Abstract
The study of the congenitally blind (CB) represents a unique opportunity to explore experience-dependant plasticity in a sensory region deprived of its natural inputs since birth. Although several studies have shown occipital regions of CB to be involved in nonvisual processing, whether the functional organization of the visual cortex observed in sighted individuals (SI) is maintained in the rewired occipital regions of the blind has only been recently investigated. In the present functional MRI study, we compared the brain activity of CB and SI processing either the spatial or the pitch properties of sounds carrying information in both domains (i.e., the same sounds were used in both tasks), using an adaptive procedure specifically designed to adjust for performance level. In addition to showing a substantial recruitment of the occipital cortex for sound processing in CB, we also demonstrate that auditory–spatial processing mainly recruits the right cuneus and the right middle occipital gyrus, two regions of the dorsal occipital stream known to be involved in visuospatial/motion processing in SI. Moreover, functional connectivity analyses revealed that these reorganized occipital regions are part of an extensive brain network including regions known to underlie audiovisual spatial abilities (i.e., intraparietal sulcus, superior frontal gyrus). We conclude that some regions of the right dorsal occipital stream do not require visual experience to develop a specialization for the processing of spatial information and to be functionally integrated in a preexisting brain network dedicated to this ability.
Keywords: blindness, cross-modal plasticity, ventral-dorsal auditory streams, modularity
When the brain is deprived of its natural sensory inputs, it can rewire itself, showing an impressive range of plastic changes (1). Early visual deprivation thus provides an exceptional model to explore the role of sensory experience in shaping the functional architecture of the brain. Based on a number of studies comparing brain activity of congenitally blind (CB) and sighted individuals (SI), the current prevailing view is that visual deafferentation results in a reliable recruitment of the occipital cortex for nonvisual sensory processing to compensate for the challenging condition that is visual deprivation (2).
Although such findings highlight the brain's remarkable ability to rewire its components, questions remain about the functional organization of the occipital cortex in CB. An important characteristic of the visual cortex in SI is domain specialization wherein specific functional activity has been found in anatomically identifiable regions (3, 4). Our main question was, therefore: does the occipital cortex of CB process the colonizing nonvisual stimuli in a global manner or does it do so using some functional modularity similar to what is observed in SI, with precise regions involved in specific cognitive functions?
Several studies have reported that the occipital cortex of CB responds quite indifferently to a variety of cognitive tasks, suggesting that some common factors (i.e., attentional) rather than specific cognitive processes may contribute to the unselective occipital activity observed in this population (5–8). In contrast, other studies do suggest that distinct regions of the visually deprived occipital cortex may show functional specialization that is to some extent comparable to what is known in SI (9). In SI, visual information is thought to be processed along two distinct (but interacting) pathways: (i) a ventral stream flowing from the primary visual cortex to the infero-temporal cortex and involved in the analysis of object properties (“what” pathway), and (ii) an occipito-parietal stream devoted to the analysis of the spatial relationship between objects (“where” pathway) (10, 11). Alternatively, another perspective on dorsal and ventral visual processing streams rather considers them to be involved in the control of object-directed actions and object recognition, respectively (12). Interestingly, recent studies in CB have reported task-specific responses in ventral (13–16) and dorsal (17–22) occipital streams in response to “what” and “where” nonvisual processing. For example, Renier et al. (22) have recently found that the middle occipital gyrus (MOG) is preferentially activated by the spatial processing of nonvisual inputs.
In the present study, we used functional MRI (fMRI) to measure brain responses of CB and SI when they processed either the identity (pitch) or the spatial (position in azimuth) attributes of exactly the same sounds; two core auditory abilities that allow us to make sense of the acoustic environment. This allowed for equal sensory input in both tasks, with only the instructions differing between the two. Additionally, using a psychophysical staircase paradigm, we ensured a level of complexity that was similar across tasks and subjects. This paradigm allowed us to precisely investigate whether specific processes map onto specialized subregions of the occipital cortex in CB and whether these regions maintain a modular organization similar to what is observed in SI.
Results
Behavioral results are presented in SI Text and show no differences between the groups.
Ventral–Dorsal Auditory Streams.
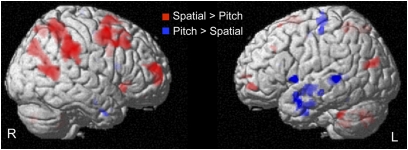
We first tested whether our paradigm allowed us to observe a dissociation between a ventral and a dorsal network for the pitch and spatial processing of sounds, respectively, as previously suggested (23, 24). A conjunction analysis (investigating what is jointly activated in both groups) revealed that the spatial processing of sounds, compared with pitch processing, elicited significantly larger brain responses in a right-sided dorsal network (including the superior frontal gyrus, the middle frontal gyrus, the inferior and superior parietal lobule, and the middle occipito-temporal gyrus; Fig. 1 and Table S1). The processing of the pitch attributes of sounds, compared with spatial processing, revealed a widespread network of temporal areas mainly localized in the left hemisphere (including inferior, middle, and superior temporal gyri and inferior frontal gyrus/insula; Fig. 1 and Table S1).
