Abstract
MicroRNAs (miRNAs) are small RNA molecules that regulate gene expression at the posttranscriptional level and are critical for many cellular pathways. The disruption of miRNAs and their processing machineries also contributes to the development of human tumors. A common scenario for miRNA expression in carcinogenesis is emerging that shows that impaired miRNA production and/or down-regulation of these transcripts occurs in many neoplasms. Several of these lost miRNAs have tumor-suppressor features, so strategies to restore their expression globally in malignancies would be a welcome addition to the current therapeutic arsenal against cancer. Herein, we show that the small molecule enoxacin, a fluoroquinolone used as an antibacterial compound, enhances the production of miRNAs with tumor suppressor functions by binding to the miRNA biosynthesis protein TAR RNA-binding protein 2 (TRBP). The use of enoxacin in human cell cultures and xenografted, orthotopic, and metastatic mouse models reveals a TRBP-dependent and cancer-specific growth-inhibitory effect of the drug. These results highlight the key role of disrupted miRNA expression patterns in tumorigenesis, and suggest a unique strategy for restoring the distorted microRNAome of cancer cells to a more physiological setting.
Keywords: non-coding RNA, transformation, therapy, pharmacogenetics
MicroRNAs (miRNAs) are small noncoding RNAs that inhibit gene expression at the posttranscriptional level. They are synthesized in the nucleus by RNA polymerase II as long primary transcripts, called primary miRNAs (1, 2). They are subsequently cleaved by DROSHA to release hairpin-shaped precursors of 70–90 nucleotides (premiRNAs) that are transported to the cytoplasm by Ran-GTP/Exportin-5, where DICER1 and TRBP process them to yield a duplex of 19–22 nt. One strand of the duplex is incorporated into the RNA-induced silencing complex, which delivers mature miRNAs to their mRNA targets. The target recognition is based on complementarity of the seed sequence of the miRNA to a specific sequence motif within the 3′ UTR of the mRNA target (3).
miRNAs play important roles in several cellular processes, by simultaneously controlling the expression levels of hundreds of genes (1, 2, 4). In human cancer, miRNA expression profiles differ between normal tissues, derived tumors, and tumor types (5, 6), and it has been shown that miRNAs can act as oncogenes or tumor suppressors (7, 8). Importantly, an miRNA expression profile of human tumors has emerged that is characterized by a defect in miRNA production and global miRNA down-regulation (5, 6, 9–11). Several mechanisms explain this miRNA deregulation in cancer, such as the failure of miRNA posttranscriptional regulation (12), CpG island promoter hypermethylation-associated transcriptional silencing (13–16), transcriptional repression by oncogenic factors (17), mutational impairment of the TARBP2 miRNA processing gene that codes for the TAR RNA-binding protein 2 (TRBP) protein (18), and down-regulation of the DICER1 miRNA biosynthesis gene (19–21). Consistent with these observations, experimental knockdown and genetic defects in miRNA-processing machinery genes, such as DICER1 and TRBP, cause miRNA global depletion and stimulate tumorigenesis (18, 22–25), suggesting that miRNA impairment actively contributes to cancer development.
Despite the impact of miRNAs on cancer biology, miRNA-based cancer therapy is still in its early stages and mostly limited to target a single miRNA (26, 27). However, because most tumors show a global down-regulation of miRNA expression (5, 6, 9–11), restoration of normal miRNA levels might represent an attractive approach in cancer therapy. Herein, we present an miRNA-based treatment of malignancies in which enoxacin, a small molecule proposed to promote RNA interference and miRNA processing (28), has a powerful cancer-specific growth-inhibitory effect mediated by a TRBP-dependent restoration of the expression of tumor suppressor miRNAs.
Results
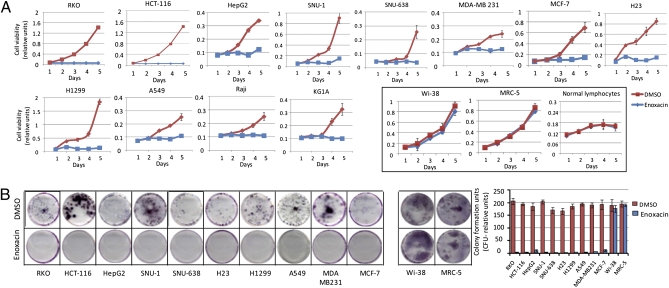
Enoxacin Treatment Has a Cancer-Specific Growth-Inhibitory Effect.
