Abstract
Synaptic release of neurotransmitters is evoked by activity-dependent Ca2+ entry into the nerve terminal. However, here it is shown that robust synaptic neuropeptide release from Drosophila motoneurons is evoked in the absence of extracellular Ca2+ by octopamine, the arthropod homolog to norepinephrine. Genetic and pharmacology experiments demonstrate that this surprising peptidergic transmission requires cAMP-dependent protein kinase, with only a minor contribution of exchange protein activated by cAMP (epac). Octopamine-evoked neuropeptide release also requires endoplasmic reticulum Ca2+ mobilization by the ryanodine receptor and the inositol trisphosphate receptor. Hence, rather than relying exclusively on activity-dependent Ca2+ entry into the nerve terminal, a behaviorally important neuromodulator uses synergistic cAMP-dependent protein kinase and endoplasmic reticulum Ca2+ signaling to induce synaptic neuropeptide release.
Keywords: GCaMP, GFP, monoamine, neuromuscular junction, neurotransmission
Neuromodulators induce presynaptic signaling to regulate fast neurotransmission triggered by activity-induced Ca2+ entry into the nerve terminal. Neuromodulators also influence the very low rate of spontaneous quantal release of classical transmitters. However, because spontaneous release is functionally relevant only in specialized cases (1), neuromodulators are not believed to typically induce physiologically significant release in the absence of extracellular Ca2+. Studies of endocrine cells suggest that release of peptides packaged in large dense-core vesicles (LDCVs) also requires Ca2+ entry because of the rarity of spontaneous LDCV fusion and the inefficient permeation of peptides through the LDCV-fusion pore (2, 3). However, because it is difficult to measure peptide release at intact synapses, a much more limited dataset supports the conclusion that Ca2+ influx into the nerve terminal is absolutely required for peptidergic transmission. Thus, it remains unclear how neuromodulators control synaptic neuropeptide release.
With this background in mind, we set out to study regulation of neuropeptide release at the Drosophila neuromuscular junction (NMJ) by octopamine-induced cAMP signaling. Octopamine, the homolog of norepinephrine in Drosophila, controls many behaviors by activating central G protein-coupled receptors that induce adenylyl cyclase activation and intracellular Ca2+ release (4, 5). Octopamine is also in NMJ boutons at muscles 12 and 13 and can have complex effects at adjacent muscle 6 and 7 NMJs: octopamine inhibits phasic transmission and facilitates tonic release induced by K+, with the latter effect requiring cAMP-dependent protein kinase (PKA) (6–8). Drosophila motoneurons also contain neuropeptides, which are released in response to nerve stimulation, depolarization, and developmental cues (9–13). However, the impact of octopamine and cAMP on synaptic neuropeptide release is unknown, even though such regulation could be relevant to the effects of octopamine on central peptidergic neurons involved in controlling metabolism, development, and circadian behavior (14, 15).
Experiments in Drosophila have established that the fluorescence from presynaptically expressed GFP-tagged atrial natriuretic factor (AnfGFP) reports native neuropeptide content and release (12, 16–19). Here, this optical assay was applied to Drosophila larvae to study regulation of neuropeptide release at the muscle 6/7 NMJ. Although the expectation was that Ca2+ entry would be required, octopamine was found to induce robust synaptic neuropeptide release in the absence of extracellular Ca2+. Genetics and pharmacology were then combined to identify the presynaptic signaling responsible for this noncanonical neurosecretion.