Fig. 1.
Ventral–dorsal auditory streams. Activations obtained in a conjunction analysis characterizing brain areas jointly activated in both groups (SI and CB) in the [Pitch > Spatial] (blue) and in the [Spatial > Pitch] (red) contrasts are overlaid at Puncorrected < 0.001 on a 3D render.
Cross-modal Plasticity in the Occipital Cortex of CB Individuals.
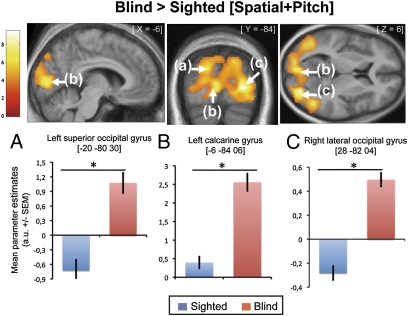
To investigate the effect of congenital blindness on the global processing of sounds, we compared the cerebral responses of blind vs. sighted participants for both tasks combined ([CB > SI] × [Spatial + Pitch]). This analysis revealed substantial activity in most of the occipital cortex in CB compared with SI (Fig. 2 and Table 1). The significance of these neuroplastic changes is supported by our calculation of the posterior probability map (25), as inferred by Bayesian statistics, showing that the probability of activation of these regions in the [Spatial + Pitch] contrast is very low in the sighted group (left MOG: 0; calcarine gyrus: 21%; right MOG: 0).
Fig. 2.
Auditory cross-modal plasticity in the blind. Upper: Activations obtained from contrasts testing the main effects of group independently of condition [Blind > Sighted] × [Spatial + Pitch]. Functional data are displayed (Puncorrected < 0.001) over a horizontal, a coronal, and a sagittal section of the mean structural image of all subjects normalized to the same stereotactic space. Lower: Mean activity estimates (arbitrary unit ± SEM) associated with sound processing (Spatial + Pitch) in the sighted (blue) and blind (red) groups for the three main activity peaks obtained with this contrast.
Table 1.
Functional results summarizing the main effect of group
| Area | Cluster size | x (mm) | y (mm) | z (mm) | Z | P |
| Group effect [Blind > Sighted] × [Spatial + Pitch] | ||||||
| Right lateral occipital gyrus | 9,289 | 28 | −82 | 4 | 5.79 | 0.000* |
| Left calcarine gyrus | — | −6 | −84 | 6 | 5.02 | 0.010* |
| Left superior occipital gyrus | — | −20 | −80 | 30 | 4.80 | 0.024* |
| Group × task interaction [Blind > Sighted] × [Spatial > Pitch] | ||||||
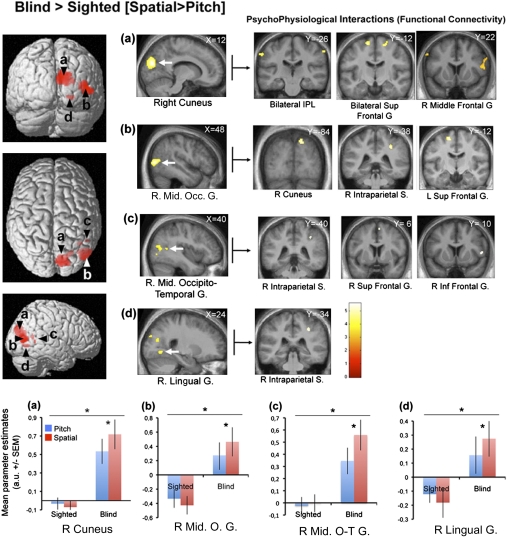
| Right cuneus† (≈hV3/V3A) | 740 | 12 | −80 | 22 | 4.28 | 0.001 |
| Right superior occipital gyrus†‡ (≈hV3/V3A) | — | 24 | −70 | 20 | 3.41 | 0.001 |
| Right middle occipital gyrus†‡ (≈hMT+/V5) | 390 | 48 | −76 | 6 | 4.20 | 0.001 |
| Right lingual gyrus§ | 538 | 24 | −48 | −8 | 3.74 | 0.011 |
| Right lingual gyrus†‡ | — | 24 | −60 | −4 | 3.49 | 0.014 |
| Right middle occipito-temporal gyrus†‡ (≈hMT+/V5) | 15 | 40 | −56 | 12 | 3.27 | 0.018 |
| Group-by-task interaction [Blind > Sighted] × [Pitch > Spatial] | ||||||
| No significant responses | ||||||
Brain activations significant after correction over the entire volume (*) or over small volume of interest (small-volume correction).
