Abstract
IgG is a major Ig subclass in mucosal secretions of the human female genital tract, where it predominates over the IgA isotype. Despite the abundance of IgG, surprisingly little is known about where and how IgG enters the lumen of the genital tract and the exact role local IgG plays in preventing sexually transmitted diseases. We demonstrate here that the neonatal Fc receptor, FcRn, is expressed in female genital tract epithelial cells of humans and mice and binds IgG in a pH-dependent manner. In vitro we show that FcRn mediates bidirectional IgG transport across polarized human endometrial HEC-1-A monolayers and primary human genital epithelial cells. Furthermore, endosomal acidification appears to be a prerequisite for FcRn-mediated IgG transcytosis; IgG transcytosis was demonstrated in vivo by translocation of systemically administered IgG into the genital lumen in WT but not FcRn-KO mice. The biological relevance of FcRn-transported IgG was demonstrated by passive immunization using herpes simplex virus-2 (HSV-2)–specific polyclonal serum, which conferred significantly higher protection against intravaginal challenge infection by the HSV-2 186 strain in WT mice than in FcRn-KO mice. These studies demonstrate that FcRn-mediated transport is a mechanism by which IgG can act locally in the female genital tract in immune surveillance and in host defense against sexually transmitted diseases.
Keywords: mucosa, humoral, uterine
Most pathogens initiate infection by making contact with polarized epithelial cells at mucosal surfaces. Immunoglobulins present in mucosal secretions function as a first line of defense against mucosally transmitted pathogens (1). In the gastrointestinal and upper respiratory tracts, the major Ig species found in the mucosal surface is secretory IgA (S-IgA), which is known to result primarily from active transport of dimeric IgA by the polymeric IgA receptor (pIgR) through polarized epithelium (2). Although S-IgA generally is considered the primary component of antibody-mediated defense in the intestine, several lines of evidence indicate that IgG also is present in mucosal secretions and support a role for IgG in host mucosal defense. First, IgG is present in secretions of the human oral mucosa, small and large intestines, and lungs (3, 4). Second, the numbers of local IgG-secreting cells in mucosal tissues and the levels of antigen-specific IgG in mucosal secretions are dramatically increased after both mucosal and systemic immunization (5–7). Third, the isotype pattern and concentrations of IgG in mucosal secretions are clearly distinct from those of serum, suggesting an active and selective representation of IgG in the mucosal site (8).
IgG is the predominant Ig subclass in the human female genital tract, where its concentration exceeds that of IgA (9). IgG also has been detected in uterine-cervical fluids (10) and vaginal washes (5, 11). The importance of IgG in genital infections has been exemplified by the predominance of human immunodeficiency virus (HIV-1)–specific IgG responses at the mucosal surfaces of HIV-1–infected women (6, 11). In these studies, there was an inverse correlation between the amount of mucosal IgG present and viral load. Therefore, HIV-specific IgG may be much more important in mucosal protection than previously thought. Unlike S-IgA, the mechanism(s) by which the IgG antibody is transported across the genital epithelium and the role of IgG in genital mucosal protection have not been investigated. Incomplete understanding of IgG transport in the genital tract and of its role in combating genital infections has hampered the design and development of vaccines and preventative treatments for sexually transmitted diseases (STDs). Historically, the source of IgG in the genital tract has been attributed to simple passive paracellular diffusion from the circulation or local production by epithelium-associated plasma cells (12). This view has been challenged by increasing evidence that IgG levels in genital mucosal secretions can be affected dramatically by genital infections (6, 13–16), the estrous cycle (17), and immunization (7, 8, 15, 18). Collectively, these observations raise the possibility of an active transport system through which IgG crosses the genital epithelium.
The neonatal Fc receptor for IgG (FcRn) is a heterodimer composed of a membrane-bound 45- to 50-kDa heavy chain associated nonconvalently with the 12-kDa β2-microglobulin (β2m) (19). FcRn was identified originally in the intestine of neonatal rodents, but it also is expressed and is functionally active in a variety of adult tissues and cells (20–22). Furthermore, FcRn is responsible for transporting maternal IgG across the placenta and colostrum IgG across the intestine to the fetal and neonatal bloodstream, respectively (20, 21). The ability of FcRn to transport IgG allows newborns to acquire humoral immunity in the form of maternal antibodies before their own immune system is fully matured. In addition, FcRn is capable of protecting IgG and albumin from catabolism (22, 23), and this protection contributes to their being the most abundant proteins in the blood. This protective function of FcRn plays an important role in prolonging the half-life and maintaining functional levels of IgG after immunization or infection and therefore promotes long-lasting protective immunity. The interaction of FcRn and IgG is markedly pH dependent, with binding of IgG at acidic (≤6.5) and release of IgG at neutral (7.0–7.5) conditions (23). As such, FcRn is likely to function primarily within the acidified endosomes where IgG binding likely occurs after fluid phase uptake.