Results
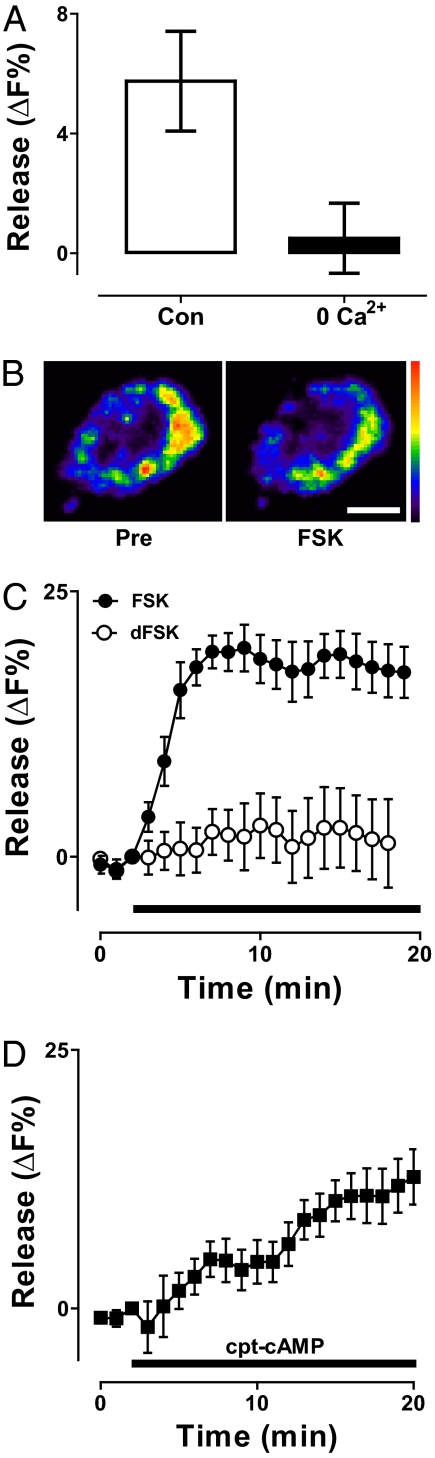
Synaptic neuropeptide release at the muscle 6/7 NMJ was measured as the percentage-change in AnfGFP fluorescence (ΔF%), with a positive number indicating a drop in content indicative of release. As expected, release induced by 70-Hz nerve stimulation for 15 s was abolished by replacing extracellular Ca2+ with the Ca2+ chelator EGTA (Fig. 1A). However, at the same terminal in the absence of extracellular Ca2+, the adenylyl cyclase activator forskolin (FSK) evoked neuropeptide release (Fig. 1 B and C, closed circles). Indeed, this release was comparable to that seen with minutes of intense electrical activity in the presence of extracellular Ca2+ (13). The inactive analog dideoxyforskolin did not mimic this response (Fig. 1C, open circles), but the membrane permeant phosphodiesterase-resistant cAMP analog 8-parachlorophenylthio-cAMP (cpt-cAMP) also induced neuropeptide release in the Ca2+-free solution (Fig. 1D). Hence, cAMP produced by adenylyl cyclase evoked robust synaptic neuropeptide release without Ca2+ entry into the nerve terminal.
Fig. 1.
Synaptic neuropeptide release is induced by cAMP in the absence of extracellular Ca2+. (A) Removing extracellular Ca2+ abolishes neuropeptide release from type Ib boutons induced by 70-Hz stimulation for 15 s. Note that the response in 0 Ca2+ (n = 4) differed from control (Con) (n = 4, P < 0.05). Release was measured as the drop in AnfGFP fluorescence (ΔF%). (B) Pseudocolor images showing neuropeptide content in 0 Ca2+ before (Pre) and after application of 50 μM FSK (FSK) for 10 min. (Scale bar, 2 μm.) The decrease in presynaptic neuropeptide signal indicative of release is shown as shift from warm colors to cool colors. (C) Time course of neuropeptide release in the absence of extracellular Ca2+ induced by FSK (n = 8) and dideoxy-forskolin (dFSK, n = 7) (application indicated by bar). (D) Time course of neuropeptide release in the absence of extracellular Ca2+ induced by 1 mM cpt-cAMP (n = 10).