†Cluster not affected by an inclusive mask (P = 0.001) of the [Blind] × [Spatial > Pitch] contrast, indicating that the [Blind > Sighted] × [Spatial > Pitch] interaction effect was driven selectively by differences observed in blind subjects.
‡Cluster not affected by an exclusive mask (P = 0.05) of the [Sighted] × [Spatial > Pitch] contrast, further indicating that the reported interaction effect was driven by differences observed in the blind subjects.
§Clusters not surviving an inclusive mask (P = 0.001) of the [Blind] × [Spatial > Pitch] contrast, thus not driven selectively by the spatial processing of sounds in blind subjects.
Several studies have emphasized that processing auditory stimuli results in a significant decrease below baseline in the occipital cortex of sighted subjects (cross-modal deactivation) (26). We investigated whether such deactivations occurred in the occipital cortex of our sighted group. Interestingly, deactivation was limited to one cluster in the right MOG (28, −82, 4) of sighted subjects, whereas no such deactivation was observed in the occipital cortex of blind subjects (Fig. S1).
Functional Specialization in the Occipital Cortex of the Congenitally Blind.
The group [Blind > Sighted] × condition [Spatial > Pitch] interaction analysis revealed significant differences in activity in the right cuneus, the right MOG, the right middle occipito-temporal gyrus, and in the right lingual gyrus (Fig. 3 and Table 1). Posterior probability of activation of these regions in SI was low, with the exception of the right middle occipito-temporal region (right cuneus: 7%; right MOG: 22%; right middle occipito-temporal region: 82%; right lingual gyrus: 4%). The group [Blind > Sighted] × condition [Pitch > Spatial] interaction analysis did not reveal any significant results (Table 1).
Fig. 3.
Network for the spatial processing of sounds in CB subjects. (Left) Activations obtained from the contrast testing which regions are specifically dedicated to the spatial processing of sounds in blind subjects: [Blind > Sighted] × [Spatial > Pitch]. Functional data are overlaid (Puncorrected < 0.001) over a 3D render of the brain and over sagittal sections of the mean structural image of all blind subjects normalized to the same stereotactic space. (Lower) Mean parameter estimates (arbitrary unit ± SEM) associated with the processing of pitch (blue) or spatial (red) attributes of sounds in the sighted and the blind groups for the four main activity peaks. (A) The right cuneus. (B) The right middle occipital gyrus. (C) The right middle occipito-temporal gyrus. (D) The right lingual gyrus. Right: Psychophysiological interaction results using the four activity peaks as seed areas.
Functional Connectivity of the Reorganized Occipital Cortex of CB Individuals.
Psychophysiological interaction (PPI) analyses were computed to identify any brain region functionally connected to the reorganized occipital regions involved specifically in the spatial processing of sounds, relative to pitch processing, in CB (Fig. 3 and Table S2). For these analyses, the right cuneus (12, −80, 22 mm), the right MOG (48, −76, 6 mm), the right middle occipito-temporal gyrus (40, −56, 12 mm), and the right lingual gyrus (24, −48, −8 mm) were selected as seed regions (these regions were found to be preferentially active in the interaction analysis [CB > SI] × [Spatial > Pitch]). We observed significant functional connectivity between the right cuneus and bilateral inferior parietal lobules, bilateral superior frontal gyri and the right middle frontal gyri. We further observed significant coupling between the right MOG and the right cuneus (this region was already observed in the contrast [CB > SI] × [Spatial > Pitch]), the right inferior parietal lobule, the left superior frontal gyrus, and the right cerebellum. We also observed that the right middle occipito-temporal gyrus was functionally connected to the right supramarginal gyrus, the right superior, and the inferior frontal gyrus. Finally, the right lingual gyrus was connected to the right inferior parietal lobule. All of the PPI analyses described above revealed stronger connectivity in CB between the seed areas and the reported brain areas for the spatial processing of sounds, compared with pitch processing. Importantly, all of the clusters reported above are not affected by an exclusion mask (P = 0.05) of the PPI carried out in the sighted subjects with the same seed areas, indicating that the reported regions present a pattern of functional connectivity that is specific to the CB.
Discussion
By contrasting BOLD signals recorded when SI and CB selectively attended to “pitch” or “spatial” attributes of sounds, we identified specific right-sided occipital subregions in CB that were preferentially activated for the spatial processing of sounds. Such a result suggests that the reorganized “visual” cortex of CB should no longer be considered as an undifferentiated structure but should rather be divided into different anatomical areas devoted to specific cognitive functions. These findings are compelling because they cannot be attributed to physical properties of the sensory inputs, nor can they be attributed to any performance differences between conditions or between groups.