Despite the enormous potential that a small molecule that enhances RNA interference might have for cancer therapeutic purposes, the effects of enoxacin in tumor proliferation have not been characterized. Thus, we first analyzed whether enoxacin could predominantly act as a cancer growth inhibitor by examining the effects of the drug in a panel of 12 cancer cell lines from seven common malignancies. The transformed cell lines studied included colorectal (RKO and HCT-116), gastric (SNU-1 and SNU-638), lung (H23, H1299, and A549), breast (MCF-7 and MDA-MB-231), liver (HepG2), leukemia (KG1a), and lymphoma (RAJI). A careful median effective concentration (EC50) study was conducted in the colorectal HCT-116 cells and demonstrated that dose-dependent growth-inhibition effect, with an EC50 of 124 μM (40 μg/mL) (Fig. S1). Thus, we treated all cancer cell lines with 40 μg/mL of enoxacin continuously for 5 d. We observed a great decrease in cell viability, measured by the MTT (methylthiazol tetrazolium) assay, in all cancer cell lines compared with carrier-(DMSO, dimethyl sulfoxide) treated cells (Fig. 1A). Most importantly, when we treated two types of normal cells, fibroblast lines (Wi-38 and MRC-5) and six primary cultures of normal peripheral blood lympho-mononuclear cells derived from healthy donors (29), we did not observe any effect on cell viability in any of these cells upon enoxacin treatment (Fig. 1A), suggesting a cancer-specific growth-suppressive activity. The antiproliferative effects of enoxacin were reinforced by the finding that the clonogenic assay capacity of all of the 10 adherent cancer cell lines was significantly inhibited by drug treatment (Fig. 1B). The cancer-selective inhibitory effect of enoxacin was confirmed by the absence of clonogenic capacity changes in the normal fibroblast cells Wi-38 and MRC-5 (Fig. 1B).
Fig. 1.
Enoxacin treatment has cancer-specific inhibitory effect. (A) Cell viability assay in 12 cancer cell lines vs. fibroblast cell cultures (Wi-38 and MRC-5) and normal lymphocytes from healthy donors. (B) Colony formation assay in the described cell lines.
Of the cellular biological explanations for the observed growth inhibition effect, the induction of cell-cycle arrest and/or apoptosis is the most likely. Thus, we assessed the cell-cycle patterns by flow cytometry in the colorectal cancer cell lines HCT-116 and RKO. We found that after 72 h of enoxacin treatment, both cancer cell lines exhibited cell-cycle arrest in the G2/M phase (Fig. S1). Enoxacin-treated HCT-116 and RKO cells accumulated 55% and 48% cells in G2/M, whereas carrier-treated cells accumulated 28.5% and 21.2%, respectively (Fig. S1). Immunocytochemistry for cyclin B1 also demonstrated an accumulation of this G2/M marker in the nucleus of enoxacin-treated cells (Fig. S1). After the arrest in G2/M phase upon enoxacin treatment, massive cell death (91.9%) due to apoptosis assessed by the APO-BrdU-Tunel assay was observed after 5 d (Fig. S1).
Enoxacin Enhances miRNA Production.
One critical matter to address is the characterization of the molecular pathways involved in the observed cancer growth-inhibitor phenotype mediated by enoxacin. Enoxacin has been characterized as an enhancer of RNA interference (28), although this mechanism is not naturally used by human cells to silence gene expression posttranscriptionally. At this last level of control, other molecules play a central role in our cells, such as miRNAs (1–3). It was also suggested that enoxacin might promote the processing of miRNAs by comparing the level of three precursor miRNAs (premiR-125a, prelet-7, and premiR-30a) (28).
We wondered whether enoxacin could enhance the production of miRNAs with putative tumor-suppressor functions that would explain the antitumoral effects of the drug. We have first addressed this question by measuring the production of miRNAs of cancer cells upon enoxacin treatment. We observed that HCT-116 and RKO enoxacin-treated cells featured an overall increase in the production of 24 mature miRNAs molecules (Fig. 2A) and a down-regulation of the corresponding precursor miRNA molecules (Fig. S2). Northern blot analyses of the tumor-suppressor miRNAs let7-a and miR-125a confirmed the enhancement of miRNA production in enoxacin-treated cells (Fig. S2). The case of let-7 is particularly exciting because it targets Cdc34 and leads to Wee1 stabilization and G2/M accumulation (30). Concordantly with these data, we observed that enoxacin use in HCT-116 and RKO cells, in addition to causing G2/M arrest and promoting the processing of let-7, induces Cdc34 down-regulation and the stabilization of the Wee1 protein (Fig. S2).