To explore the roles of cAMP effectors, analogs that specifically stimulate PKA or epac (exchange protein activated by cAMP) were applied in the absence of extracellular Ca2+. Both the PKA activator N6-Benzoyl-cAMP (N6) and the epac activator 8-(4-methoxyphenylthio)-2′-O-methyl-cAMP (Me) evoked synaptic neuropeptide release (Fig. 2A). Although the epac activator produced a larger response in WT NMJs, this could reflect its greater concentration (200 vs. 100 μM) and membrane permeability. Therefore, we set out to determine whether epac and PKA are necessary for neuropeptide release induced by the adenylyl cyclase activator. There is no known specific chemical epac inhibitor, but there is a fly line with a P element insertion near the C-terminal cyclic nucleotide binding domain (allele EpacKG00434). The epac activator failed to induce neuropeptide release in this line (Fig. 2B, compare WT+Me to mut+Me), showing that the mutation disrupts epac function. Nevertheless, FSK still evoked robust release in the epac mutant (Fig. 2B, mut+FSK). In contrast, H89 (which inhibits PKA, but not epac) markedly reduced FSK-evoked release (Fig. 2B, compare WT+FSK to WT+FSK+H89). Hence, epac had at most a minor contribution to cAMP-evoked synaptic neuropeptide release without Ca2+ entry. Furthermore, these results are consistent with the conclusion that cAMP-induced release was mediated mainly by PKA.
Fig. 2.
Roles of PKA and epac in cAMP-evoked synaptic neuropeptide release. (A) Time course of release evoked by specific activators of PKA (100 μM N6, n = 7) and epac (200 μM Me, n = 6). (B) PKA-mediated neuropeptide release in the absence of epac function. Release was quantified after application of drugs for 10 min. Release induced by the epac activator (WT+Me, n = 5) was abolished in epac mutant flies (mut+Me, n = 5), but FSK-evoked release in WT animals (WT+FSK, n = 8) was still apparent in the epac mutant (mut+FSK, n = 3). In contrast, FSK-evoked neuropeptide release was inhibited by 5 μM H89 (WT+FSK+H89, n = 7). *P < 0.05, **P < 0.01.
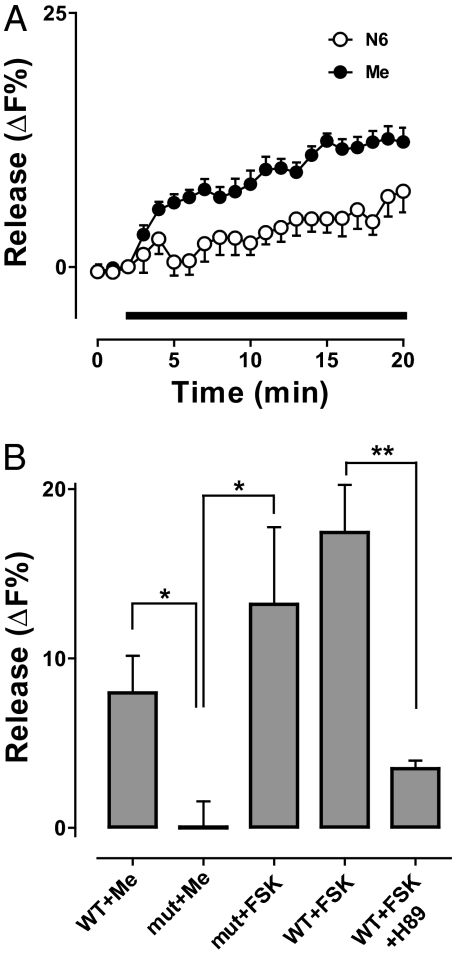
Induction of synaptic neuropeptide release without Ca2+ entry by pharmacological PKA activators led us to study octopamine, a native neuromodulator that acts presynaptically via PKA at the Drosophila NMJ (8). Octopamine also evoked neuropeptide release from motoneuron boutons in Ca2+-free medium (Fig. 3 A and B, filled circles). Furthermore, release elicited by octopamine was inhibited with H89 (Fig. 3 B and C) (P < 0.001). However, there are two limitations associated with bath applied H89: it could have acted on both sides of the synapse or had nonspecific effects in addition to inhibiting PKA. Therefore, a selective dominant-negative regulatory PKA subunit (20) was expressed only in neurons to specifically inhibit presynaptic PKA. With two chromosomal insertions of the dominant-negative gene (BDK22 and BDK35), octopamine-induced release was inhibited (Fig. 3C). Hence, presynaptic PKA is required for Ca2+ entry-independent neuropeptide release by the native neuromodulator.
Fig. 3.