Although FcRn plays critical roles in the acquisition of maternal humoral immunity in early life and in the regulation of IgG catabolism in adults, its function in IgG-mediated immunity in the female genital tract remains to be established. Because IgG is the major Ig isotype in the genital tract, we hypothesized that FcRn in polarized genital epithelial cells functions as an intracellular trafficking receptor and mediates transcytosis of IgG across genital tract epithelium, leading to enriched IgG in genital mucosal secretions. Our findings show that FcRn in the female genital epithelial of both humans and mice is capable of transporting IgG both apically and basolaterally. Moreover, FcRn-transcytosed viral-specific IgG conferred protection against mucosal challenge with the model pathogen HSV-2. These results reveal an unexpected and important role for FcRn acting at the female genital epithelium in immune surveillance and host defense against STDs.
Results
Analysis of FcRn Expression in Human Female Genital Epithelial Cells.
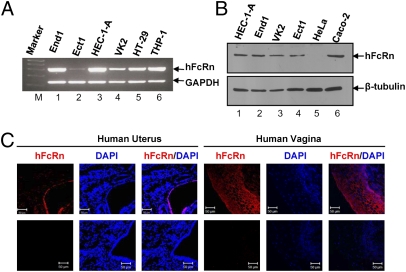
To determine if FcRn is expressed in female genital epithelial cells, RT-PCR analysis was performed on total RNA obtained from four human female genital epithelial cell lines (End1, endocervical; Ect1, exocervical; HEC-1-A, uterine; and VK2, vaginal) and two control human cell lines (the intestinal epithelial cell line HT29 and macrophage-like cell line THP-1). Human FcRn cDNA was amplified as a single 296-bp band in all cell lines examined, and the sequences of the PCR products were confirmed by DNA sequencing (Fig. 1A). Moreover, the FcRn cDNA sequence we detected was identical to the predicted sequence expressed by human placenta as reported previously (24). FcRn protein expression was assessed by Western blot using a rabbit anti-human FcRn (anti-hFcRn) antibody (25). The FcRn-expressing human intestinal epithelial cell line Caco-2 was used as a positive control (20), and the FcRn-negative human cervical epithelial cell line HeLa was used as a negative control (25). The 45-kDa band detected in all four genital epithelial cell lines (Fig. 1B) was consistent with the known molecular weight of the hFcRn heavy chain and comigrated with FcRn heavy chain expressed by Caco-2 cells (Fig. 1B, lane 6).
Fig. 1.
Detection of hFcRn gene expression in genital epithelial cell lines. (A) Semiquantitative RT-PCR of hFcRn mRNA prepared from cell lines of human female genital tract, intestine, or macrophage origin. Primer pairs amplified the α2 domain of hFcRn. RNA from human intestinal epithelial cell line HT-29 and macrophage-like cell line THP-1 were used as positive controls. Negative control RT-PCR was done without RT. GAPDH was used as an internal control for RNA quality and loading. Ect1, exocervical cells; End1, endocervical cells; HEC-1-A, human uterine epithelial cells; VK2, vaginal cells. (B) Western blot analysis of hFcRn expression in human genital epithelial cells. Cell lysates from HeLa (lane 5) and human intestinal epithelial cell Caco-2 (lane 6) were used as negative and positive controls, respectively. Blots were probed with affinity-purified rabbit anti-hFcRn–specific antibody (Upper) or β-tubulin–specific antibody (Lower) followed by incubation with HRP-conjugated anti-IgG antibody. (C) Immunohistochemical staining of hFcRn expression in human uterine and vaginal tissues. Tissue sections were stained with affinity-purified rabbit anti-hFcRn antibody followed by Alexa Fluor 555-conjugated IgG (red). FcRn staining was not observed in the presence of normal IgG. DAPI (blue) indicates nuclear staining. Data are representative of at least three sections. Images were captured with a 40× water objective lens. (Scale bars: 50 μm.)
The expression of FcRn in human uterine and vaginal tissues was confirmed further by immune staining using anti-hFcRn antibodies (25). FcRn was localized to both uterine and vaginal tissues, with the most intense staining in the epithelium (Fig. 1C). Immune staining in the stroma was weak in the uterus and almost undetectable in the vagina. Many scattered cells in the uterine lamina propria also showed weak cytoplasmic staining (Fig. 1C). No staining was seen using an irrelevant rabbit IgG (Fig. 1C), illustrating the specificity of the anti-hFcRn antibodies. Together these results show that human vaginal, ectocervical, endocervical, and endometrial epithelia express the 45-kDa heavy chain of FcRn. The presence of abundant FcRn mRNA and protein in human female genital epithelial cells and its observed cellular distribution are consistent with data from enterocytes of human small intestine (20).
Receptor-Mediated Transcytosis of IgG in HEC-1-A Cells.
