Abstract
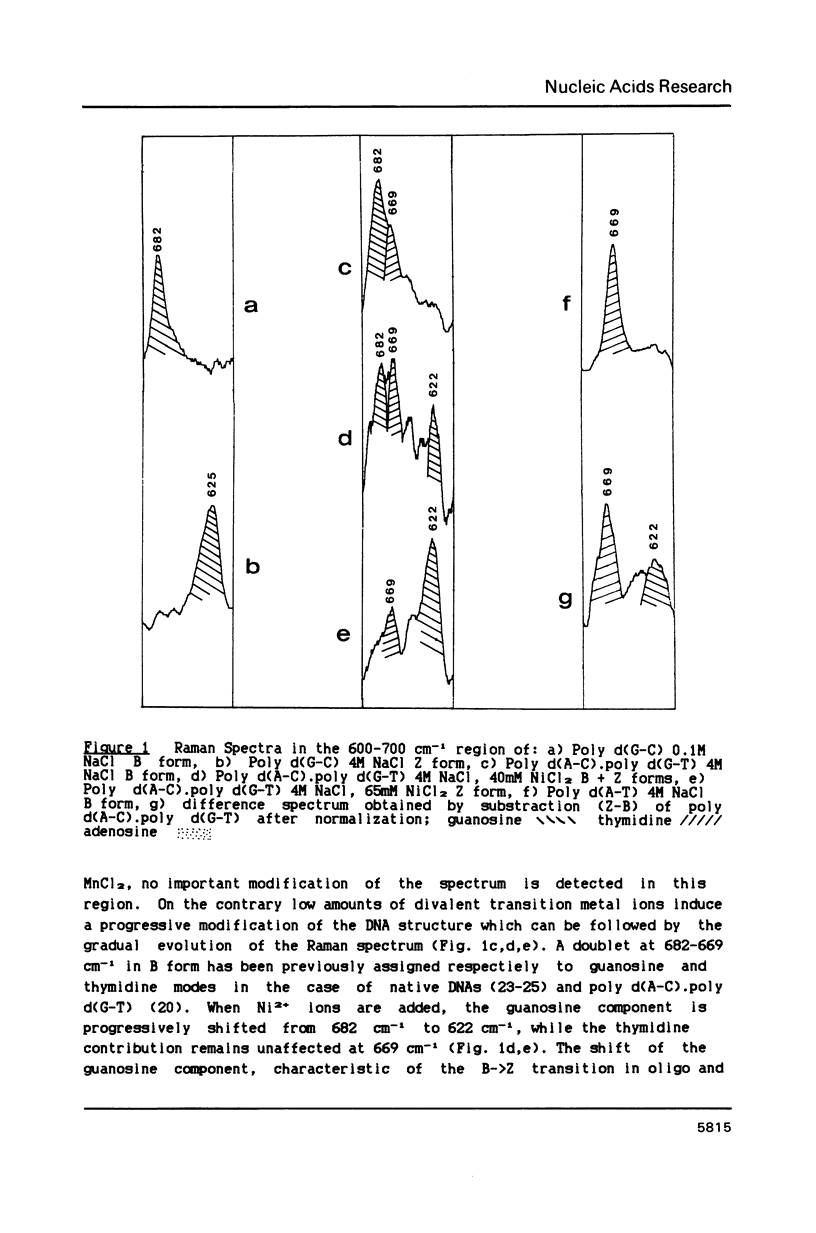
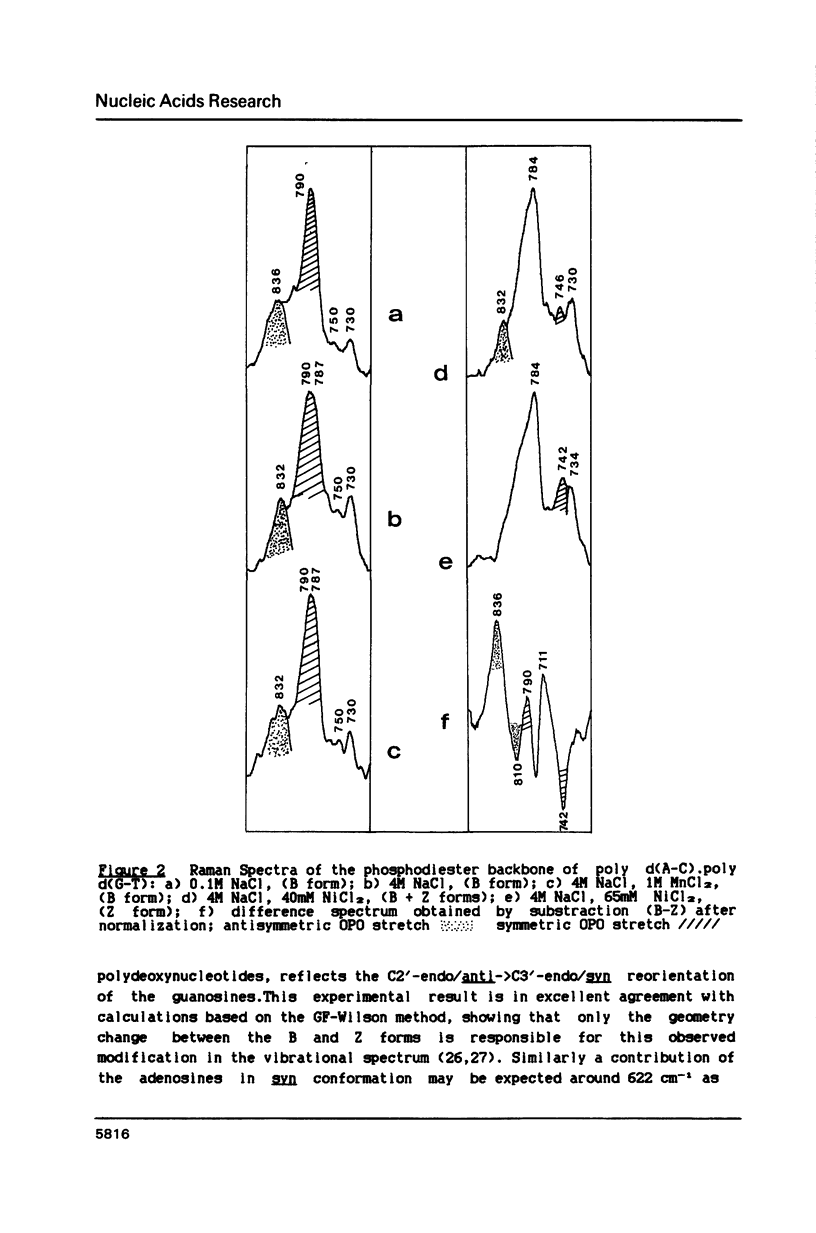
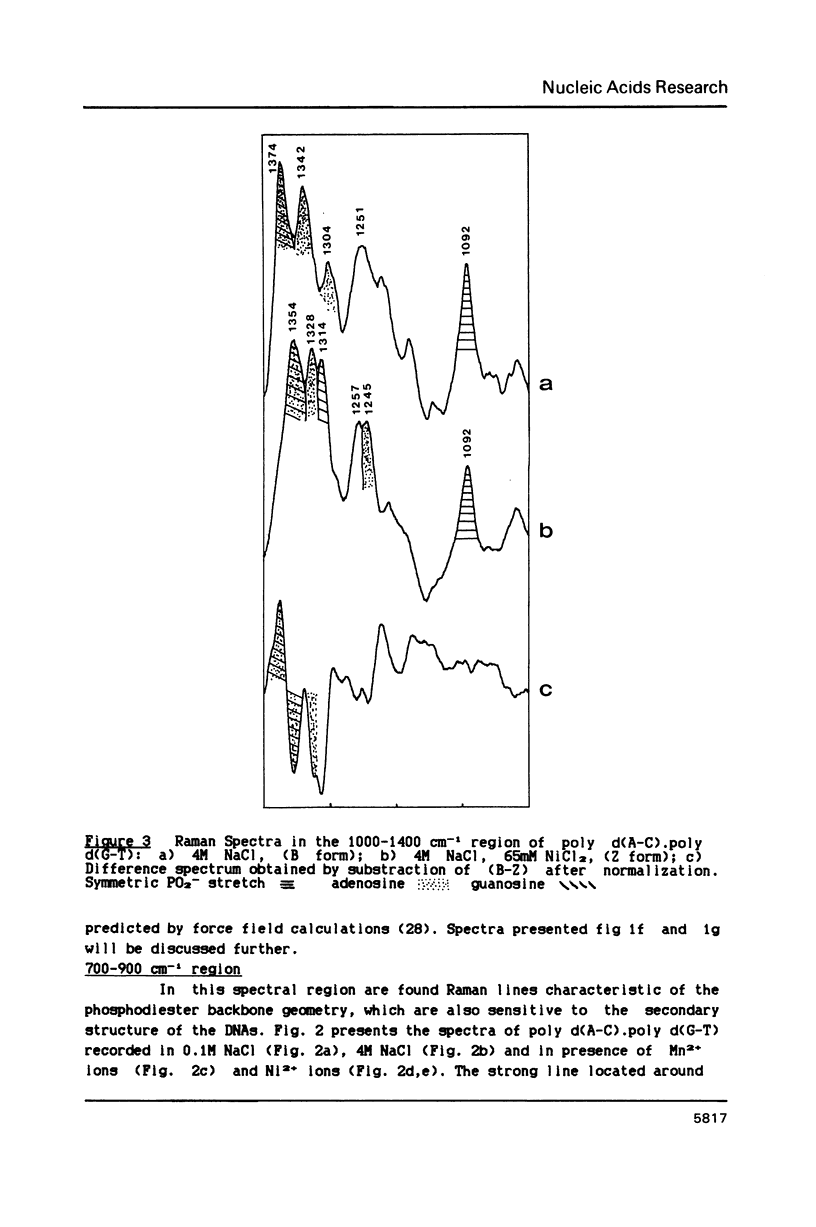
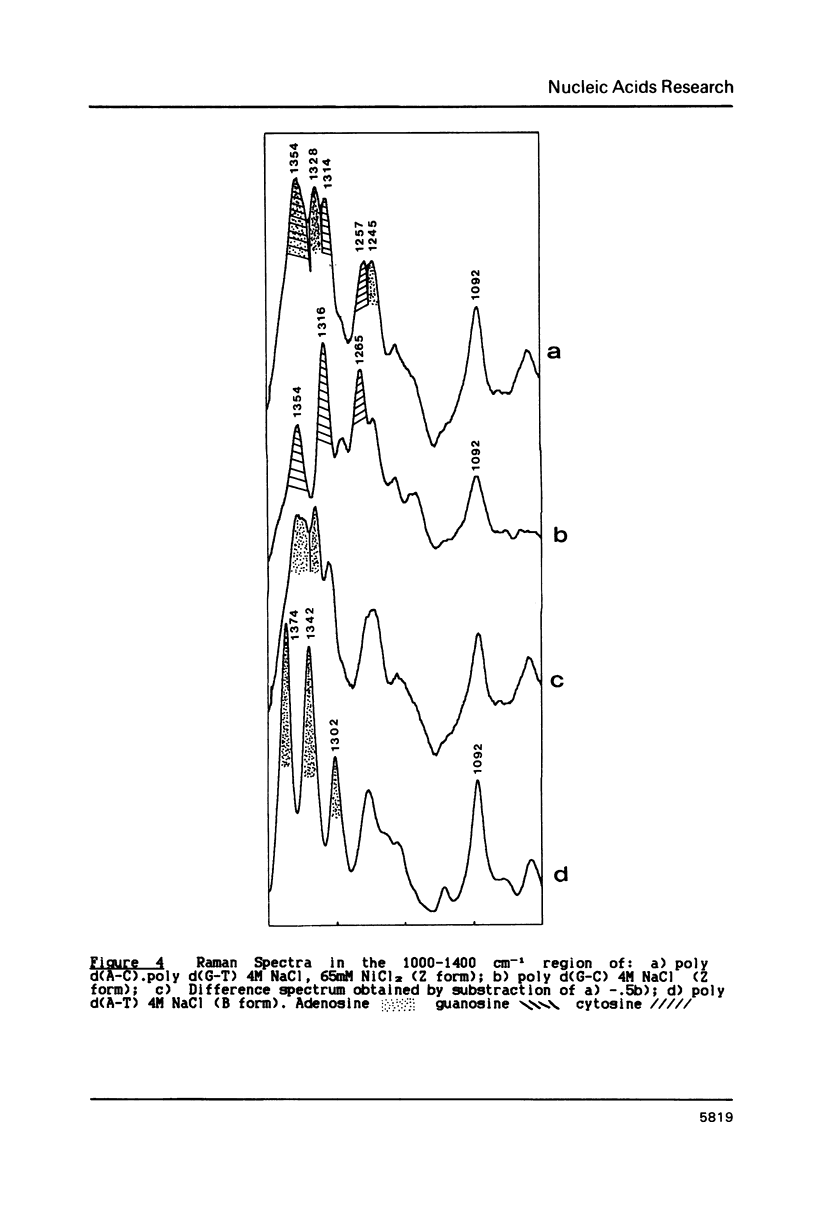
Poly d(A-C).poly d(G-T) structures have been studied in solution by Raman spectroscopy, in presence of Na+, Mn2+ and Ni2+ counterions. Increase of the Na+ concentration or addition of Mn2+ ions up to 1M MnCl2 does not modify the B geometry of the polynucleotide. On the contrary, in conditions of low water activity (4M NaCl), the presence of small amounts of nickel ions (65 mM) induces a left-handed geometry of the DNA. The shift of the guanine line located at 682 cm-1 in B form to 622 cm-1 reflects unambiguously the C2'-endo/anti-greater than C3'-endo/syn reorientation of the deoxyribose-purine entities. Moreover modifications in the phosphate backbone lines indicate that the polymer is in a Z conformation. New or displaced lines corresponding to adenosine vibrations are correlated with the left-handed structure. An interaction of the Ni2+ ions specifically with the N7 site of purines, combined with a low water activity is necessary to promote the B-greater than Z transition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S., Bourtayre P., Liquier J., Taillandier E. Interaction of transition metal ions with Z form poly d(A-C).poly d(G-T) and poly d(A-T) studied by I.R. spectroscopy. Nucleic Acids Res. 1986 Apr 25;14(8):3501–3513. doi: 10.1093/nar/14.8.