Remarkably, most of the regions showing functional preference for auditory–spatial processing are regions that are known to show preference for visuospatial processing in sighted subjects (11), suggesting that cross-modal plasticity in CB may be constrained by the innate disposition of a specific cortical area to selectively serve a particular function. Moreover, we further show that these reorganized regions in CB are part of an extended network of higher-order brain regions known to be important in processing the spatial attributes of sensory inputs.
Ventral–Dorsal Auditory Streams.
The existence of separate hierarchical visual pathways for the analysis of object properties (the occipito-temporal “what” stream) and for the analysis of the spatial relationship between objects (the occipito-parietal “where” stream) is arguably one of the most influential theories about the organization of the visual system (9). Similarly, it was later postulated that auditory-cortical processing might follow such a dual principle of organization (23, 24, 27).
The present results confirm that the processing of pitch attributes preferentially maps onto a “ventral-what” stream (here mainly composed of distributed left temporal areas) and that the spatial processing of sounds are more localized within a “dorsal-where” stream (here mainly composed of distributed right parietal and frontal areas) (Fig. 1 and Table S1). One of the strengths of the present study is the direct comparison of brain activity elicited by the pitch and the spatial task in a well-controlled paradigm adjusting in real time the difficulty level of the tasks throughout the experiment, while the physical stimuli were kept identical regardless of the task. Indeed, both tasks share exactly the same sensory stimulation, require the same motor response, share similar working memory or attentional load, and the performance level never differed between them; the only difference resides in the perceptual process at play (either “process pitch” or “process location”).
Functional Specialization in the Occipital Cortex of CB for Spatial Processing.
In accordance with the literature on cross-modal processing after blindness, we demonstrate here a substantial level of activity in the occipital cortex of CB in response to sounds (Fig. 2 and Table 1). Previous studies have suggested that blindness may lead to a general-purpose functional reorganization of the occipital cortex due to the undifferentiated pattern of activity observed during different tasks (5–8). Indeed, the auditory activity we observe in the primary visual cortex of CB seems equal in both tasks, supporting the notion that “early” areas of occipital cortex in the CB (e.g., V1/V2) support more generalized functions (28, 29). However, we also demonstrate that occipital regions involved in auditory spatial discrimination partially differ from those involved in auditory pitch discrimination (Fig. 3).
Spatial hearing in CB is shown here to preferentially map onto specialized subregions mainly located in the right dorsal occipital stream. The two primarily activated regions (Fig. 3 and Table 1) are the right cuneus [in the vicinity of what has been described as dorsal hV3/V3A in SI (30)] and the right MOG [in the vicinity of what has been described as hMT+/V5 in SI (31)]. Because these regions have been extensively documented as subserving visuospatial/motion abilities in SI (11, 32), we suggest that they might maintain their functional role in CB for the processing of a preserved modality, in this case audition. It is also worth noting that these two regions were also reliably active at an individual level (Table S3). Regarding the right cuneus, our results are in agreement with a previous study of Collignon et al. (17) demonstrating that the application of transcranial magnetic stimulation (TMS) over the right superior occipital gyrus (in the vicinity of the right cuneus/superior occipital clusters observed in the present study; see figure 2 in ref. 17) selectively interfered with sound localization abilities in CB, whereas TMS did not interfere with pitch and intensity discriminations in CB and had no effect on any auditory ability in SI. Regarding the right MOG, our results replicate those of Renier et al. (22), who also found this region preferentially active for the processing of spatial over nonspatial nonvisual stimuli in CB. In our study, the identified MOG was located posterior to the meeting point of the ascending limb of the inferior temporal sulcus and the lateral occipital sulcus (region B in Fig. 3), matching the anatomical location of hMT+/V5 in SI (31, 33). Anatomical localization of functional activations based on probabilistic cytoarchitectonic maps as implemented in Anatomy Toolbox (34) and quantifying structure–function correspondences showed that the cluster of interest covered 73% of hMT+/V5 region. Because our spatial task has the potential to induce an apparent motion percept (Materials and Methods), further studies should selectively address whether the MOG could be differentially activated by location and motion processing (either real or apparent).
The lingual gyrus, a primary visual region, was also found to be preferentially activated in CB for the spatial processing of sounds, even if to a lesser extent than the dorsal clusters (Table 1). This finding is not entirely unexpected: Gougoux et al. (35) found a similar region to be active during an auditory–spatial task in an early blind group. In the visual domain, the right lingual gyrus has often been reported to be specifically activated during direction/motion discriminations (32, 36, 37). This region may contribute to more “object-like” processing important for extracting spatial position, as postulated by Gougoux et al. (35), although this remains speculative.
