Abstract
Carbonaceous chondrites are asteroidal meteorites that contain abundant organic materials. Given that meteorites and comets have reached the Earth since it formed, it has been proposed that the exogenous influx from these bodies provided the organic inventories necessary for the emergence of life. The carbonaceous meteorites of the Renazzo-type family (CR) have recently revealed a composition that is particularly enriched in small soluble organic molecules, such as the amino acids glycine and alanine, which could support this possibility. We have now analyzed the insoluble and the largest organic component of the CR2 Grave Nunataks (GRA) 95229 meteorite and found it to be of more primitive composition than in other meteorites and to release abundant free ammonia upon hydrothermal treatment. The findings appear to trace CR2 meteorites’ origin to cosmochemical regimes where ammonia was pervasive, and we speculate that their delivery to the early Earth could have fostered prebiotic molecular evolution.
Carbonaceous chondrites (CC) are asteroidal meteorites containing organic materials as diverse as kerogen-like macromolecules and soluble biomolecular analogs such as amino acids. Because the resources, means, and environments by which life emerged are utterly unknown, the evidence from meteorites of an active cosmochemical evolution of the biogenic elements has raised questions of a possible exobiology, i.e., of whether the molecular evolution that led to Earth life was aided by exogenous biomolecule precursors.
The delivery of cometary and asteroidal materials is undoubted; it is observed today and known to have showered the Earth throughout its history (1). However, the detailed analyses of the Murchison meteorite, the most studied of CC, have not been encouraging for this hypothesis. Murchison’s possible prebiotic precursors, in fact, are found mixed in a complex organic suite that is highly heterogeneous, the apparent product of random processes and rich in stable end products, e.g., hydrocarbons (2, 3). Such composition seems to offer little prebiotic potential of finding an evolutionary path toward the functional specificity needed by even the simplest biochemistry. Given that the number of CC analyzed so far for soluble molecular species has been relatively small, this point of view was often extended to abiotic processes in general.
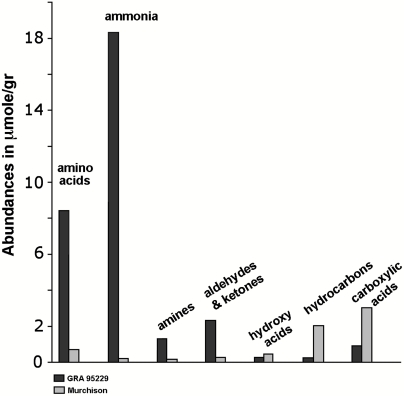
Recent analyses of several pristine stones found in Antarctica and belonging to a previously undescribed CC family, the Renazzo-type or CR, have offered a yet unknown view of the synthetic capabilities of extraterrestrial environments and revealed soluble organic suites that, unlike Murchison’s, are made up of mainly water-soluble compounds, with predominant N-containing species (4, 5) (Fig. 1). Ammonia and amino acids are the most abundant single molecule and compound group in CR2† extracts, whereas several of the CR2 compounds display high deuterium enrichments that allow one to trace their origin to cold cosmic regimes (5, 6). Also, uniquely between CC analyzed so far, CR2 have lower homologs as their major constituents; i.e., the extended cosmic processes leading to their asteroidal parent-bodies resulted in a de facto selection of simple prebiotic compounds such as glycine, alanine, and glycolic acid.
Fig. 1.
Overview of abundance distributions for the major soluble compound groups in the GRA 95229 and Murchison meteorites; in μmoles/g of meteorite (3–6).
As for all CC, the largest portion of the CR2-carbon is contained as a complex insoluble organic material (IOM), whose heterogeneous composition is not well defined (3). Because it is inaccessible to solvents, the IOM can be analyzed as a whole only by microscopy and solid-state spectroscopy, by decomposition techniques, such as chemical degradation and pyrolysis, or by combustion for its elemental composition. CR2-IOM has so far shown H/C and N/C elemental ratios that are, on the average, similar to those of Murchison-type IOM but to be depleted in O/C (7).