Octopamine acts via PKA to evoke synaptic neuropeptide release. (A) Pseudocolor image showing neuropeptide content before (Pre) and after application of 100 μM octopamine for 10 min (Oct). (Scale bar, 2 μm.) (B) Time course of neuropeptide release induced by octopamine (indicated by bar) in the absence (Con, ●, n = 9) and presence of H89 (○, n = 8). (C) Release induced by octopamine in 5 min (Con, n = 9) was inhibited by H89 (n = 8) or expression of a dominant-negative PKA subunit [BDK22 (n = 5) and BDK35 (n = 6)]. *P < 0.05, ***P < 0.001.
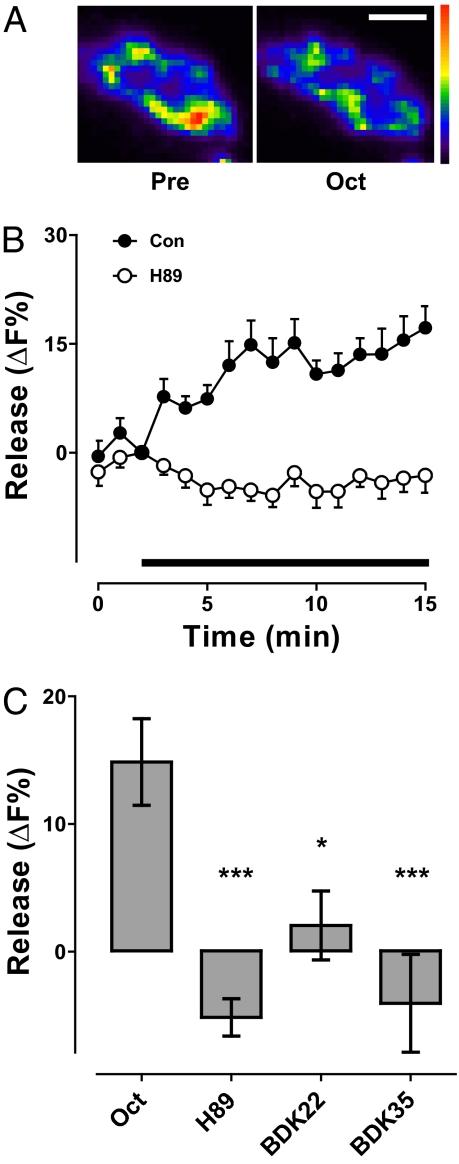
In addition to activating PKA, octopamine can evoke Ca2+ release from internal stores (4). Releasing presynaptic endoplasmic reticulum (ER) Ca2+ with caffeine (21) for 3 min elicited release of 24 ± 4% of synaptic neuropeptide content, suggesting a possible role for ER Ca2+ in presynaptic octopamine action. Therefore, to genetically probe whether ER Ca2+ stores participated in the octopamine effect, neuropeptide release was studied in the CaP60AKum170 (Kum170) mutant, in which the sarco-ER Ca2+ ATPase (SERCA) is persistently inhibited by brief exposure to 40 °C (22). Although octopamine evoked release under permissive conditions, heating Kum170 animals for 8 min to deplete ER Ca2+ stores abolished release measured subsequently at room temperature (Fig. 4A). A similar effect was produced by acute application of the SERCA inhibitor thapsigargin (Tg) (Fig. 4B), excluding developmental or pleiotropic effects of the mutation. Hence, ER Ca2+ was required for octopamine-induced synaptic neuropeptide release.
Fig. 4.
ER Ca2+ is required for octopamine-induced neuropeptide release. (A) Time course of octopamine-induced neuropeptide release in unheated or briefly heated Kum170 flies. Octopamine application indicated by bar. (B) ER Ca2+ channels are required for octopamine-induced release. Neuropeptide release evoked by octopamine for 10 min (Con, n = 9) was evident in the presence of DMSO (n = 3), but was abolished by 20 μM Tg (n = 3), RyR RNAi (n = 6), IP3R RNAi (n = 5), 100 μM Ryan (n = 5), and 0.1 μM Xesto (n = 4). DMSO served as the control for Tg and Xesto, and Con was the control for statistical analysis of Ryan and the RNAis. *P < 0.05, **P < 0.01.