None of the brain clusters showing specific activations in CB for the spatial processing of sounds correlated with the spatial resolution level of CB. This may seem puzzling given that a previous study reported that the degree of activation of several foci in the occipital cortex of CB correlated with sound localization accuracy (35). However, the use of an adaptive staircase method, as was the case here, obviously results in the absence of performance differences between subjects. Consequently, the measure used to correlate with brain occipital activity is the “auditory–spatial threshold,” reflecting the mean interaural level difference (ILD) and interaural time difference (ITD) required to discriminate the left or right target from the centrally presented probe (Materials and Methods). In audition, computation steps for the spatial perception of sounds in azimuth are mainly processed by the superior olivary nuclei in the brainstem (38). We therefore suggest that the activity in the occipital cortex of CB reflects higher-level perceptual functions rather than the extraction of ILD-ITD cues, which possibly explains the absence of correlation between auditory spatial threshold and occipital recruitment.
The fact that no occipital regions showed preferential activations for the processing of pitch in CB is not so surprising in light of the results of a recent study carried out in deaf cats (39). The authors propose that “supramodal” functions, or attributes that are shared across senses, have a greater potential to engage specific cross-modal plasticity mechanisms after the loss of a sensory input. Indeed, pitch processing, which is specific to audition in contrast to the ability to spatially locate information, which is shared by both vision and audition, may have less potential for specialized cross-modal recruitment of occipital regions in the blind.
Functional Connectivity of the Reorganized Occipital Cortex of CB.
We also demonstrate here that the occipital regions preferentially activated for auditory–spatial processing in CB are part of an extended network of brain areas, including multisensory regions (i.e., inferior parietal lobules, intraparietal sulcus, and superior frontal gyrus; Fig. 3) traditionally considered as important for spatial attention and awareness (40). In fact, most of these regions are activated in both groups, as revealed by the conjunction analysis based on the [Spatial > Pitch] contrast (Fig. 1). Interestingly, we found reliable functional connectivity between the three main peaks of activity located in the right dorsal occipital stream and the posterior superior frontal sulcus (Fig. 3), a region known as the frontal eye field and thought to play a role in the control of spatial attention in SI (41). Strikingly, it has been shown that this region is actively engaged during auditory spatial attention in CB (42). Taken together, these results suggest that the reorganized occipital regions in CB are inherently part of the network involved in auditory localization. Indeed, specific connectivity of precise occipital regions (i.e., dorsal) into an extended brain network wired to serve a specific function (i.e., spatial processing) might constrain the cross-modal reorganization in CB to regions with similar functional specificity. Supporting this hypothesis are the results of a diffusion tensor imaging study showing limited changes in the occipito-parieto-frontal white matter tracts of CB subjects relative to SI (43) and also from a recent study demonstrating that the prefrontal cortex shows massive functional connectivity with the hMT+/V5 area in CB (44).
Mechanisms.
These findings raise questions regarding the developmental mechanisms through which auditory inputs massively invade occipital regions in CB. In early life, the brain is sculpting itself on the basis of experience, with some synaptic connections eliminated and others strengthened (45). The human occipital cortex undergoes such changes in synaptic density during its normal development (46). After a peak of development ending approximately at the age of 8 mo, approximately 40% of the synapses of the visual cortex are gradually removed to reach a stable synaptic density at approximately the age of 11 y (45). It has been suggested that the maintenance of normally transient intermodal connections may underlie, at least in part, the plastic changes observed in CB (2, 9, 47). Moreover, recent anatomical studies showed direct connections between auditory and visual cortical regions in adult sighted monkeys (48, 49), suggesting that some intermodal connections might not be pruned in early infancy. In the present study, as previously observed (26), we found clusters of deactivation in the occipital cortex of sighted subjects during sound processing (Fig. S1). Because both activations and deactivations by nonvisual tasks indicate the presence of nonvisual input in the occipital cortex of sighted individuals (22), these observations suggest that connections between auditory and visual cortices (48) may subserve cross-modal inhibition and/or multisensory integration in the early stages of sensory processing in individuals without visual deprivation (50, 51). In the absence of competitive visual input during development, these connections may provide the pathways for occipital processing of auditory inputs after visual deprivation.
Klinge et al. (52) recently used dynamic causal modeling of an fMRI dataset to investigate the effective connectivity underlying auditory activations in the primary visual cortex of CB. They found clear evidence for stronger corticocortical connections from primary auditory cortex to primary visual cortex in the blind compared with sighted controls, whereas their results regarding thalamocortical tracts (from medial geniculate nucleus to V1) were inconsistent. These results suggest that plastic changes in corticocortical connectivity probably play a crucial role in allowing auditory information to elicit the participation of the primary visual cortex of blind individuals.
Conclusion.