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam S., Liquier J., Taboury J. A., Taillandier E. Right- and left-handed helixes of poly[d(A-T)].poly[d(A-T)] investigated by infrared spectroscopy. Biochemistry. 1986 Jun 3;25(11):3220–3225. doi: 10.1021/bi00359a021. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides J. M., Thomas G. J., Jr Characterization of DNA structures by Raman spectroscopy: high-salt and low-salt forms of double helical poly(dG-dC) in H2O and D2O solutions and application to B, Z and A-DNA. Nucleic Acids Res. 1983 Aug 25;11(16):5747–5761. doi: 10.1093/nar/11.16.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides J. M., Wang A. H., van der Marel G. A., van Boom J. H., Rich A., Thomas G. J., Jr The Raman spectra of left-handed DNA oligomers incorporating adenine-thymine base pairs+. Nucleic Acids Res. 1984 Jul 25;12(14):5913–5925. doi: 10.1093/nar/12.14.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms S., Vergne J., Brahms J. G., Di Capua E., Bucher P., Koller T. Natural DNA sequences can form left-handed helices in low salt solution under conditions of topological constraint. J Mol Biol. 1982 Dec 5;162(2):473–493. doi: 10.1016/0022-2836(82)90539-3. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Kiser E. J., Peticolas W. L. Determination of the backbone structure of nucleic acids and nucleic acid oligomers by laser Raman scattering. Proc Natl Acad Sci U S A. 1972 Apr;69(4):938–941. doi: 10.1073/pnas.69.