Fig. 2.
Enoxacin enhances miRNA production through binding to TRBP protein. (A) Expression fold change of 24 quantified mature miRNAs in HCT-116 and RKO colon cancer cell lines upon enoxacin treatment. (B) Antihistidine protein blot showing expression of TRBPWT and TRBPMut recombinant proteins (Left). Enoxacin steady-state kinetics for binding to recombinant TRBP wild-type that was immobilized on a CM5 Biacore chip (Center). SPR assay with chip immobilized TRBPWT and TRBPMut proteins (Right). (C) Isothermal titration data for TRBPWT and TRBPMut with enoxacin. Left shows the calorimetric titrations for the TRBP wild-type protein, and Center represents the integrated heat values as a function of the protein/enoxacin molar ratio where the solid line represents the best fit. (Right) TRBPMut fails to bind enoxacin.
We also analyzed the global miRNA expression profile of RKO cells upon enoxacin treatment by using a comprehensive expression miRNA microarray platform (9). The expression profile of 731 miRNAs demonstrated that, among the differentially expressed miRNAs (n = 122), enoxacin-treated RKO cancer cells exhibited an overall up-regulation of miRNAs, 81% (100 of 122), whereas only 18% (22 of 122) underwent down-regulation. Strikingly, for the 26 enoxacin-up-regulated miRNAs in which a role in cancer development has been proposed (31, 32), 84.6% had potential tumor-suppressor features (Fig. S2). Most importantly, the restoration of the expression of tumor-suppressor miRNAs in RKO cells upon enoxacin treatment was associated with the down-regulation of their respective target oncoproteins, as we observed for MYC (let7-a and let7-b) and K-ras (miR-18a*, let7-a, let7-b, miR-143, and miR-205) (Fig. S2). Interestingly, we did not observe down-regulation of tumor suppressor genes regulated by the few oncogenic miRNAs induced by enoxacin treatment, such as the miR-21 target proteins PTEN and PDCD4 (Fig. S2).
Enoxacin Binds to TRBP.
It is also important to establish a mechanistic link that could explain how enoxacin promotes miRNA processing. Once we ruled out the possibility that enoxacin treatment increased the expression of the miRNA machinery proteins (Fig. S3), a clue about the mechanism was provided by the observation that enoxacin increases the binding affinity of the TRBP protein to prelet-7 (28). TRBP is an integral component of a DICER1-containing complex, in which it plays a critical role in miRNA processing (33, 34). Thus, it is possible that the observed enhancement of miRNA production upon enoxacin treatment is mediated by a direct effect of the drug on the TRBP protein. Indeed, we have identified a physical interaction between enoxacin and the TRBP protein by using two independent methods, surface plasmon resonance (SPR) and isothermal titration calorimetry. In the first approach, we synthesized the whole TRBP wild-type protein in bacteria, in addition to a TRBP-mutated protein that has altered amino acids 149–179 and lacks the last 187 amino acids (18) (Fig. 2B). The binding of wild-type and mutant TRBP proteins to enoxacin was first measured by SPR using the BIAcore CM5 sensor chip, which analyzes the ratio of actual to theoretical binding resonance units (RUactual and RUtheor, respectively). Enoxacin had an RUactual to RUtheor ratio of 0.9, signifying monomeric binding of enoxacin to the immobilized TRBP wild-type protein (Fig. 2B). The mutant form of TRBP was unable to bind to enoxacin (Fig. 2B). We calculated an affinity constant (KD) of 12.56 μM for the affinity of enoxacin for TRBP wild-type by SPR (Fig. 2B). Time-course experiments confirmed specific enoxacin binding to TRBP, in which a sequential reduction of enoxacin concentration reduced binding response units to the same magnitude (Fig. 2B). The binding kinetics revealed a very stable interaction with a dissociation constant (Kd) of 2.2 × 10−3 s−1 (Student's t test P ≤ 0.01) (Fig. 2B). These time-course and kinetics experiments confirmed the absence of binding of enoxacin to the TRBP mutant form (Fig. 2B). Most importantly, isothermal titration calorimetry experiments corroborated the findings obtained with the SRS BIAcore 2000 assays: The binding of enoxacin to the TRBP wild-type protein was entropically driven (ΔS = 138.4 J/K·mol−1), whereas there was no binding between enoxacin and the TRBP-mutant form (Fig. 2C). Thus, there is a direct physical interaction between enoxacin and TRBP that connects the drug and the miRNA-processing pathway.