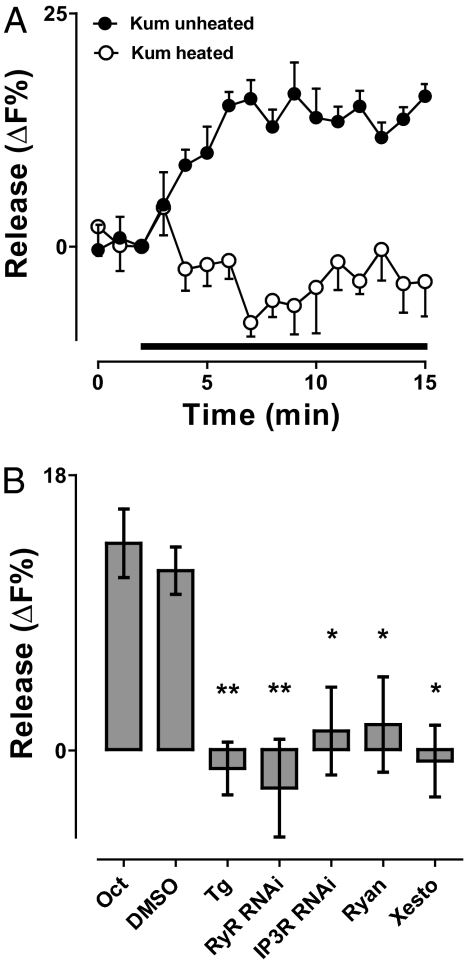
Therefore, we tested whether octopamine-induced release depended on ER Ca2+ channels [i.e., the ryanodine receptor (RyR) and the inositol trisphosphate receptor (IP3R)]. Because null RyR and IP3R mutants are lethal and dominant-negative subunits are not available, RNA interference was used initially. Specifically, neuronal expression of RyR RNAi or IP3R RNAi each inhibited the octopamine response (Fig. 4B). To exclude off-target or developmental effects, ER Ca2+ channels were acutely blocked by specific inhibitors known to be effective in Drosophila (23). Inhibiting RyRs with ryanodine (Ryan) or IP3Rs with xestospongin C (Xesto) blocked octopamine-induced neuropeptide release in the absence of extracellular Ca2+ (Fig. 4B). Hence, independent pharmacology and genetic experiments showed that octopamine required presynaptic RyRs and IP3Rs. Finally, to test whether octopamine elevates presynaptic Ca2+, the GCaMP3 Ca2+ indicator (24) was expressed in neurons and imaged in the nerve terminal in the absence of extracellular Ca2+. Under these conditions, octopamine induced a transient increase in cytoplasmic Ca2+ (Fig. 5A). Taken together, the above results show that octopamine activated presynaptic ER Ca2+ channels to induce synaptic neuropeptide release.
Fig. 5.
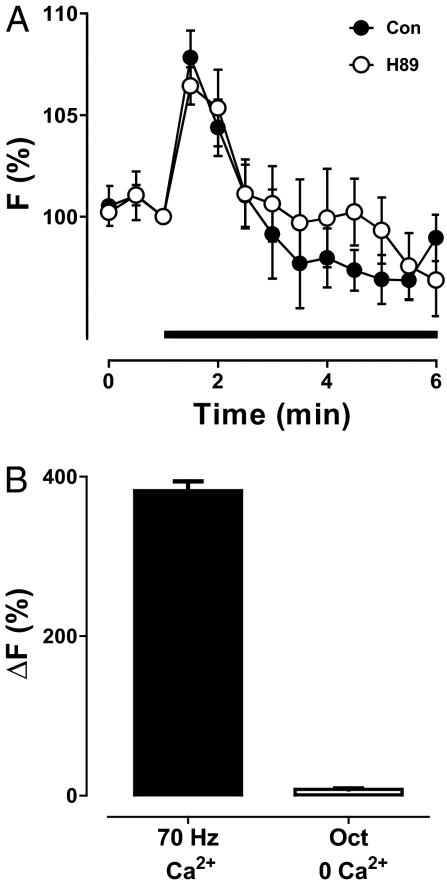
Octopamine induced mobilization of intracellular Ca2+. (A) Time course of GCaMP3 fluorescence (F) without extracellular Ca2+ induced by octopamine (indicated by bar) in the presence (n = 4) and absence (n = 4) of the PKA inhibitor H89. (B) Comparison of the peak changes in Ca2+ (ΔF) induced by octopamine in the absence of extracellular Ca2+ (n = 4) or by 15 s of 70-Hz stimulation in the presence of extracellular Ca2+ (n = 8).