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Starr P. A., Spiro T. G. Raman spectroscopic elucidation of DNA backbone conformations for poly(dG-dT).poly(dA-dC) and poly(dA-dT).poly(dA-dT) in CsF solution. Biopolymers. 1985 Aug;24(8):1493–1500. doi: 10.1002/bip.360240806. [DOI] [PubMed] [Google Scholar]
- Ghomi M., Taboury J. A., Taillandier E. Experimental and calculated study of the vibrational modes of poly(dG-dC).poly(dG-dC) in B and Z conformations. Biochimie. 1984 Feb;66(2):87–92. doi: 10.1016/0300-9084(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Ho P. S., Frederick C. A., Saal D., Wang A. H., Rich A. The interactions of ruthenium hexaammine with Z-DNA: crystal structure of a Ru(NH3)6+3 salt of d(CGCGCG) at 1.2 A resolution. J Biomol Struct Dyn. 1987 Feb;4(4):521–534. doi: 10.1080/07391102.1987.10507657. [DOI] [PubMed] [Google Scholar]
- Letellier R., Ghomi M., Taillandier E. Interpretation of DNA vibration modes. II--The adenosine and thymidine residues involved in oligonucleotides and polynucleotides. J Biomol Struct Dyn. 1987 Feb;4(4):663–683. doi: 10.1080/07391102.1987.10507667. [DOI] [PubMed] [Google Scholar]
- Letellier R., Ghomi M., Taillandier E. Interpretation of DNA vibration modes: I--The guanosine and cytidine residues involved in poly(dG-dC).poly(dG-dC) and d(CG)3.d(CG)3. J Biomol Struct Dyn. 1986 Feb;3(4):671–687. doi: 10.1080/07391102.1986.10508455. [DOI] [PubMed] [Google Scholar]