We selected the HEC-1-A cell to study IgG transcytosis because it expresses FcRn (Fig. 1A) and had been used to demonstrate pIgR-mediated IgA transcytosis (26). FcRn-dependent IgG transport was measured by transepithelial flux of human IgG. As shown in Fig. 2A, intact human IgG applied to apical (lane 4) or basolateral (lane 3) cell surfaces was transported in both luminal and ablumenal directions across HEC-1-A cell monolayers, as assessed by Western blot. Transport of IgG, as indicated by the presence of its heavy chain (55 kDa), was detected in monolayers incubated at 37 °C (lanes 3 and 4) but not at 4 °C (lanes 5 and 6). To show whether IgG transcytosis was not mediated by FcγRIII (SI Results and Fig. S1), IgG transport was inhibited by FcRn knockdown or competitive inhibition of FcRn function by fragment B of Staphylococcal protein A (Fig. S2). In contrast, chicken IgY, which is structurally similar to IgG but does not bind FcRn, did not cross HEC-1-A cell monolayers in either direction at 37 °C (Fig. S3A, lanes 3 and 4). Thus, the transepithelial flux of biotin-labeled IgG did not occur by passive diffusion through intercellular tight junctions or monolayer leaks. These data indicate that IgG can enter both apical- and basolateral-directed transcytotic pathways in HEC-1-A cells. In a separate experiment, the apically directed transport pathway was determined to be approximately twofold more efficient in IgG transport than the basolaterally directed pathway (Fig. S3B). In this experiment, the amount of IgG transported in either direction was measured by a quantitative ELISA using a control IgG as a standard.
Fig. 2.
Bidirectional transcytosis of IgG. (A) HEC-1-A cells (5 × 105 cells per well) were grown in 12-well Transwell inserts. When the transepithelial resistance of the monolayer reached 300–400 Ω/cm2, cells were loaded with 500 μg/mL human IgG in either the apical (lanes 4 and 6) or basolateral (lanes 3 and 5) chamber and incubated at 37 °C or 4 °C. Three hours later culture medium was collected from the opposite chamber and subjected to Western blot analysis. Lane 1 represents an IgG. Results are representative of three individual experiments. (B) HEC-1-A cells were incubated in the presence (lane 3) or absence (lane 4) of bafilomycin A1 (0.1 μM), and basolaterally directed transport of IgG-biotin (100 μg/mL) was measured after 3 h of incubation. Transcytosed IgG was measured by Western blot. (C) IgG transcytosis in the EpiVaginal tissue model. EpiVaginal tissue was grown in a 12-well Transwell insert. When the transepithelial resistance of the monolayer reached 300–400 Ω/cm2, cells were loaded with human IgG-biotin (100 μg/mL) (lanes 3–6) or chicken IgY-biotin (lanes 8 and 9) in either the apical (lanes 4, 6, and 9) or basolateral (lanes 3, 5, and 8) chamber and incubated at 37 °C or 4 °C. Lanes 1 and 7 represent an IgG or IgY standard, respectively. Cells were warmed to 37 °C to stimulate transcytosis; medium was collected from the nonloading compartment 3 h later and was subjected to avidin blot analysis. A negative control was performed without adding IgG (lane 2). The results are representative of at least three independent experiments. A, apical; B, basolateral; HC, heavy chain; LC, light chain.
To demonstrate pH dependency in IgG transcytosis, HEC-1-A cells were incubated in the presence or absence of bafilomycin A1 (0.1 μM). Bafilomycin A1 is a specific inhibitor of the vacuolar H+ ATPase that collapses pH gradients in intracellular vesicles but does not interfere with membrane trafficking (20). Fig. 2B shows that bafilomycin A1 completely inhibited apical-to-basolateral transport of IgG (lane 3). Incubations at 37 °C and 4 °C in the absence of bafilomycin A1 provided positive and negative internal controls (lanes 2 and 4, respectively). Identical results were obtained in studies of apically directed IgG transport. Thus, FcRn-mediated transepithelial transport of IgG in HEC-1-A cells requires acidic conditions in intracellular compartments.
Transport of IgG by Primary Human Genital Epithelial Cells.
To test whether the IgG transcytosis seen in HEC-1-A cells was a general property of human genital epithelial tissues, a commercially available primary human genital epithelial tissue, Human EpiVaginal Tissue Model (VEC-100-FT), that closely reflects in vivo conditions was studied. Human FcRn expression was verified in these primary human genital epithelial tissues by RT-PCR amplification with FcRn-specific primers (Fig. S4A) and Western blot with FcRn-specific antibodies (Fig. S4B). Specificity of FcRn detection was confirmed with either FcRn-negative HeLa cells (lane 2) or FcRn-positive HEC-1-A cells (lane 1) (Fig. S4B). When biotin-labeled human IgG (100 μg/mL) was loaded in either the apical or the basolateral chamber, the human primary genital epithelial cells transcytosed IgG in either the apical-to-basolateral direction (lane 4) or the basolateral-to-apical direction (lane 3) (Fig. 2C). IgG transport did not occur when tissues were incubated at 4 °C (lanes 5 and 6). In addition, biotin-labeled IgY was not transcytosed in either direction at 37 °C (Fig. 2C, lanes 8 and 9). Overall, the results indicate that human IgG was selectively and bidirectionally transported across primary human female genital epithelial cells and that redistribution was not caused by passive diffusion through paracellular pathways or leakiness in the tissue model.