Given that Ca2+ can induce neuropeptide release directly, why is PKA also required for octopamine-induced neuropeptide release? One potential explanation could be that PKA promotes activation of RyRs and IP3Rs, as occurs in muscle (25, 26). If this happened in the nerve terminal, then inhibiting PKA would reduce octopamine-induced Ca2+ mobilization. However, in contrast to neuropeptide release (Fig. 3B), the octopamine Ca2+ response was unaffected by H89 (Fig. 5A). Hence, ER Ca2+ mobilization was independent of PKA. The basis of the PKA requirement became evident, however, with quantitative consideration of the Ca2+ data. First, the octopamine Ca2+ response, although detectable, was minuscule compared with electrical activity in the presence of extracellular Ca2+ (Fig. 5B). Second, the octopamine-induced increase in Ca2+ was brief compared with evoked neuropeptide release (compare Figs. 3B and 4A to 5A). Apparently, the extent and duration of octopamine-induced Ca2+ mobilization are not sufficient, suggesting that synergistic signaling with PKA is required to evoke robust neuropeptide release in the absence of Ca2+ entry into the nerve terminal.
Discussion
Neuromodulators regulate synaptic release induced by Ca2+ entry into the nerve terminal. However, here a monoamine was found to evoke neuropeptide release without Ca2+ entry into the terminal. Given that neuropeptides and other LDCV cargoes, such as neurotrophins, activate postsynaptic signaling, neuropeptide release induced by signaling instead of activity may serve to alter postsynaptic function. It is also possible that the effect of cAMP on synaptic development (27) may be caused in part by neuromodulator-induced synaptic release of neuropeptides and other bioactive proteins packaged in LDCVs. Furthermore, the induction of synaptic neuropeptide release in the absence of activity-dependent Ca2+ entry at central synapses could be important in regulation of neuropeptide-dependent insect behavior by octopamine (5, 14, 15). Indeed, given the importance of monoamines and neuropeptides in the control of mood and behavior in mammals, a similar mechanism could operate in the brain.
Synaptic neuropeptide release induction by a neuromodulator is reminiscent of presynaptic facilitation of spontaneous quantal release of classical transmitters, which also occurs without Ca2+ entry. However, quantal “minis”, which are produced by rare exocytosis of individual small synaptic vesicles, are typically insufficient for conventional neurotransmission (1). Nevertheless, it is possible that the same mechanism is responsible for neuromodulator-induced synaptic neuropeptide release and spontaneous minis, but with LDCVs being more sensitive to presynaptic signaling than small synaptic vesicles. According to this reasoning, there may be significant release of neuropeptide cotransmitters at mammalian synapses with known presynaptic facilitation of spontaneous minis. Such unconventional peptidergic transmission, which could now be revealed with GFP imaging, may have been undetected because neuropeptides do not typically evoke obvious postsynaptic electrophysiological responses.
It is surprising that a small, transient ER Ca2+ response is required for sustained octopamine-induced neuropeptide release. Of course, bulk cytoplasmic Ca2+ measurements may not accurately reflect local Ca2+ levels at sites of exocytosis. However, an alternative explanation is that Ca2+ from the ER does not directly evoke exocytosis, but rather promotes PKA action. For example, such a synergistic effect could occur on a PKA substrate that is phosphorylated more efficiently after binding Ca2+. In this case, the nonphysiological direct adenylyl cyclase activator FSK could on its own induce substrate phosphorylation, but the native neuromodulator, which acts via receptors and G proteins to more weakly activate adenylyl cyclase, could require synergistic activation of ER Ca2+ channels and PKA to produce a significant effect.