We report here on a study of the CR2 Grave Nunataks (GRA) 95229 IOM, which was undertaken to ascertain whether the distinctive organic composition of CR2 precursor environments could also be reflected in the molecular makeup of this complex material. GRA-IOM was treated with weight-equivalent amounts of water at conditions of high temperature and pressure (300 °C and 100 MPa). The experimental parameters were selected to be in a range where diagenesis of clays is known to ensue in nature and to plausibly mimic, within laboratory time scales, the hydrothermal activity in both asteroidal parent bodies (e.g., ref. 8) and the early Earth (e.g., ref. 9). Analyses of the resulting hydrothermal extracts revealed the release of abundant free ammonia, an unexpected finding that is indicative of pervasive ammonia availability in some asteroidal precursor environments.
Results and Discussion
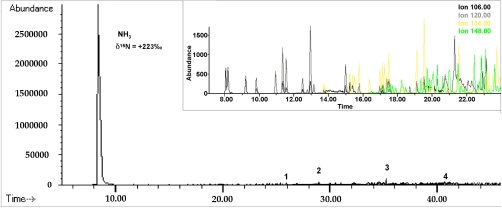
As summarized in Fig. 2, the hydrothermolytic treatment of GRA demineralized powders led to the near exclusive release of ammonia in the water extract, whereas extraction of the residue IOM with solvents produced low abundances of diverse and highly branched hydrocarbons. Ammonia was found in the amount of 10 μg mg-1 IOM and to account for near 60% of the nitrogen measured in the IOM by elemental analysis prior to the treatment, 14.7 μg mg-1. Ammonia was identified by gas chromatography–mass spectrometry (GC-MS), as were the hydrocarbon species (Fig. 2). Ammonia isotope ratio was determined by GC-isotope ratio mass spectrometry (IRMS; Fig. S1) and to have a δ15N of +223‰. This value is not in the terrestrial range (the terrestrial atmosphere is the reference standard in the δ15N scale‡), but is comparable to one we measured for the IOM powders prior to treatment (+200‰) and higher than the +134‰ measured for the soluble ammonia of a similar meteorite’s water extracts (5). The large number of highly substituted alkyl and small aromatic molecules released by solvent extraction of the treated GRA-IOM powders were found in nanomole and subnanomole amounts (Tables S1 and S2).
Fig. 2.
Gas chromatogram of hydrothermally treated GRA-IOM water and solvent extracts. Mass-spectrometric detection, combined to scale: 1-naphthalene, 2-pentadecane, 3-heptadecane, 4-phenanthrene. (Inset) Expanded view of the chromatographic elution of C2–C5 branched benzenes (C2 black, C3 gray, C4 yellow, and C5 green).
The abundant release of ammonia from a meteoritic macromolecular material is unprecedented, because similar treatment of other CC-IOM produced mainly aromatic hydrocarbons (10). It is also surprising because the complexity, condensed nature, and evident insolubility in acids of all CC-IOM, GRA-IOM included, would have seemed to exclude the reversible retention of a simple molecule such as ammonia.
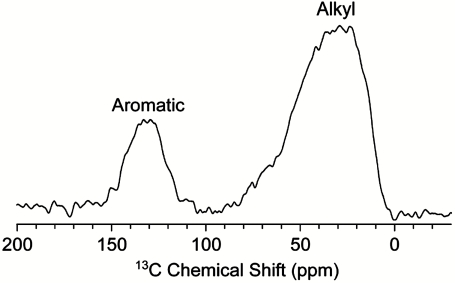
Although the IOM of carbonaceous meteorites is not known in molecular detail, their solid state 13C NMR analyses show two main broad features characteristic of sp2 and sp3 hybridized carbon atoms (11), i.e., olefinic/aromatic and aliphatic, respectively. In spite of the inherent approximation of the technique for inhomogeneous sample [for example, the population of CH3, CH2, and CH cannot be individually accounted for; (12) and references therein], the ratios between the aromatic∶aliphatic domains appear to remain consistent in repeat studies and have been interpreted to indicate a general IOM structure of condensed aromatic macromolecules plus their alkyl branching and bridges; e.g., see ref. 3 for a review.