IgG Transcytosis in the Mouse Genital Tract.
Previous reports indicated that IgG is the major Ig present in human vaginal washings (27). We reasoned that the differential transport of Igs into the genital tract might result from local expression of FcRn and efficient FcRn-mediated basolateral-to-apical IgG transfer across epithelium. We thus examined the level of mouse FcRn expression in the uterus and vagina of adult mice by Western blot and immunofluorescence with the liver and intestine as positive and negative controls, respectively. We found that FcRn was expressed in the epithelial cells of both uterus and vagina (Fig. 3A). As expected, mouse FcRn was detected in the liver but not in the small intestine of adult mice, consistent with findings that mouse FcRn transcription in intestinal epithelium is greatly diminished following weaning (22, 23). Using IgG-agarose beads as a ligand, mouse FcRn heavy chain was specifically pulled down at pH 6.0 but not at pH 7.4 (Fig. 3B) from cell lysates of both uterine (lanes 1 and 2) and vaginal (lanes 3 and 4) tissues. Furthermore, frozen tissue sections of mouse vaginal (Fig. 3C, Upper) and uterine (Fig. 3C, Lower) mucosa showed a specific epithelial staining pattern of FcRn. Taken together, these data indicate that, as in humans, the epithelial cells of the mouse genital tract express high levels of functionally active FcRn.
Fig. 3.
Mouse FcRn (mFcRn) expression in the genital tract. (A) Detection of mFcRn protein in freshly isolated epithelial cells by Western blot. Membranes were probed with an affinity-purified rabbit anti-mFcRn or β-tubulin antibody and HRP-conjugated donkey anti-rabbit IgG. Arrows indicate the location of the mFcRn heavy chain and β-tubulin. (B) pH-dependent IgG binding in mouse epithelial cells from vaginal and uterine tissues. Epithelial cells from mouse uterine (lanes 1 and 2) and vaginal (lanes 3 and 4) tissues were isolated and lysed in PBS (pH 6.0 or 7.4) with 0.5% 3[(3-cholamidoprobyl) dimethylammonio]-1-propanesulfonate (CHAPS) and protease inhibitors. Approximately 0.5 mg of the soluble proteins was incubated with human IgG-Sepharose at 4 °C. The eluted proteins or cell lysates were subjected to Western blot. Cell lysates from uterus (lane 5) or vagina (lane 6) were used as positive controls. Membranes were probed with affinity-purified rabbit anti-mFcRn antibody and HRP-conjugated donkey anti-rabbit IgG. The mFcRn heavy chain is indicated by an arrow. (C) Immunostaining of mFcRn in the vaginal and uterine tissues of mice. Cryosections were prepared from vaginal and uterine tissues of C57L/B6 female mice. Sections were stained for mFcRn antibody plus Alexa Fluor 488-conjugated IgG (green). Rabbit IgG was used as the negative control. Vagina (Upper) and uterus (Lower) of normal adult mouse showed punctuate staining visible at the apical cytoplasmic membrane and in the apical cytoplasm. The nucleus is stained with DAPI (blue). Images were captured using a 40× water objective lens. Arrowheads indicate the lumen. (Scale bars: 50 μm.)
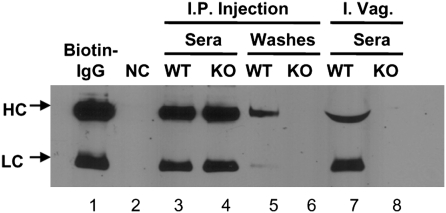
Because FcRn is highly expressed in mouse genital epithelial cells, we next asked if murine FcRn could mediate IgG transport across the genital mucosal barrier in vivo. First, we addressed whether FcRn influenced the amount of endogenous IgG in the female genital tract lumen. As Fig. S5 A and B shows, significantly higher IgG levels were present in vaginal washings of FcRn-WT mice than in washings from FcRn-KO mice, even with large variations caused by estrous cycling and furthermore, analysis of IgG-to-IgA titer ratios revealed a predominance of IgG (Fig. S5B). Second, we tested whether IgG administered systemically required FcRn to gain access to the genital tract lumen. Prepubertal mice were used to minimize variability caused by estrous cycling. Biotin-labeled mouse IgG was administered i.p. into WT (100 μg) or FcRn-KO (200 μg) mice (twice as much IgG was used in FcRn-KO mice to compensate for the reduction in circulating IgG that occurs in the absence of FcRn) (28). Twelve hours after administration, vaginal washings were sampled and subjected to avidin blot analysis. Fig. 4 shows that IgG serum concentrations were similar in WT and FcRn-KO mice (lanes 3 and 4). However, IgG was detectable only in the vaginal washings of WT mice (lane 5), not in vaginal washings of FcRn-KO mice (lane 6). These results indicate FcRn mediates basolateral-to-apical transfer of IgG in the female genital tract. Finally, to address whether FcRn-dependent transport at this site is bidirectional, we performed a parallel experiment in which IgG was administered intravaginally (i.vag.) into WT and FcRn-KO mice. IgG was readily detected in the sera of WT mice but not of FcRn-KO mice (Fig. 4, lane 7). Taken together, these data strongly support the notion that FcRn mediates both apical-to-basolateral and basolateral-to-apical transport of IgG in the female genital tract.