Genetic and pharmacological experiments established that PKA plays a much larger role in the octopamine effect than epac, even though either cAMP effector can induce Ca2+ entry-independent synaptic neuropeptide release. Because the cAMP affinities of epac and PKA are similar (28), the prominence of PKA may reflect that phosphorylation can be long lasting compared with more transient interactions of epac with its targets. Alternatively, PKA may be positioned to interact more efficiently with adenylyl cyclase, neuropeptide-containing LDCVs, or sites of neuropeptide release. Accordingly, it will be interesting to determine whether epac is significant in other presynaptic cAMP effects, including regulating conventional Ca2+-dependent neurotransmission and neuropeptide release induced by activity.
Materials and Methods
Unless otherwise indicated, Drosophila melanogaster third instar larvae were filleted and imaged in Ca2+-free HL3 in which 1.5 mM Ca2+ was substituted with 0.5 mM EGTA, a Ca2+ chelator (70 mM NaCl, 5 mM KCl, 0.5 mM Na3EGTA, 20 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose, 115 mM sucrose, 5 mM sodium Hepes, pH 7.2). AnfGFP fluorescence was detected with wide-field epifluorescence microscopy in muscle 6 and 7 type Ib boutons of elav-GAL4 UAS-AnfGFP flies using an Olympus BX microscope equipped with a 60× 1.1 NA water-immersion objective, fluorescein filters, and a Hamamatsu digital-cooled CCD camera, as described previously (13, 19). The same optics were used to measure Ca2+ in flies generated by crossing Ok6-GAL4 with UAS-GCaMP3 (24), which were kindly provided by Loren Looger (Janelia Farm, Ashburn, VA). Epac function was disrupted by generating elav-GAL4 UAS-AnfGFP flies that were homozygous for a P-element insertion in the gene (allele EpacKG00434; Bloomington stock 13663). Single crosses were performed with elav-GAL4 UAS-AnfGFP flies and RNAi lines targeting the RyR (VDRC 109631) and the IP3R (Bloomington stock 25937). Similarly, presynaptic expression of the dominant-negative PKA subunit was accomplished with single crosses of BDK22 and BDK35 flies (20), which were kindly provided by Daniel Kalderon (Columbia University, New York, NY), to flies expressing AnfGFP driven by elav-Gal4 or Geneswitch 3550–2 with RU486 feeding (28, 29). Nerve stimulation was performed in Ca2+-free HL3 or Ca2+-containing HL3, as described previously (13, 19). Inhibitors of signaling, such as H89, Tg, Xesto, and Ryan, were applied for 15 to 25 min before application of octopamine or FSK. SERCA was also inactivated by heating Kum170 larvae for 8 min at 40 °C. Signaling chemicals were purchased from Biolog Life Sciences, Axxora, and Sigma.
Release was quantified as the percent-change in AnfGFP fluorescence, with a positive number indicating a decrease in AnfGFP content indicative of release [i.e., 100*(Fo − F)/Fo, where Fo was the initial fluorescence and F was the fluorescence after stimulation]. Pseudocolor scales are presented in arbitrary units, with warm colors indicating greater content. Thus, release is shown as a shift to cooler colors. Statistical significance was determined with Student's t test for two experimental groups and ANOVA and the Bonferroni posttest for more experimental groups.