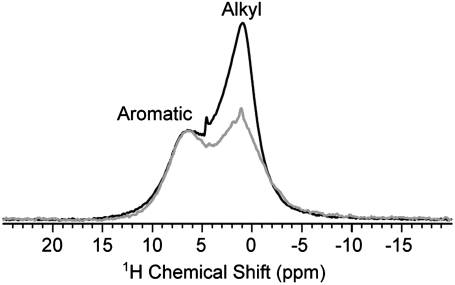
The relative abundance ratio of these components changes significantly between meteorites: It was found to be largest for Tagish Lake, a meteorite rich in oxidized molecular species, and lowest for the CR2 EET 92049, where the alkyl component is about equal to the aromatic content (13). Our NMR analyses show (Figs. 3 and 4) that the GRA-IOM composition contains aromatic and alkyl functionalities that, when determined from fitting the two resonances observed in the 1H spectrum, have a ratio of approximately 2∶3. Together with the detection of highly branched hydrocarbons in the solvent extract after hydrothermal treatment (HT), these data suggest that GRA-IOM is composed of loosely condensed aromatic macromolecules with particularly abundant alkyl portions of branches, linkages, or both. By microscopy, this IOM appears largely amorphous without any clear resolution in its continuity but, as for other CC, it also contains about 5–10% of distinct nano-sized entities such as aromatic flakes, solid or hollow spheres, and tubes (14, Fig. S2) of heterogeneous composition.
Fig. 3.
C-13 Solid-state NMR spectra of GRA-IOM.
Ammonia or its precursor molecules, therefore, could have resided in diverse chemical settings within the GRA-IOM and their sources are challenging to trace. Reasonably, we may assume that chemical regimes producing soluble N-rich compositions as seen in CR2s were reflected in their insoluble materials as well; for example, that the condensing of macromolecules hydrocarbons in an NH3-rich environment led to the formation of amines- and imine-containing alkyl branches, which could then hydrothermally protonate and hydrolyze, with the elimination of free ammonia. 1H NMR spectra appear to confirm this assumption by indicating a labile GRA-IOM alkyl component, which was significantly reduced upon HT treatment (Fig. 4). Anomalously high δ15N values of up to +4,500 were detected spectroscopically in micron-sized inclusions of several meteorites’ IOMs (15) and could also be considered sources of the released ammonia; however, these particles’ chemical composition was not sufficiently characterized to confirm the possibility.
Fig. 4.
H-1 Solid-state NMR spectra of GRA-IOM before (black) and after (gray) hydrothermal treatment.
Regardless of precise chemical source, the exclusive detection of ammonia in our analyses, its large abundance relative to total nitrogen and isotopic correspondence to soluble ammonia mainly point to pervasive ammonia in CR2 precursor environments and its availability as the most abundant single molecule in CR2 parent asteroids. In perspective to previous studies, our findings also stress the diversity of chemical and prebiotic environments to reach the solar system, the range of their possible transformation upon processing as well as offer analytical insights vis-à-vis the astronomical observations of main-belt comets (16) and ice-covered asteroids (17,18).
It is interesting to speculate how these data might influence the discourse on the origins of life. Besides the noble gases, nitrogen is the fourth most abundant element in the Sun and the universe overall. On the Earth, it is an indispensable complement of the biosphere as a constituent of DNA, RNA, and proteins; i.e., it is necessary for life’s information transfer and catalytic processes. In its reduced form as ammonia, we should expect it to have participated in numerous abiotic as well as prebiotic reactions. In CR2 meteorites, for example, ammonia abundance led preferentially to the formation of amino over hydroxyacids (6), syntheses that could have further benefited in icy planetesimals by ammonia’s ability to reduce the freezing point of water. On the early Earth, on the other hand, the prebiotic inventory of reduced nitrogen necessary for the formation of N-containing biomolecules has been difficult to predict. Although the hypotheses of a reducing atmosphere had initially allowed one to envision a considerable ammonia abundance as well as evolutionary pathways for the production of amino acids (e.g., by Miller-type processes, 19), the current geochemical evidence of a neutral early Earth atmosphere (20 and references therein) combined with the known photochemical destruction of ammonia (21) has left prebiotic scenarios struggling to account for a constant provision of ammonia (22, 23). An abundant exogenous delivery of ammonia, therefore, might have been significant in aiding early Earth’s molecular evolution toward prebiotic syntheses and the data in this study, showing the capability of some asteroidal bodies to provide it, would make a reasonable case for exobiology.