TRBP Mutant Cancer Cells Are Resistant to the Growth-Inhibitory and miRNA-Processing Effects Mediated by Enoxacin.
We decided to investigate an additional link between enoxacin, TRBP protein, miRNA processing, and cellular growth by taking advantage of human cancer cells harboring genetic defects in the TARBP2 gene that encodes the TRBP protein (18). The presence of TARBP2 frameshift mutations in a subset of colorectal, gastric, and endometrial malignancies causes diminished TRBP protein expression and a defect in the processing of miRNAs (18). The reintroduction of TRBP in the mutant cells restores the efficient production of miRNAs and inhibits tumor growth (18).
Thus, we assessed the effects of enoxacin in a colorectal cancer cell line harboring an inactivating heterozygous TARBP2 frameshift mutation (Co115), a reconstituted Co115 cell line that it is stably transfected with the TRBP protein (Co115.TRBPWT), and a Co115 cell line stably transfected with the inert TRBP mutant protein (Co115.TRBPMut) (18). In addition, we used another TRBP-defective model by stably transfecting the colorectal cancer cell line RKO with a short hairpin that silences the TRBP protein (RKO.shTRBP). We first confirmed that the three TRBP-impaired cell lines (Co115, Co115.TRBPMUT, and RKO.shTRBP) had lower levels of wild-type protein expression than the proficient cells (Co115.TRBPWT, RKO, and HCT-116) (Fig. S4). We observed that enoxacin administration led to a small reduction in cell viability in the three cell lines in which TRBP function was defective (Co115, Co115.TRBPMUT, and RKO.shTRBP) (Fig. S4). Almost no effect on the clonogenic capacity (Fig. 3A) was observed, relative to the marked reduction of cellular growth for both assays in the TRBP-reconstituted (Co115.TRBPWT) or naturally proficient cells (RKO and HCT-116) (Fig. S4 and Fig. 3A). The EC50 value for enoxacin of the TRBP mutant Co115 cells, EC50 = 238 μM (76.2 μg/mL) (Fig. S4), double the one observed in TRBP wild-type HCT-116 cells (Fig. S1).
Fig. 3.
Enoxacin growth-inhibitory and miRNA-enhancing expression is TRBP-dependent. Colony formation (A) and BrdU-TUNEL (B) assays in TRBP-reconstituted (Co115.TRBPWT), TRBP mutant (Co115 and Co115.TRBPMut), and TRBP-depleted (RKO.shTRBP) cells. (C) Mean of fold change expression of 24 quantified mature miRNAs in Co115, Co115.TRBPWT, Co115.TRBPMut, and RKO.shTRBP cells upon enoxacin use.
We also examined whether the described resistance to growth inhibition upon enoxacin use in TRBP-impaired cells was reflected in the cell cycle. Flow cytometry demonstrated that upon enoxacin treatment, TRBP-reconstituted cells (Co115.TRBPWT) exhibited cell-cycle arrest in G2/M phase with an increase from 6 to 38% cells in this stage (Fig. S4). However, enoxacin treatment of TRBP-impaired cells (Co115, Co115.TRBPMUT, and RKO.shTRBP) did not significantly increase the G2/M cellular fraction (Fig. S4). The cell-death values followed a similar pattern: TRBP-reconstituted cells (Co115.TRBPWT) underwent massive apoptosis (97.7%) after enoxacin use that was not observed in mutant TRBP cells (Co115, 6.7%) or cells stably transfected with the inert mutant form (Co115.TRBPMUT, 3.1%) (Fig. 3B). Conversely, depletion of TRBP in RKO cells (RKO.shTRBP) rendered these cells more resistant to enoxacin-mediated cell death than the control cells (RKO) (Fig. S4). Thus, these data imply that the inhibition of cancer-cell growth, the induction of G2/M cell-cycle arrest and cell death by apoptosis upon enoxacin use is mediated by the miRNA-processing protein TRBP.