Acknowledgments
We thank Dr. Daniel Kalderon for the BDK22 and BDK35 fly lines, Dr. Loren Looger for the GCaMP3 flies, and Man Yan Wong for assistance with Geneswitch experiments. This research was supported by National Institutes of Health Grant NS32385 (to E.S.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Zucker RS. Minis: Whence and wherefore? Neuron. 2005;45:482–484. doi: 10.1016/j.neuron.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Stenovec M, Kreft M, Poberaj I, Betz WJ, Zorec R. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. FASEB J. 2004;18:1270–1272. doi: 10.1096/fj.03-1397fje. [DOI] [PubMed] [Google Scholar]
- 3.Barg S, et al. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron. 2002;33:287–299. doi: 10.1016/s0896-6273(02)00563-9. [DOI] [PubMed] [Google Scholar]
- 4.Evans PD, Maqueira B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- 5.Roeder T. Tyramine and octopamine: Ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 6.Monastirioti M, et al. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa K, Kidokoro Y. Octopamine inhibits synaptic transmission at the larval neuromuscular junction in Drosophila melanogaster. Brain Res. 1999;837:67–74. doi: 10.1016/s0006-8993(99)01676-5. [DOI] [PubMed] [Google Scholar]
- 8.Hou D, et al. Presynaptic impairment of synaptic transmission in Drosophila embryos lacking Gs(alpha) J Neurosci. 2003;23:5897–5905. doi: 10.1523/JNEUROSCI.23-13-05897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson MS, Halpern ME, Keshishian H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: Characterization of muscle fiber-specific neuromuscular endings. J Neurosci. 1988;8:242–255. doi: 10.1523/JNEUROSCI.08-01-00242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorczyca M, Augart C, Budnik V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J Neurosci. 1993;13:3692–3704. doi: 10.1523/JNEUROSCI.13-09-03692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong Y, Peña LA. A novel synaptic transmission mediated by a PACAP-like neuropeptide in Drosophila. Neuron. 1995;14:527–536. doi: 10.1016/0896-6273(95)90309-7. [DOI] [PubMed] [Google Scholar]
- 12.Loveall BJ, Deitcher DL. The essential role of bursicon during Drosophila development. BMC Dev Biol. 2010;10:92. doi: 10.1186/1471-213X-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci. 2005;8:173–178. doi: 10.1038/nn1377. [DOI] [PubMed] [Google Scholar]
- 14.Kula-Eversole E, et al. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc Natl Acad Sci USA. 2010;107:13497–13502. doi: 10.1073/pnas.1002081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao S, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- 17.Husain QM, Ewer J. Use of targetable gfp-tagged neuropeptide for visualizing neuropeptide release following execution of a behavior. J Neurobiol. 2004;59:181–191. doi: 10.1002/neu.10309. [DOI] [PubMed] [Google Scholar]
- 18.Kula E, Levitan ES, Pyza E, Rosbash M. PDF cycling in the dorsal protocerebrum of the Drosophila brain is not necessary for circadian clock function. J Biol Rhythms. 2006;21:104–117. doi: 10.1177/0748730405285715. [DOI] [PubMed] [Google Scholar]
- 19.Levitan ES, Lanni F, Shakiryanova D. In vivo imaging of vesicle motion and release at the Drosophila neuromuscular junction. Nat Protoc. 2007;2:1117–1125. doi: 10.1038/nprot.2007.142. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Ohlmeyer JT, Lane ME, Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80:553–562. doi: 10.1016/0092-8674(95)90509-x. [DOI] [PubMed] [Google Scholar]
- 21.Shakiryanova D, et al. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci. 2007;27:7799–7806. doi: 10.1523/JNEUROSCI.1879-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanyal S, et al. Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics. 2005;169:737–750. doi: 10.1534/genetics.104.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vázquez-Martínez O, Cañedo-Merino R, Díaz-Muñoz M, Riesgo-Escovar JR. Biochemical characterization, distribution and phylogenetic analysis of Drosophila melanogaster ryanodine and IP3 receptors, and thapsigargin-sensitive Ca2+ ATPase. J Cell Sci. 2003;116:2483–2494. doi: 10.1242/jcs.00455. [DOI] [PubMed] [Google Scholar]
- 24.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 26.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 27.Zhong Y, Budnik V, Wu CF. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: Role of cAMP cascade. J Neurosci. 1992;12:644–651. doi: 10.1523/JNEUROSCI.12-02-00644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dao KK, et al. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem. 2006;281:21500–21511. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson L, et al. Spatial and temporal control of gene expression in Drosophila using the inducible GeneSwitch GAL4 system. I. Screen for larval nervous system drivers. Genetics. 2008;178:215–234. doi: 10.1534/genetics.107.081968. [DOI] [PMC free article] [PubMed] [Google Scholar]