Materials and Methods
GRA powders weighing 3.994 g were extracted with water and dichloromethane (DCM)/MeOH (9∶1, vol∶vol) and demineralized as previously described (11). GRA-IOM weighed 45 mg, approximately 1.1% of the starting powders. Water was triply distilled; 6N HCl, 48% HF solution, DCM, and MeOH were twice distilled.
Hydrothermal Treatment.
The chosen aliquots of IOM powders (15 mg) were extracted before each HT experiment with 2 mL of water at 100 °C for 24 h and, after drying, with 2 mL of DCM/MeOH at room temperature for 2 h. The extracts were then decanted and the powders dried in a desiccator. HT experiments are conducted in pure gold tubes, 5 mm i.d. (0.125-mm wall) by ∼30 mm, which were annealed at 500 °C in air overnight before use. Prior to loading the powders, one side of the tube was sealed by an Auto Arc TIG 50A Precision Welder. The IOM powders were loaded into the Au tubes and degassed water was added in equal proportion by weight (15–20 μL) with a syringe. The tubes were loaded at about 0 °C, sealed by arc welding under argon atmosphere, then heated to 110 °C for 10 min and reweighed to test that no weight was lost, verifying the seal. The sealed Au tubes were then placed in a stainless steel pressure vessel sealed with a Teflon Bridgman seal (24), fitted with a thermocouple at each end of the capsule and a Bourdon-type pressure gauge. The vessel was put into a clam-shell furnace and pumped up to pressure. Water was used as the pressurizing medium within the vessel. The experiments were conducted at 300 °C and 100 MPa for 6 d. The temperature had a measured gradient of ≤ 5 °C, whereas the pressure measurements had an accuracy of ± 5 MPa.
After the treatment, the hydrothermal vessel was quenched in ice and Au tubes were removed, weighed to certify no weight loss or gain, rinsed with water and solvents, and then opened by slicing their midsection with a previously cleaned razor blade. The IOM powders’ suspension was transferred with several rinses (≥1 mL) to a vial and centrifuged. The extract had a pH = 5, 5, was decanted, dried by rotary evaporation and derivatized for gas chromatographic analyses. The powders were then dried in a desiccator and extracted with 1 mL of DCM/MeOH. The solvent extracts were also centrifuged, decanted, and dried.
Molecular Analyses by Mass Chromatography–Mass Spectrometry.
The dry HT IOM water extracts were reacted in succession with isopropanol (3N HCl) and trifluoroacetic anhydride (e.g., see ref. 25); derivatized samples were dissolved in DCM for analysis. GC-MS analyses were performed with an Agilent 6890N GC-5973N Mass Selective Detector Network System equipped with either of two chiral columns: a CP (cross-polarization) Chirasil-dex CB (CHROMPACK), 25 m × 0.25 mm, 0.25-μm df, and a Chirasil-val (ALLTECH ASS. INC), 50 m × 0.25 mm, 16-μm df. The GC oven temperature programs were, respectively, 70 °C initial, 5 min hold, 70 to 85 °C @ 1 °C min-1, 85 to 200 °C @ 5 °C min-1, and 45 min hold; 70 °C initial, 1 min hold, 70 to 100 °C @ 2 °C min-1, 100 to 200 °C @ 4 °C min-1, and 45 min hold. Acidic counterions, whose presence was indicated by the slightly acidic pH of the extract, would not have been detected with this procedure and were not searched for.
The HT IOM solvent extracts were dried and the residues redissolved in DCM; their GC-MS analyses were performed utilizing a DB-17 capillary column (J&W Scientific, 60 m × 0.25 mm, 0.25-μm df). The GC oven temperature program was 70 °C initial, 1 min hold, 70 to 100 °C @ 2 °C min-1, from 100 to 300 °C @ 4 °C min-1, and 60 min hold. Aliquots from all the sample solutions were injected into the GC by Solids Injector (SGE Inc.).