Fig. 4.
IgG transcytosis in the genital tract of mouse. FcRn-mediated IgG transport in vivo. Biotin-labeled mouse IgG was administered i. p. (lanes 3–6) or i.vag. (lanes 7 and 8) into WT (lanes 3, 5, and 7) or FcRn-KO (lanes 4, 6, and 8) mice. Lane 2 is a negative control. The sera and vaginal washes were sampled as indicated and subjected to avidin blot analysis. Results are representative of three individual experiments. HC, heavy chain; LC, light chain.
Prophylactic Efficacy of IgG Antibody Against Genital Infections.
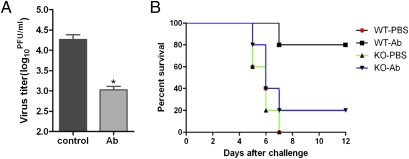
Because we found that FcRn mediates IgG transport into the lumen of the genital tract, we next asked whether this mechanism could protect against genital infection. HSV-2 was chosen as our model pathogen because vaginal infection of prepubertal mice with WT HSV-2 causes severe neurological disorders and lethality within 8–14 d (29). First, the Transwell system was used to investigate whether HSV-2–specific antibodies were able to inactivate live HSV-2 viral particles after transcytosis across a polarized HEC-1-A cell monolayer. HSV-2–specific rabbit antibody or normal rabbit serum was added to the basolateral chamber, and HSV-2 virus subsequently was added to the opposing apical chamber to allow apical infection. Virus yield was measured from the apical medium after 24 h by a standard plaque assay. HSV-2–specific polyclonal antibody reduced HSV-2 virus yield by 10-fold relative to cells treated with normal rabbit serum (Fig. 5A). We next determined whether transcytosed pathogen-specific IgG provided host protection from infection in the genital tract in vivo. Prepubertal WT or FcRn-KO mice were inoculated i.vag. with a lethal dose of HSV-2 strain 186 (1.4 × 106 pfu) 12 h after i.p. administration of HSV-2–specific antibody or normal control serum. The survival rate (Fig. 5B) for WT animals that received HSV-2–specific serum was 80%, whereas only 20% of the animals in the FcRn-KO group survived (P = 0.0437). All animals that received normal serum succumbed to infection within 6–7 d after challenge (P = 0.0079 for WT mice receiving viral-specific serum versus control serum). Importantly, there was no survival difference between FcRn-KO mice that received normal serum vs. viral-specific serum (P = 0.3255). Therefore, the systemic administration of HSV-2–specific antibodies conferred protection from genital infection in an FcRn-dependent manner.
Fig. 5.
Transcytosis of HSV-2–specific antibodies into the vaginal lumen and protection against HSV-2 virus challenge infection. (A) Transcytosis of rabbit anti–HSV-2 antibodies across polarized HEC-1-A monolayers and neutralization of HSV-2 virus. Viral titers were measured by standard plaque assay on Vero cell monolayers. Virus titer in apical compartments was compared in groups treated with normal rabbit serum or rabbit anti–HSV-2 serum. Asterisks indicate a significant difference as compared with normal rabbit serum control (P < 0.05). (B) Survival rates of mice following FcRn-mediated transfer of rabbit anti–HSV-2 antibodies. Mean survival rates after adoptive serum transfer and genital HSV-2 challenge are shown. Survival rate was calculated as the percent of prepubertal mice that survived divided by total number of mice in the group. Results are representative of three individual experiments. n = 15 mice per group.
Discussion
The present study clearly demonstrates that IgG transcytosis in female genital epithelial cells is bidirectional and FcRn dependent. As predicted from the defined functions of hFcRn in placental trophoblast and intestinal epithelial cells (22, 23), our results confirmed that FcRn transports IgG across HEC-1-A epithelial cell monolayers. In addition, we established that FcRn is expressed abundantly in the human uterine and vaginal epithelia and that primary female human genital epithelial tissues are able to engage in robust bidirectional IgG transcytosis via FcRn. Although whether FcRn mediates IgG transport in the adult human female genital tract remains unknown, this possibility is likely based on our results showing that murine epithelial cells lining both the vagina and the uterus express high levels of FcRn and mediate efficient IgG transport. Overall, these data demonstrate an FcRn-dependent mechanism for bidirectional IgG transport across the genital epithelium that may provide immunity against local infection.