The present study sheds light on mechanisms of cross-modal plasticity by demonstrating that domain specialization, wherein specific functional processing is found to involve specific cortical regions (3), seems to be a ubiquitous property of the occipital cortex, even when deprived of its “natural” inputs since birth. In particular, we have shown that the spatial processing of sounds in CB is performed in specific occipital regions overlapping areas well known to process the spatial attributes of visual inputs in SI. These results suggest that the dorsal stream innately designates its computational role for processing space independently of sensory developmental experience. Moreover, because these reorganized regions are part of an extended brain network, the maintenance of their functional specificity may help a colonized area to keep its functional role within a system of multiple cortical regions. We therefore postulate that cross-modal plasticity in CB allows nonvisual processes to find a “neuronal niche” into a set of circuits that perform functions that are sufficiently close to the ones required by the remaining senses (9, 22).
Materials and Methods
Subjects.
Eleven CB [four female, age range 28–56 y (mean ± SD, 36 ± 13 y)] and 11 SI [four female, age range 26–56 y (mean ± SD, 39 ± 11 y)] participated in the study. Both groups were blindfolded throughout the fMRI acquisition and were matched for age, sex, handedness, educational level, and musical experience. None of the blind subjects had ever had functional vision allowing pattern recognition or visually guided behavior, and all were totally blind except for one who had only rudimentary sensitivity for brightness with no pattern vision. In all cases, blindness was attributed to peripheral deficits with no neurological impairment (Table S4). For all subjects, pure-tone detection thresholds at octave frequencies ranging from 250 to 8,000 kHz were within normal limits in both ears. All of the procedures were approved by the research ethic and scientific boards of the Centre for Interdisciplinary Research in Rehabilitation of Greater Montreal and the Quebec Bio-Imaging Network. Experiments were undertaken with the understanding and written consent of each subject.
Task and General Experimental Design.
Subjects were scanned in a single fMRI session using a block design. The run consisted of 30 successive blocks (20.4-s duration each) separated by rest periods ranging from 6 to 12.4 s (median, 7.34 s), during which the subjects had to alternatively process the spatial or the pitch attributes of the sounds. The duration of the rest blocks were jittered between the start of the sampling of brain volume images relative to the start of the task blocks. This method was used to avoid time-locked sampling whereby all brain images would be acquired at the same time points. A short verbal instruction (1,300 ms) was delivered 2 s before the start of each block to instruct participants which task they would have to carry out (spatial or pitch). The starting condition (either spatial or pitch) was counterbalanced across subjects. In the “spatial” condition, participants had to determine whether the second sound of a pair was left- or right-sided compared with a constant central probe sound, regardless of the variation in pitch of these sounds. In the “pitch” condition, participants had to determine whether the second sound of a pair was lower- or higher-pitched compared with the same probe sound, regardless of the position of these sounds. Therefore, in both conditions and irrespective of the instructions given, the probe was a central sound (simulating zero degrees azimuth) of 1,000 Hz with a 150-ms duration (10-ms rise/fall times). The target sounds always appeared 200 ms after the probe and also had a 150-ms duration (10-ms rise/fall times). It is worth noting that, in the spatial blocs, the presentation in rapid succession of two spatially separate auditory stimuli can induce the perception of auditory movement, a phenomenon called “apparent motion” (53). Each pair of sounds was separated by a 1,200-ms response period. Each block, either spatial or pitch, consisted of 12 successive pairs of sounds (Fig. S2). The same response buttons (right index and right major) were used in both two-alternative forced choice tasks.
The difficulty level of both tasks was controlled throughout the scan by adjusting the gap between the probe and the target using a dynamic psychophysical staircase procedure (one-down for correct response/six-up for wrong response), with the subject performance converging at ≈90% correct. Given the age heterogeneity of the participants and the age-related decline in spatial or pitch acuity, the staircase procedure adjusted the pairings to generate equal task difficulty for all subjects. Moreover, the target locations in a new pitch block were determined by the locations obtained in the preceding spatial block, and vice versa (e.g., pitch in spatial task), so that the same sounds were included in both conditions. This methodology ensures that when contrasting the two tasks, no effect can be attributable to the difference in difficulty level or to difference between physical attributes of the stimuli between the two tasks.
The experimental run was preceded by a short sound calibration run, during which the volume level was adjusted for each subject so as to ensure optimal auditory perception during scanning. The task was coded using Cogent2000v1.24 (http://www.vislab.ucl.ac.uk/cogent.php) implemented in MATLAB (Mathworks), and the auditory stimuli were delivered by means of circumaural, fMRI-compatible headphones (MR Confon).
All auditory stimuli were created using Audition 2.0 (Adobe Systems). A matrix of 6,400 sounds using 40 left and right “spatial gaps” (created by jointly varying steps of 0.2% ILD with steps of 20-μ ITD from the probe sound; two primary cues for sound localization in azimuth) and 40 high and low “pitch gaps” (created using steps of 5 cents from the probe sound). When using the term ‘‘spatial processing of sound’’ in this experiment, we refer to the ability to lateralize sounds perceived along a line joining the two ears (51).