Following our discovery that enoxacin enhances the overall production of miRNAs with putative tumor-suppressor functions in the TRBP-proficient HCT-116 and RKO cells (Fig. 2A and Fig. S2), we wondered whether cells with defects in TRBP would be more refractory to the aforementioned effect. We addressed this matter by measuring the expression levels of the 24 described mature and precursor miRNAs. Co115 TRBP-deficient cells display an impaired expression of mature miRNAs that is improved by TRBP transfection (Co115.TRBPWT), but is not enhanced in cells transfected with the TRBP-mutant form (Co115.TRBPMUT) (18). We observed that enoxacin treatment significantly increased the production of mature miRNAs only in reconstituted Co115 cells, whereas the effect on untransfected-deficient Co115 cells or those transfected with the inert TRBP-mutant form was lower (Fig. 3C and Fig. S5). The same phenomenon was found upon stable depletion of TRBP in the proficient RKO cell line: The enhancement of miRNA production upon enoxacin use was lower in RKO.shTRBP (Fig. 3C and Fig. S5). As expected, a significant down-regulation of the corresponding precursor miRNA molecules upon enoxacin use was observed only in TRBP-proficient cells (Co115.TRBPWT) (Fig. S5). Thus, these results reinforce the idea that the small molecule enoxacin exerts its antiproliferative effects in cancer cells by promoting miRNA biogenesis in a TRBP-mediated manner.
Enoxacin Inhibits the Growth of Xenografted Cancer Cells by Promoting TRBP-Mediated Processing of miRNAs.
By following the description above of the antiproliferative effect of enoxacin in cultured cancer cells, we translated these results to in vivo animal tumor models. We first used xenografted nude mice maintained for 4 wk with daily i.p. injections of 10 mg/kg enoxacin in 5% DMSO, or saline solution in 5% DMSO. We found that the implanted colorectal cancer cell lines RKO and HCT-116 showed potent tumor-growth inhibition upon enoxacin use (Student's t test: P = 3.12 × 10−6 and P = 2.05 × 10−6, respectively) as indicated by tumor weight (Fig. 4A) and growth (Fig. S6). Interestingly, when the stably TRBP-depleted RKO cell line (RKO.shTRBP) was xenografted, enoxacin treatment was unable to inhibit tumor weight (Fig. 4A) and growth (Fig. S6). The evidence of TRBP-mediated growth suppression upon enoxacin administration was reinforced by the use of the TRBP mutant cell line Co115. The weight and growth of the Co115 cell xenografts was almost unaffected by enoxacin (Fig. 4A and Fig. S6) and Co115 cells transfected with the mutant TRBP protein (Co115.TRBPMUT) were equally insensitive (Fig. 4A and Fig. S6). However, Co115 TRBP-reconstituted (Co115.TRBPWT) xenografts experienced potent growth inhibition (Student t test: P = 6.84 × 10−5), as reflected by tumor weight and growth upon enoxacin treatment (Fig. 4A and Fig. S6). We next conducted a pathological examination of all xenografted tumors with the different enoxacin and control treatments. We observed that all TRBP–wild-type tumor tissues, including RKO, HCT-116, and Co115.TRBPWT, showed significant necrosis upon enoxacin use (Fig. 4B). However, minimal necrosis upon enoxacin use was observed in the TRBP-deficient (Co115, Co115.TRBPMUT, and RKO.shTRBP) xenografts (Fig. 4B). Most importantly, at the time of sacrifice, colon, lung, liver, and kidney tissues were resected for pathological analysis and no toxicity was detected in any of the mice used in the assay (Fig. S6). We wondered whether enoxacin also promoted miRNA processing in the described xenografted nude mice model. We found that enoxacin use significantly increased the production of the described 24 mature miRNAs only in TRBP-proficient xenografts (RKO, HCT-116, and Co115.TRBPWT), whereas the effect in impaired TRBP tumors (Co115, Co115.TRBPMUT, and RKO.shTRBP) was minimal (Fig. 4C and Fig. S7). Conversely, a significant down-regulation of the corresponding precursor miRNA molecules upon enoxacin use was observed only in TRBP-proficient cells (RKO, HCT-116, and Co115.TRBPWT) (Fig. S7). Overall, these experiments confirm a role for enoxacin as a small molecule with in vivo and in vitro antiproliferative effects mediated by a TRBP-associated enhancement of miRNA production.
Fig. 4.
Enoxacin-treated xenografted nude mice. (A) Enoxacin treatment on TRBP wild-type (HCT-116, RKO, and Co115.TRBPWT) and TRBP-impaired (Co115, Co115.TRBPMut, and RKO.shTRBP) xenograted cells. Tumor weight at 30 d is shown. Arrows indicate the location of the xenografted cells. (B) Tumors stained for hematoxilin-eosin after excision. TRBP wild-type (RKO, HCT-116) and reconstituted (Co115.TRBPWT) tumors treated with enoxacin showed a significant increase in necrosis. (C) Mean of fold change expression of 24 mature miRNAs quantified by real-time PCR from xenografts treated with enoxacin or DMSO. (D) Lung (Left) and liver (Right) metastasis after HCT-116 colon cancer cell injection in nude mice treated with enoxacin or DMSO.