The female genital mucosa is a dynamic tissue in which hormonal fluctuations dramatically influence the structure, function, and protein expression of the genital tract epithelium (30). Indeed, our results showed that local IgG levels are affected substantially by the stage of the estrous cycle. How hormonal changes in the estrous cycle affect IgG transport across genital mucosa is currently unknown; we have preliminary data that suggest hormones modulate IgG transport by regulating the expression of FcRn in female genital epithelium. Future studies will be required to address this possibility formally.
The female genital mucosa varies, because the upper tract, including the cervix, uterus, and fallopian tubes, is composed mainly of polarized simple columnar epithelia (9, 31), which have a neutral pH extracellular environment under normal physiological conditions. We provided evidence that, at steady state in HEC-1-A cells, FcRn is localized within the intracellular vesicles (Fig. S6), a distribution similar to that seen in enterocytes of the human small intestine and the vascular endothelium. Because IgG binding to FcRn is pH dependent, IgG could access the transcytotic pathway in such cells only by fluid-phase endocytosis followed by binding to FcRn in acidic intracellular compartments rather than at the cell surface, because both ligand–receptor binding in acidic vesicles and bafilomycin A1-sensitive membrane traffic proved to be required for IgG transcytosis. In contrast, the lower tract (the vagina) has a partially keratinized pseudostratified epithelium, which presumably is necessary to provide a stronger physical barrier (9, 31). Although how FcRn transports IgG across a simple columnar epithelium seems straightforward, it is less clear whether FcRn is able to transport IgG across the pseudostratified epithelium, even though FcRn is amply expressed in this tissue. It is possible that FcRn progressively transports IgG across this stratified layer or that the FcRn in vaginal epithelium is not involved in active transport of IgG. Hysterectomized women have an ~95% decrease in Ig, including IgG, in their vaginal secretions (32), suggesting that the uterus is the main source of immunoglobulins in female genital tract secretions.
The vaginal lumen is acidic (pH 4.0 for humans and pH 6.2–6.5 for mice) (33) because of acidic products arising from the anaerobic metabolism of vaginal glycogen (34). Given this low vaginal pH, cell surface FcRn could capture IgG efficiently through receptor-mediated endocytosis, protecting IgG from degradation and discharging it at local sites where the physiological pH is near neutral. Moreover, immunization with attenuated HSV-2 virus induced an 86-fold increase in the number of IgG plasma cells in the vaginal mucosa (16). Therefore, the acidic vaginal environment may provide a physiological milieu in which FcRn sequesters IgG supplied not only from the circulation but also by local plasma cells. Infection by sexually transmitted pathogens can be rapid and massive. IgG held in local reserve in the female genital tract by FcRn may arm this site for coping with such infections.
The bidirectional IgG transport that we document for the genital mucosa suggests a potential route for sensing and responding to genital infections. Antigen trafficking across epithelial barriers is the first step required to generate effective mucosal and systemic immune responses after mucosal infection (16). Highly specialized microfold (M) cells within the follicle-associated epithelium in both gut and nasal tract are known to transport antigens (31). However, the genital tracts of both males and females lack follicle-associated epithelium and typical M cells (9) and therefore must rely on alternative mechanisms for mucosal immunity. The bidirectional transport of IgG across the genital endothelium by FcRn may contribute to both local and systemic recall responses by delivering antigen-specific IgG across the genital epithelial barrier to the genital lumen (apical side) and then recovering captured cognate antigens as part of immune complexes in the lamina propria (basolateral side) for uptake by antigen-presenting cells. An analogous bidirectional mechanism has been shown to control IgG immunity to luminal bacteria in the intestine (35).
Finally, our results showing that FcRn transpors neutralizing HSV-2–specific polyclonal antibody across epithelial cells and significantly protects mice after intravaginal challenge with lethal HSV-2 clearly demonstrate that FcRn is an efficient conduit for supplying neutralizing IgG from the systemic circulation to combat genital infection. The observation that Rhesus macaques passively administered a mixture of neutralizing IgGs are protected from vaginal transmission of HIV-1 (36, 37) could be explained by a similar FcRn-dependent mechanism. It is possible that, by intercepting microbes before mucosal contact and attachment and/or by blocking the entry into the targeted epithelium, IgG may prove more important than IgA for host protection within the female genital tract. IgG also may eliminate locally infected cells through antibody-dependent, cell-mediated cytotoxic reactions. Therefore, systemic and local induction of high levels of IgG antibodies that are transported to mucosal surfaces by FcRn provides an important component of immune surveillance and host defense at the genital mucosa.