Before the fMRI acquisition, all participants underwent a 30-min training session in a mock scanner, with recorded scanner noise played in the bore of the simulator to familiarize them with the fMRI environment and to ensure that the participants understood and could perform the tasks.
Behavioral Analysis.
Performances in the scanner were analyzed by separately submitting accuracy scores and reaction times to a 2 (Groups: CB vs. SI; between-subjects factor) × 2 (Tasks: Spatial vs. Pitch; within-subjects factors) repeated-measures ANOVA. Moreover, we also separately submitted the auditory–spatial and auditory–pitch resolution level (calculated as the mean gap separating the target from the probe for an entire run) to a simple ANOVA with the factor Groups (Blind vs. Sighted) as a between-subjects factor. A threshold of P < 0.05 was used for assessing the significance of the results. Behavioral results are presented in SI Text and show no differences between groups.
fMRI Data Acquisition and Analysis.
The fMRI series were acquired using a 3-T TRIO TIM system (Siemens) equipped with a 12-channel head coil. Multislice T2*-weighted fMRI images were obtained with a gradient echo-planar sequence using axial slice orientation [time to repetition (TR) 2,200 ms; time to echo (TE) 30 ms; functional anisotropy (FA) 90°; 35 transverse slices; 3.2-mm slice thickness; 0.8-mm interslice gap; field of view (FoV) 192 × 192 mm2; matrix size 64 × 64 × 35; voxel size 3 × 3 × 3.2 mm3]. The four initial scans were discarded to allow for steady-state magnetization.
A structural T1-weigthed 3D magnetization prepared rapid gradient echo sequence (voxel size 1 × 1 × 1.2 mm3; matrix size 240 × 256; TR 2,300 ms; TE 2.91 ms; TI 900 ms; FoV 256; 160 slices) was also acquired for all subjects. Functional volumes were preprocessed and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/; Welcome Department of Imaging Neuroscience, London), implemented in MATLAB R2008a (Mathworks). Preprocessing included the realignment of functional time series, the coregistration of functional and anatomical data, a spatial normalization to an echo planar imaging template conforming to the Montreal Neurological Institute space, and a spatial smoothing (Gaussian kernel, 8 mm FWHM).
Details of the fMRI data analysis can be found in SI Text.
Supplementary Material
Acknowledgments
We thank the Institut Nazareth et Louis Braille for their help in recruiting the blind participants. This research was supported in part by the Fond de Recherches en Santé du Québec (G.V., M.L., and F.L.), the Canada Research Chair Program (M.L. and F.L.), the Canadian Institutes of Health Research (G.A., M.L., and F.L.), and the Natural Sciences and Engineering Research Council of Canada (P.V., G.C., M.L., and F.L.). O.C. was a postdoctoral researcher at the Belgian National Funds for Scientific Research at the time of the testing.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013928108/-/DCSupplemental.
References
- 1.Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- 2.Bavelier D, Neville HJ. Cross-modal plasticity: Where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 3.Grill-Spector K, Malach R. The human visual cortex. Annu Rev Neurosci. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- 4.Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Stevens AA, Snodgrass M, Schwartz D, Weaver K. Preparatory activity in occipital cortex in early blind humans predicts auditory perceptual performance. J Neurosci. 2007;27:10734–10741. doi: 10.1523/JNEUROSCI.1669-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kujala T, Alho K, Näätänen R. Cross-modal reorganization of human cortical functions. Trends Neurosci. 2000;23:115–120. doi: 10.1016/s0166-2236(99)01504-0. [DOI] [PubMed] [Google Scholar]
- 7.Lewis LB, Saenz M, Fine I. Mechanisms of cross-modal plasticity in early-blind subjects. J Neurophysiol. 2010;104:2995–3008. doi: 10.1152/jn.00983.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton H, Sinclair RJ, Dixit S. Working memory for vibrotactile frequencies: Comparison of cortical activity in blind and sighted individuals. Hum Brain Mapp. 2010;31:1686–1701. doi: 10.1002/hbm.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collignon O, Voss P, Lassonde M, Lepore F. Cross-modal plasticity for the spatial processing of sounds in visually deprived subjects. Exp Brain Res. 2009;192:343–358. doi: 10.1007/s00221-008-1553-z. [DOI] [PubMed] [Google Scholar]
- 10.Mishkin M, Lewis ME, Ungerleider LG. Equivalence of parieto-preoccipital subareas for visuospatial ability in monkeys. Behav Brain Res. 1982;6:41–55. doi: 10.1016/0166-4328(82)90080-8. [DOI] [PubMed] [Google Scholar]
- 11.Haxby JV, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 13.Pietrini P, et al. Beyond sensory images: Object-based representation in the human ventral pathway. Proc Natl Acad Sci USA. 2004;101:5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. Category-specific organization in the human brain does not require visual experience. Neuron. 2009;63:397–405. doi: 10.1016/j.neuron.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amedi A, et al. Shape conveyed by visual-to-auditory sensory substitution activates the lateral occipital complex. Nat Neurosci. 2007;10:687–689. doi: 10.1038/nn1912. [DOI] [PubMed] [Google Scholar]
