 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczepanowski R, Pessoa L. Fear perception: Can objective and subjective awareness measures be dissociated? J Vis. 2007;7:11–17. doi: 10.1167/7.4.10. [DOI] [PubMed] [Google Scholar]
- 21.Lau HC. A higher order Bayesian decision theory of consciousness. Prog Brain Res. 2008;168:35–48. doi: 10.1016/S0079-6123(07)68004-2. [DOI] [PubMed] [Google Scholar]
- 22.Cleeremans A. Consciousness: The radical plasticity thesis. Prog Brain Res. 2008;168:19–33. doi: 10.1016/S0079-6123(07)68003-0. [DOI] [PubMed] [Google Scholar]
- 23.Schurger A, Pereira F, Treisman A, Cohen JD. Reproducibility distinguishes conscious from nonconscious neural representations. Science. 2010;327:97–99. doi: 10.1126/science.1180029. [DOI] [PubMed] [Google Scholar]
- 24.Clifford CW. Consciousness: Reading the neural signature. Curr Biol. 2010;20:R61–R62. doi: 10.1016/j.cub.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Del Cul A, Dehaene S, Reyes P, Bravo E, Slachevsky A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain. 2009;132:2531–2540. doi: 10.1093/brain/awp111. [DOI] [PubMed] [Google Scholar]
- 26.Summerfield C, Jack AI, Burgess AP. Induced gamma activity is associated with conscious awareness of pattern masked nouns. Int J Psychophysiol. 2002;44:93–100. doi: 10.1016/s0167-8760(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 27.Boehler CN, Schoenfeld MA, Heinze HJ, Hopf JM. Rapid recurrent processing gates awareness in primary visual cortex. Proc Natl Acad Sci USA. 2008;105:8742–8747. doi: 10.1073/pnas.0801999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastner S, et al. Modulation of sensory suppression: Implications for receptive field sizes in the human visual cortex. J Neurophysiol. 2001;86:1398–1411. doi: 10.1152/jn.2001.86.3.1398. [DOI] [PubMed] [Google Scholar]
- 29.Boussaoud D, Desimone R, Ungerleider LG. Visual topography of area TEO in the macaque. J Comp Neurol. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- 30.Gattass R, Sousa AP, Gross CG. Visuotopic organization and extent of V3 and V4 of the macaque. J Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178:363–380. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- 32.Kondo H, Komatsu H. Suppression on neuronal responses by a metacontrast masking stimulus in monkey V4. Neurosci Res. 2000;36:27–33. doi: 10.1016/s0168-0102(99)00104-2. [DOI] [PubMed] [Google Scholar]
- 33.Bar M, Biederman I. Localizing the cortical region mediating visual awareness of object identity. Proc Natl Acad Sci USA. 1999;96:1790–1793. doi: 10.1073/pnas.96.4.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainer G, Asaad WF, Miller EK. Memory fields of neurons in the primate prefrontal cortex. Proc Natl Acad Sci USA. 1998;95:15008–15013. doi: 10.1073/pnas.95.25.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H, Azuma M. Topographic studies on visual neurons in the dorsolateral prefrontal cortex of the monkey. Exp Brain Res. 1983;53:47–58. doi: 10.1007/BF00239397. [DOI] [PubMed] [Google Scholar]
- 36.Rainer G, Miller EK. Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron. 2000;27:179–189. doi: 10.1016/s0896-6273(00)00019-2. [DOI] [PubMed] [Google Scholar]
- 37.Seitz AR, Nanez JE, Sr, Holloway S, Tsushima Y, Watanabe T. Two cases requiring external reinforcement in perceptual learning. J Vis. 2006;6:966–973. doi: 10.1167/6.9.9. [DOI] [PubMed] [Google Scholar]
- 38.Herzog MH, Fahle M. The role of feedback in learning a vernier discrimination task. Vision Res. 1997;37:2133–2141. doi: 10.1016/s0042-6989(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 39.Petrov AA, Dosher BA, Lu ZL. Perceptual learning without feedback in non-stationary contexts: Data and model. Vision Res. 2006;46:3177–3197. doi: 10.1016/j.visres.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Fahle M, Edelman S. Long-term learning in vernier acuity: Effects of stimulus orientation, range and of feedback. Vision Res. 1993;33:397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.