In conclusion, this study documents the operation of an FcRn-mediated IgG transcytosis system in the female genital epithelium that resembles the systems found in other mucosal epithelia (38). This local system appears to be the major source of IgG in genital mucus and provides an effective protective mechanism against genital infection. Our results suggest that vaccines that elicit high levels of broadly neutralizing IgG antibodies may provide effective protection against mucosal infection and transmission. Further efforts to understand how human IgG antibodies mediate this protection could yield insights into mucosal immunity and facilitate the development of safe and effective mucosal vaccines against STDs. Furthermore, the discovery of FcRn-mediated IgG transport in the genital tract may provide the basis for a passive immunoprophylaxis approach for preventing mucosal transmission of HIV and other sexually transmitted pathogens.
Methods
Cell Lines, Virus, and Animals.
The Vk2, Ect1, and End1 cell lines were described previously (39). The tissue sections of human vaginal and epithelial tissues were purchased from Abcam or Integrated Laboratory Services-Biotech. The human EpiVaginal tissue model VEC-100-FT was purchased from MatTek. HSV-2 strain 186 was provided by Lawrence Stanberry (Columbia University, New York). FcRn-KO mice (28) were purchased from the Jackson Laboratory. HSV-2–specific polyclonal serum with a neutralization titer of 1:32 was purchased from Dako. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee.
Isolation of RNA and Semiquantitative RT-PCR.
Total RNA was isolated from cells using TRIzol (Invitrogen). Primers for amplification of FcRn and GAPDH have been described elsewhere (40).
Collection of Vaginal Fluid and Determination of Mouse Estrous Cycles.
Vaginal washes were collected as described previously (15). Vaginal smears were used to determine the stage of estrous cycle as previously described (41). May–Grünwald–Giemsa staining of vaginal smears of the four different stages of estrous cycle (diestrus, proestrus, estrus, and metestrus 1 and 2) was performed.
Vaginal and Uterine Epithelial Cell Isolation.
At necropsy, vaginal and uterine tissues were collected and washed in PBS to remove possible blood contamination. Vaginal or uterine epithelial cells were isolated by a method described by Wira et al. (30), with some modifications. In brief, the vaginal or uterine tissue was minced carefully into small pieces and digested for 20 min at 37 °C with agitation in HBSS supplemented with 200 U/mL hyaluronidase, 1 mg/mL collagenase type IV, 0.2 mg/mL DNase I, and 1 mg/mL BSA. The digested mixture was collected and passed through a 100-μm mesh and washed with RPMI containing 10% FBS. Epithelial cells were separated on discontinuous Percoll gradients (Pharmacia) with centrifugation at 453 × g for 20 min. For additional details see SI Methods.
IgG Binding Assay and IgG Transcytosis.
An IgG binding assay was performed as described (27). Purified human IgG or chicken IgY was biotinylated with sulfo-NHS-biotin (Pierce). IgG transport was performed as described with modifications (20). The same procedure was used to perform IgG transcytosis in VEC-100-FT primary human female genital tissue. For in vivo IgG transport, 200 μg of biotinylated mouse IgG in 100 μL of PBS was administered i.p. to WT or FcRn-KO mice. In addition, biotinylated IgG in 30 μL PBS was administered i.vag. to WT mice (50 μg per mouse) and FcRn-KO mice (100 μg per mouse). Vaginal flushing or serum was collected 12 h after treatment. The IgG was analyzed by SDS/PAGE and avidin blot-ECL.
Immunofluorescence, Confocal Microscopy, and Western Blot.
Immunofluorescent staining of cells or frozen tissue sections was performed as described (25). For Western blot, cell lysates (50 μg) were separated by electrophoresis on 12% SDS/PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat milk and probed with affinity-purified rabbit anti-human or mouse FcRn Ab, mouse anti–β-tubulin Ab, or rabbit anti-β2m Ab. Protein bands were visualized with ECL.
Passive Antibody Transfer and Virus Challenge.