- Martin J. C., Wartell R. M. Changes in raman vibrational bands of calf thymus DNA during the B-to-A transition. Biopolymers. 1982 Mar;21(3):499–512. doi: 10.1002/bip.360210303. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Tsuboi M., Sato T. Structure-spectrum correlations in nucleic acids. I. Raman lines in the 600-700 cm-1 range of guanosine residue. Nucleic Acids Res. 1984 Sep 11;12(17):6901–6908. doi: 10.1093/nar/12.17.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Prescott B., Steinmetz W., Thomas G. J., Jr Characterization of DNA structures by laser Raman spectroscopy. Biopolymers. 1984 Feb;23(2):235–256. doi: 10.1002/bip.360230206. [DOI] [PubMed] [Google Scholar]
- Soumpasis D. M., Wiechen J., Jovin T. M. Relative stabilities and transitions of DNA conformations in 1:1 electrolytes: a theoretical study. J Biomol Struct Dyn. 1987 Feb;4(4):535–552. doi: 10.1080/07391102.1987.10507658. [DOI] [PubMed] [Google Scholar]
- Taboury J. A., Adam S., Taillandier E., Neumann J. M., Tran-Dinh S., Huynh-Dinh T., Langlois d'Estaintot B., Conti M., Igolen J. The B----Z transition in two synthetic oligonucleotides: d(C-2-amino-ACGTG) and d(m5CGCAm5CGTGCG) studied by IR, NMR and CD spectroscopies. Nucleic Acids Res. 1984 Aug 10;12(15):6291–6305. doi: 10.1093/nar/12.15.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboury J. A., Taillandier E. Right-handed and left-handed helices of poly(dA-dC) X (dG-dT). Nucleic Acids Res. 1985 Jun 25;13(12):4469–4483. doi: 10.1093/nar/13.12.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier E., Taboury J. A., Adam S., Liquier J. Left-handed helical structure of poly[d(A-C)].poly[d(G-T)] studied by infrared spectroscopy. Biochemistry. 