We then decided to investigate the role of enoxacin in the metastatic process of distal seeding and growth in the two main organs affected by colorectal cancer dissemination. Thus, we developed two in vivo experimental HCT-116–based models as a successful means of obtaining metastases in the liver and lungs after spleen and tail vein injection, respectively. In both models, treatment with enoxacin significantly reduced the number of macro- and micrometastases at mice killing (Fig. 4D and Fig. S8). Thus, our results also suggest a role for enoxacin in the inhibition of tumor dissemination.
Enoxacin Inhibits the Growth of Human Primary Colorectal Tumors Orthotopically Implanted in Mice by Promoting TRBP-Mediated miRNA Processing.
The establishment of suitable mouse models of cancer showing human-like tumor progression is essential to develope unique therapeutic approaches. In this regard, models of orthotopic implantation of primary human tumors may be more valuable for clinical validation of new drugs than pure s.c. implantation models (35). Thus, we complemented our enoxacin in vivo mouse studies by generating orthotopic models of implanted human primary colorectal tumors. On the basis of the aforementioned results about the TRBP-mediated effect of the drug, we selected a subset of colon tumors that might carry TARBP2 mutations, microsatellite unstable tumors (MSI+) (18). Seven MSI+ tumors were identified from a previously established collection of 84 human primary colorectal tumors. The seven MSI+ colorectal tumors were genetically screened for mutations in TARBP2 and one (14%) had the same TARBP2 mutation as the colorectal cancer cell line Co115: A deletion in a (C)5 repeat in exon 5 that creates a premature stop codon and truncates TRBP (Fig. 5A). The mutation was heterozygous and associated with a decrease in TRBP protein levels (Fig. 5B). When the seven MSI+ tumors were orthotopically reimplanted in the ceacum of three nude mice per patient, only three tumors grew: two TARBP2 wild-type (CRC43 and CRC56) and the TARBP2 mutant (CRC64) (Fig. 5C). When palpable intraabdominal masses for these three tumors were detected, 60 mice were randomized into two groups: A control group (n = 10 for each tumor) treated with saline solution supplied with 5% DMSO and a group (n = 10 for each tumor) treated daily by i.p. injection of a 10 mg/kg enoxacin dose over 15 d. Notably, we observed that enoxacin caused a significant reduction in tumor weight in the human primary colorectal tumors with wild-type TRBP (CRC43 and CRC56) at the time of killing, compared with the DMSO-treated ones (Student's t test: P = 0.018) (Fig. 5D). Conversely, no significant differences in tumor growth were observed in the TRBP-mutant tumor (CRC64) upon enoxacin use (Student's t test: P = 0.26). The careful pathological examination of the TRBP wild-type tumor tissues (CRC56 and CRC43) from enoxacin-treated animals showed significant necrosis (65% and 72%, respectively) compared with DMSO-treated mice (5% and 16%) (Student's t test: P = 0.003 and P = 0.001, respectively) (Fig. 5E).
Fig. 5.
Orthotopic mouse colorectal cancer model for enoxacin use. (A) Sequence (C)5 repeat in exon 5 of TARBP2 in CRC56, CRC43, and CRC64 colorectal tumors. (B) Down-regulation of TRBP protein in CRC64 TRBP mutated colorectal tumor by Western Blot. (C) Hematoxilin-eosin staining of mice ceacum showing normal colon mice mucosa (N) and orthotopic inserted human colorectal tumors (T). (D) Orthotopic tumors excised from mice treated with enoxacin or DMSO. (E) Necrosis evaluation of orthotopic tumors from mice treated with enoxacin or DMSO. (F) Mean of fold change expression of 24 mature miRNAs from the orthotopic tumors upon enoxacin or DMSO use. (G) Clustering of the miRNA expression profile of orthotopic colorectal tumors excised from enoxacin- or DMSO-treated mice compared with four normal colon tissues. Enoxacin treatment of the CRC43 wild-type TRBP tumor restores a normal-like miRNA expression profile.