Identification of compounds were made by comparison of their peak retention times and mass spectra with those of standard compounds. For unknowns, identification was made by comparison of the mass spectra with library data (NIST98) and individual examination of the suggested match reliability.
Elemental Analysis Isotope Ratio Mass Spectrometry.
The C and N concentration and 13C and 15N isotopic composition of IOM residue were measured on a Costech Elemental Analyzer combined to a Finnigan Termo Delta Plus Advantage mass spectrometer. The following weights and values were obtained from three aliquots of powders: C, 632 μg mg-1, δ13C = -22.7 ± 0.4; N, 27.9 μg mg-1, δ15N = 196 ± 0.7. C∶N (mol∶mol) = 26.4.
Gas Chromatography–Combustion–Isotope Ratio Mass Spectrometry (GC-C-IRMS).
The stable isotope ratio of 15N in ammonia-TFA derivative was measured with a Thermo GC-C-IRMS system consisting of Thermo Trace GC Ultra gas chromatograph coupled to a Delta Plus XP isotope ratio mass spectrometer through a GC/C-III interface (Thermo Electron Corp.). Aliquots (0.4–3 uL) of ammonia derivative in dichloromethane were injected in splitless mode (12.8 psi). The compounds were separated on a Chirasil-Val capillary column employing a helium flow of 1 mL min-1. Following GC separation, amino nitrogen was converted to N2 in an oxidation reactor at 980 °C. Any nitrous oxides formed by combustion were reduced to N2 in a reduction reactor at 650 °C. The δ15N = 223 ± 3.8 value for CR2-ammonia was obtained from three repeat injections.
Solid-State NMR.
Carbon-13 and proton solid-state NMR spectra were collected on a Varian VNMRS 800-MHz spectrometer equipped with a 1.6-mm triple-resonance MAS (magic angle spinning) probe. The experimental parameters for obtaining 13C CP-MAS spectra were a 2-μs 1H π/2 pulse, a 1-ms ramped (∼20%) 1H spin-lock pulse with a rf field strength of 125 kHz at the ramp maximum and a 13C square contact pulse. The MAS frequency (νR) was 40 kHz and the 1H → 13C CP condition was matched to the -1 spinning side band in the Hartmann–Hahn profile on the 13C channel. Two-pulse phase-modulated 1H decoupling with 130-kHz rf field strength was applied during acquisition with an 11° phase shift. Typical acquisition parameters for 13C CP-MAS spectra were 100-kHz sweep width, 512 points, 143,360 scan averages, and a 1-s recycle delay. A background spectrum was collected under identical experimental conditions and subtracted from the sample spectrum to eliminate contributions from probe background. Proton NMR spectra were obtained with a spin-echo pulse sequence with a 200-μs delay between the π/2 and π-pulse and 40-kHz MAS. Typical acquisition parameters for 1H MAS spectra were 100-kHz sweep width, 512 points, 8 scan averages, and a 1-s recycle delay. A d1 array (1 s, 5 s, and 10 s) was collected to determine the optimal recycle delay for both 13C CP-MAS and 1H MAS experiments. All three spectra displayed identical signal intensity for both the aromatic and alkyl regions in both cases indicating that a d1 of 1 s is sufficient for obtaining fully relaxed spectra. The 1H and 13C chemical shift were indirectly referenced to tetramethylsilane by setting the chemical shift of adamantane to 1.63 and 38.56 ppm, respectively.
Supplementary Material
Acknowledgments.
The authors thank George Cooper [National Aeronautics and Space Administration (NASA) Ames Research Center, Moffet Field, CA] for providing part of the GRA powders, Mathew McCarthy for making isotopic analyses possible, and Natalya Zolotova for EA-IRMS analyses. Edward Skibo and Arthur Weber helped with useful discussions. This work was supported by grants from the NASA Exobiology Program and the NASA Astrobiology Institute Director’s Fund.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014961108/-/DCSupplemental.