1984 Nov 20;23(24):5703–5706. doi: 10.1021/bi00319a007. [DOI] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. A., Peticolas W. L. Sequence dependence of conformations of self-complementary duplex tetradeoxynucleotides containing cytosine and guanine. Biochemistry. 1984 Jul 3;23(14):3202–3207. doi: 10.1021/bi00309a014. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Gessner R. V., van der Marel G. A., van Boom J. H., Rich A. Crystal structure of Z-DNA without an alternating purine-pyrimidine sequence. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3611–3615. doi: 10.1073/pnas.82.11.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Hakoshima T., van der Marel G., van Boom J. H., Rich A. AT base pairs are less stable than GC base pairs in Z-DNA: the crystal structure of d(m5CGTAm5CG). Cell. 1984 May;37(1):321–331. doi: 10.1016/0092-8674(84)90328-3. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Harrell J. T. Characteristics and variations of B-type DNA conformations in solution: a quantitative analysis of Raman band intensities of eight DNAs. Biochemistry. 1986 May 6;25(9):2664–2671. doi: 10.1021/bi00357a056. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]
- Woisard A., Fazakerley G. V. Ultrapolymorphic DNA: B, A, Z, and Z* conformations of poly(dA-dC).poly(dG-dT). Biochemistry. 1986 May 6;25(9):2672–2676. doi: 10.1021/bi00357a057. [DOI] [PubMed] [Google Scholar]