Tissue samples from the tumors were also collected for analysis of miRNA expression profiles. Upon enoxacin treatment, we observed increased expression of the 24 studied mature miRNAs in the TRBPwt implanted colorectal cancer orthotopic primary tumors (CRC56 and CRC43) relative to DMSO-treated mice (Student's t test: CRC56, P = 0.012; CRC43, P = 0.023) (Fig. 5F and Fig. S9). Importantly, no significant changes in miRNA production were detected when the implanted orthotopic TRBPMut tumor (CRC64) was treated with enoxacin (Student t test: P = 0.78) (Fig. 5F and Fig. S9). As expected, a significant down-regulation of the corresponding precursor miRNA molecules upon enoxacin use was only observed in CRC56 and CRC43 wild-type TRBP tumors (Fig. S9). Finally, we used the expression miRNA microarray platform (9) to analyze two primary human orthotopically transplanted tumors (TRBP wild-type CRC43 and mutant CRC64). The expression profile of 731 miRNAs demonstrated that, upon enoxacin use, there was an overall up-regulation of miRNA expression levels in the TRBP wild-type tumor (CRC43) shifting the microRNAome to a more “normal colon expression profile” that clustered its miRNA transcriptome within the primary normal colon mucosa branch (four samples were used) (Fig. 5G). Among the enoxacin-up-regulated miRNAs in the CRC43 tumor with a proposed role in cancer, 74% (49 of 66) had potential tumor suppressor features (Fig. S9). The restoration of the expression of tumor-suppressor miRNAs in the tumors upon enoxacin treatment was associated with the down-regulation of their respective target oncoproteins, such as we observed for MYC (let7-a, and let7-b) and K-ras (miR-18a*, let7-a, let7-b, miR-143, and miR-205) (Fig. S9). Conversely, we observed a minimal effect on the miRNA expression profile of the TRBP mutant CRC64 tumor upon enoxacin use: It was unable to “shift” its microRNAome to the normal colon expression signature branch (Fig. 5G). Only 10 miRNAs (1.3%) were up-regulated (Fig. S9) and the downstream target oncoproteins of the miRNAs remained unchanged (Fig. S9). These findings emphasize the central role of TRBP-mediated miRNA processing in mediating the cancer-specific growth-inhibitor effect of enoxacin reported here.
Discussion
Human tumors have aberrant miRNA expression profiles (microRNAomes) that occur in the context of genetic (18, 24, 36) and epigenetic (13–16) lesions in miRNA loci and the miRNA processing machinery, or associated with upstream events in transforming and growth-inhibitor genes, such as MYC (17). From a functional standpoint, some of the cancer-related miRNAs can act as oncogenes or tumor suppressors (7, 8, 31, 32), opening up the possibility of searching for drugs that might regulate miRNA expression or use artificial miRNAs as potential antitumoral agents.
Most of the achievements in this area concern miRNAs with known oncogenic roles. miRNAs can be inhibited in several ways, such as using complementary nucleic acid analogs that block the unique signature of miRNAs (antagomirs) by “base-pairing” (37). Covalent modifications of the analog inhibitor include locked nucleic acids (LNAs) 2′-O-methyl and 2′-O-methoxyethyl (26). Alternatively, it is possible to use a sponge vector expressing miRNA target sites to saturate the endogenous miRNA (38). For miRNAs with tumor-suppressor roles, fewer examples exist, a prime example being the systemic adeno-associated virus-mediated delivery of miR-26a in a hepatocellular carcinoma mouse model, which suppresses tumorigenesis (39). Similar results have recently been obtained in a mouse model of lung cancer and xenografted prostate tumors for the exogenous delivery of let-7 and miR-16, respectively (40, 41). However, if most human tumors are characterized by a defect in miRNA production and global miRNA down-regulation (5, 6, 9–11), it is tempting to propose that restoring the global microRNAome can also have a therapeutic effect. This is the same line of reasoning as for DNA demethylating agents and histone deacetylase inhibitors that, even without the existence of any target specificity, have received clinical approval for the treatment of certain hematological malignancies (42, 43). Enoxacin belongs to the family of synthetic antibacterial compounds based on a fluoroquinolone skeleton (44). Fluoroquinolones are commonly used broad-spectrum antibiotics (44) that are relatively nontoxic and inhibit type II DNA topoisomerase in mammalian cells and bacterial DNA gyrase. Enoxacin has been used to treat bacterial infections such as urinary tract infections (45). Most importantly, of 10 fluoroquinolones analyzed, enoxacin was the only one capable of enhancing RNAi induced by either shRNAs or siRNA duplexes and of stimulating miRNA expression (28), suggesting that the miRNA biogenesis-enhancing activity of enoxacin does not depend on general fluoroquinolone activity, but rather on the unique chemical structure of the molecule.