- 16.Gougoux F, et al. Voice perception in blind persons: A functional magnetic resonance imaging study. Neuropsychologia. 2009;47:2967–2974. doi: 10.1016/j.neuropsychologia.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Collignon O, Lassonde M, Lepore F, Bastien D, Veraart C. Functional cerebral reorganization for auditory spatial processing and auditory substitution of vision in early blind subjects. Cereb Cortex. 2007;17:457–465. doi: 10.1093/cercor/bhj162. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardi E, et al. The effect of visual experience on the development of functional architecture in hMT+ Cereb Cortex. 2007;17:2933–2939. doi: 10.1093/cercor/bhm018. [DOI] [PubMed] [Google Scholar]
- 19.Saenz M, Lewis LB, Huth AG, Fine I, Koch C. Visual motion area MT+/V5 responds to auditory motion in human sight-recovery subjects. J Neurosci. 2008;28:5141–5148. doi: 10.1523/JNEUROSCI.0803-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weeks R, et al. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirier C, et al. Auditory motion perception activates visual motion areas in early blind subjects. Neuroimage. 2006;31:279–285. doi: 10.1016/j.neuroimage.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 22.Renier LA, et al. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68:138–148. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alain C, Arnott SR, Hevenor S, Graham S, Grady CL. “What” and “where” in the human auditory system. Proc Natl Acad Sci USA. 2001;98:12301–12306. doi: 10.1073/pnas.211209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friston KJ, Penny W. Posterior probability maps and SPMs. Neuroimage. 2003;19:1240–1249. doi: 10.1016/s1053-8119(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 26.Laurienti PJ, et al. Deactivation of sensory-specific cortex by cross-modal stimuli. J Cogn Neurosci. 2002;14:420–429. doi: 10.1162/089892902317361930. [DOI] [PubMed] [Google Scholar]
- 27.Lomber SG, Malhotra S. Double dissociation of ‘what’ and ‘where’ processing in auditory cortex. Nat Neurosci. 2008;11:609–616. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- 28.Amedi A, Floel A, Knecht S, Zohary E, Cohen LG. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat Neurosci. 2004;7:1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- 29.Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci. 2003;6:758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- 30.Tootell RB, et al. Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tootell RB, et al. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunaert S, Van Hecke P, Marchal G, Orban GA. Motion-responsive regions of the human brain. Exp Brain Res. 1999;127:355–370. doi: 10.1007/s002210050804. [DOI] [PubMed] [Google Scholar]
- 33.Watson JD, et al. Area V5 of the human brain: Evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- 34.Eickhoff SB, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 35.Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F. A functional neuroimaging study of sound localization: Visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005;3:e27. doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornette L, et al. Human brain regions involved in direction discrimination. J Neurophysiol. 1998;79:2749–2765. doi: 10.1152/jn.1998.79.5.2749. [DOI] [PubMed] [Google Scholar]
- 37.Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tollin DJ, Yin TC. The coding of spatial location by single units in the lateral superior olive of the cat. I. Spatial receptive fields in azimuth. J Neurosci. 2002;22:1454–1467. doi: 10.1523/JNEUROSCI.22-04-01454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 2010;13:1421–1427. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- 40.Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci. 2010;30:148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paus T. Location and function of the human frontal eye-field: A selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 42.Garg A, Schwartz D, Stevens AA. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia. 2007;45:2307–2321. doi: 10.1016/j.neuropsychologia.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimony JS, et al. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb Cortex. 2006;16:1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedny M, Konkle T, Pelphrey K, Saxe R, Pascual-Leone A. Sensitive period for a multimodal response in human visual motion area MT/MST. Curr Biol. 2010;20:1900–1906. doi: 10.1016/j.cub.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- 46.Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- 47.Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 48.Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. Int J Psychophysiol. 2003;50:19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 50.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Collignon O, et al. Time-course of posterior parietal and occipital cortex contribution to sound localization. J Cogn Neurosci. 2008;20:1454–1463. doi: 10.1162/jocn.2008.20102. [DOI] [PubMed] [Google Scholar]
- 52.Klinge C, Eippert F, Röder B, Büchel C. Corticocortical connections mediate primary visual cortex responses to auditory stimulation in the blind. J Neurosci. 2010;30:12798–12805. doi: 10.1523/JNEUROSCI.2384-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lakatos S, Shepard RN. Constraints common to apparent motion in visual, tactile, and auditory space. J Exp Psychol Hum Percept Perform. 1997;23:1050–1060. doi: 10.1037//0096-1523.23.4.1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.