For in vitro neutralization, rabbit anti–HSV-2 serum or normal rabbit serum was added into the basolateral chamber of the polarized HEC-1-A cells and incubated for 12 h. HSV-2 strain 186 was inoculated into the apical chamber at 0.1 multiplicity of infection (MOI). The medium in the apical chamber was collected 24 h later, and viral titers were determined by plaque assay. To test FcRn function in vivo, 4-wk-old female mice were given a single i.p. injection of rabbit anti–HSV-2 IgG (100 μg) in 100 μL PBS 12 h before infection. Anesthetized mice were infected i.vag. with 1.4 × 106 pfu of HSV-2. FcRn-KO mice received i.p injections of 20–40 μg IgG every 24 h for 7 consecutive days following challenge to compensate for IgG degradation. Mice were monitored for 12 d for pathology and death.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical significance was determined by Student's t test (two tailed). For animal survival data, statistical significance was determined by log-rank test (Kaplan–Meier survival analysis). P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We are grateful to Dr. Raina Fichorova, Dr. Lawrence Stanberry, and Dr. Richard Blumberg for supplying cell lines and reagents, to Dr. Neil Simister for helpful discussions of mouse IgG catabolism, and to Dr. Lilin Ye, Yu Bai, Dr. Xindong Liu, and Dr. Li Lu for technical help. This work was supported in part by National Institutes of Health Grants AI67965, AI65892, and AI73139 (to X.Z.) and DK56597 (to D.C.R.) and by the Maryland Agricultural Experiment Station competitive grants from the University of Maryland (to X.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012861108/-/DCSupplemental.
References
- 1.Corthésy B. Roundtrip ticket for secretory IgA: Role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Kaetzel CS. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 3.Haimovici F, Mayer KH, Anderson DJ. Quantitation of HIV-1-specific IgG, IgA, and IgM antibodies in human genital tract secretions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:185–191. doi: 10.1097/00042560-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds HY. Identification and rôle of immunoglobulins in respiratory secretions. Eur J Respir Dis Suppl. 1987;153:103–116. [PubMed] [Google Scholar]
- 5.Bouvet JP, Bélec L, Pirès R, Pillot J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect Immun. 1994;62:3957–3961. doi: 10.1128/iai.62.9.3957-3961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bélec L, et al. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berneman A, Belec L, Fischetti VA, Bouvet JP. The specificity patterns of human immunoglobulin G antibodies in serum differ from those in autologous secretions. Infect Immun. 1998;66:4163–4168. doi: 10.1128/iai.66.9.4163-4168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson M, Lycke NY. Immunology of the human genital tract. Curr Opin Infect Dis. 2003;16:43–49. doi: 10.1097/00001432-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Kutteh WH, Mestecky J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31:40–46. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 11.Lu FX. Predominate HIV1-specific IgG activity in various mucosal compartments of HIV1-infected individuals. Clin Immunol. 2000;97:59–68. doi: 10.1006/clim.2000.4910. [DOI] [PubMed] [Google Scholar]
- 12.Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997;36:23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MR, Brais LJ, Evelegh MJ. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J Gen Virol. 1990;71:1497–1504. doi: 10.1099/0022-1317-71-7-1497. [DOI] [PubMed] [Google Scholar]
- 14.Milligan GN, Bernstein DI. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology. 1995;206:234–241. doi: 10.1016/s0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 15.Parr EL, Parr MB. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109–8115. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parr EL, Bozzola JJ, Parr MB. Immunity to vaginal infection by herpes simplex virus type 2 in adult mice: Characterization of the immunoglobulins in vaginal mucus. J Reprod Immunol. 1998;38:15–30. doi: 10.1016/s0165-0378(97)00081-8. [DOI] [PubMed] [Google Scholar]
- 17.Kozlowski PA, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: Influence of the menstrual cycle. J Immunol. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 18.Artenstein AW, et al. Mucosal immune responses in four distinct compartments of women infected with human immunodeficiency virus type 1: A comparison by site and correlation with clinical information. J Infect Dis. 1997;175:265–271. doi: 10.1093/infdis/175.2.265. [DOI] [PubMed] [Google Scholar]
- 19.Burmeister WP, Huber AH, Bjorkman PJ. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994;372:379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson BL, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, et al. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–546. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 23.Ward ES, Ober RJ. Multitasking by exploitation of intracellular transport functions the many faces of FcRn. Adv Immunol. 2009;103:77–115. doi: 10.1016/S0065-2776(09)03004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: Possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29:158–163. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball JM, et al. A polarized human endometrial cell line that binds and transports polymeric IgA. In Vitro Cell Dev Biol Anim. 1995;31:196–206. doi: 10.1007/BF02639434. [DOI] [PubMed] [Google Scholar]
- 27.Mestecky J. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J Reprod Immunol. 2007;73:86–97. doi: 10.1016/j.jri.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Roopenian DC, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 29.McDermott MR, Smiley BJ, Brais PLJ, Rudzroga H, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:247–253. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wira CR, Sandoe CP. Specific IgA and IgG antibodies in the secretions of the female reproductive tract: Effects of immunization and estradiol on expression of this response in vivo. J Immunol. 1987;138:4159–4164. [PubMed] [Google Scholar]
- 31.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 32.Jalanti R, Isliker H. Immunoglobulins in human cervico-vaginal secretions. Int Arch Allergy Appl Immunol. 1977;53:402–408. doi: 10.1159/000231778. [DOI] [PubMed] [Google Scholar]
- 33.De Bernardis F, Mühlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67:5170–5175. doi: 10.1128/iai.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida M, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 37.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 38.Spiekermann GM, et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.