Overall, our data indicate that enoxacin specifically inhibits the growth of a broad spectrum of cancer cells by enhancing the miRNA-processing machinery, particularly at the TRBP-mediated stage. These results also provide a proof-of-concept basis for identifying further small activators of miRNA biogenesis and represent a unique step toward the potential application of miRNA-based therapy in the treatment of human cancer.
Materials and Methods
Human cancer cell lines were obtained from the American Type Culture Collection. Total RNA was isolated by TRIzol (Invitrogen). TaqMan MiRNA assays were used to quantify the levels of mature miRNAs (18). SuperScript III Platinum One-Step RT-qPCR kit (Invitrogen) was used to quantify precursor miRNAs (18). miRNA expression study by microarray analysis, protein blotting, and confocal microscopy were developed as described (18). For the in vivo nude mice xenografts, orthotopics, and lung/liver metastases experiments, 5-wk-old male nu/nu Swiss mice (Harlam) were used. Additional experimental details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by Grants SAF2007-00027-65134, Consolider CSD2006-49, The Dr. Josef Steiner Cancer Research Foundation, and the Advanced Grant EPINORC from the European Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014720108/-/DCSupplemental.
References
- 1.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 6.Volinia S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 8.Hammond SM. MicroRNAs as tumor suppressors. Nat Genet. 2007;39:582–583. doi: 10.1038/ng0507-582. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Gaur A, et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 11.Melo SA, Esteller M. Dysregulation of microRNAs in cancer: Playing with fire. FEBS Lett. August 11, 2010 doi: 10.1016/j.febslet.2010.08.009. 10.1016/j.febslet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Thomson JM, et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito Y, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Lujambio A, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. , and erratum (2007) 67:3492. [DOI] [PubMed] [Google Scholar]
- 15.Toyota M, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68:4123–4132. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 16.Lujambio A, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang TC, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melo SA, et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nat Genet. 2009;41:365–370. doi: 10.1038/ng.317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Karube Y, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–115. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merritt WM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martello G, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 23.Kumar MS, et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009;23:2700–2704. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill DA, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambertz I, et al. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ. 2010;17:633–641. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duchaine TF, Slack FJ. rna interference and micro rna-oriented therapy in cancer: Rationales, promises, and challenges. Curr Oncol. 2009;16:61–66. doi: 10.3747/co.v16i4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70:7027–7030. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan G, et al. A small molecule enhances RNA interference and promotes microRNA processing. Nat Biotechnol. 2008;26:933–940. doi: 10.1038/nbt.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuurman RK, Gelfand EW, Dosch HM. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980;125:820–826. [PubMed] [Google Scholar]
- 30.Legesse-Miller A, et al. let-7 Overexpression leads to an increased fraction of cells in G2/M, direct down-regulation of Cdc34, and stabilization of Wee1 kinase in primary fibroblasts. J Biol Chem. 2009;284:6605–6609. doi: 10.1074/jbc.C900002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina PP, Slack FJ. microRNAs and cancer: An overview. Cell Cycle. 2008;7:2485–2492. doi: 10.4161/cc.7.16.6453. [DOI] [PubMed] [Google Scholar]
- 32.Spizzo R, Nicoloso MS, Croce CM, Calin GA. SnapShot: MicroRNAs in cancer. Cell. 2009;137:586. doi: 10.1016/j.cell.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Chendrimada TP, et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haase AD, et al. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Pimpec-Barthes F, et al. Pleuro-pulmonary tumours detected by clinical and chest X-ray analyses in rats transplanted with mesothelioma cells. Br J Cancer. 1999;81:1344–1350. doi: 10.1038/sj.bjc.6693248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krützfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 38.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kota J, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trang P, et al. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2010;29:1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeshita F, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010;18:181–187. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 44.Bhanot SK, Singh M, Chatterjee NR. The chemical and biological aspects of fluoroquinolones: reality and dreams. Curr Pharm Des. 2001;7:311–335. doi: 10.2174/1381612013398059. [DOI] [PubMed] [Google Scholar]
- 45.Schaeffer AJ. The expanding role of fluoroquinolones. Am J Med. 2002;113(Suppl 1A):45S–54S. doi: 10.1016/s0002-9343(02)01059-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.