†The number classifies CC groups’ petrographic type and level of asteroidal parent body secondary processes.
‡δ15N‰ = [Rsample - Rstandard/Rstandard] × 103.
References
- 1.Thomas PJ, et al., editors. Comets and the Origin and Evolution of Life. New York: Springer; 1997. [Google Scholar]
- 2.Schmitt-Kopplin P, et al. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc Natl Acad Sci USA. 2010;107:2763–2768. doi: 10.1073/pnas.0912157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzarello S, Cooper GW, Flynn GJ. In: Meteorites and the Early Solar System II. Lauretta DS, McSween HY, editors. Tucson: Univ Arizona Press; 2006. pp. 625–651. [Google Scholar]
- 4.Pizzarello S, Huang Y, Alexandre MDR. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc Natl Acad Sci USA. 2008;105:3700–3704. doi: 10.1073/pnas.0709909105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizzarello S, Holmes W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim Cosmochim Acta. 2009;73:2150–2162. [Google Scholar]
- 6.Pizzarello S, Wang Y, Chaban GM. A comparison study of the hydroxy acids from the Murchison GRA 9529 and LAP 02342 meteorites. Geochim Cosmochim Acta. 2010;74:6206–6217. [Google Scholar]
- 7.Alexander CMO’D, Fogel M, Yabuta H, Cody GD. The origin and evolution of chondrites recorded in the elemental and isotopic composition of their macromolecular organic matter. Geochim Cosmochim Acta. 2007;71:4380–4403. [Google Scholar]
- 8.Zolensky ME, et al. CM chondrites exhibit the complete petrologic range from type 2 to 1. Geochim Cosmochim Acta. 1997;61:5099–5115. [Google Scholar]
- 9.Kelley DS, Baross JA, and Delaney JR. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu Rev Earth Pl Sc. 2002;30:385–491. [Google Scholar]
- 10.Yabuta H, Williams LB, Cody GD, Alexander CMO’D, Pizzarello S. The insoluble carbonaceous material of CM chondrites: A possible source of discrete organic compounds under hydrothermal conditions. Meteorit Planet Sci. 2007;42:37–48. [Google Scholar]
- 11.Cronin JR, Pizzarello S, Frye JS. 13C NMR spectroscopy of insoluble carbon in carbonaceous chondrites. Geochim Cosmochim Acta. 1987;51:299–303. doi: 10.1016/0016-7037(87)90242-0. [DOI] [PubMed] [Google Scholar]
- 12.Cody GD, Alexander CMO’D, Tera F. Geochim Cosmochim Acta. 2002;66:1851–1865. [Google Scholar]
- 13.Cody GD, Alexander CMO’D. NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrites groups. Geochim Cosmochim Acta. 2005;69:1085–1097. [Google Scholar]
- 14.Pizzarello S, Garvie LAJ. 38th Lunar and Planetary Conference; Houston, TX: Lunar and Planetary Institute; 2007. http://www.lpi.usra.edu, Abstract 1236. [Google Scholar]
- 15.Busemann H, et al. Interstellar chemistry recorded in organic matter from primitive meteorites. Science. 2006;312:727–730. doi: 10.1126/science.1123878. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh HH, Jewitt D. A population of comets in the main asteroid belt. Science. 2006;312:561–563. doi: 10.1126/science.1125150. [DOI] [PubMed] [Google Scholar]
- 17.Campin H, et al. Water ice and organics on the surface of the asteroid 24 Thamis. Nature. 2010;464:1320–1321. doi: 10.1038/nature09029. [DOI] [PubMed] [Google Scholar]
- 18.Rivkin AS, Emery JP. Detection of ice and organics on an asteroidal surface. Nature. 2010;464:1322–1323. doi: 10.1038/nature09028. [DOI] [PubMed] [Google Scholar]
- 19.Stribling R, Miller SL. Energy yelds for hydrogen cyanide and formaldehyde syntheses: The HCN and amino acids concentrations in the primitive ocean. Origins Life Evol B. 1987;17:261–273. doi: 10.1007/BF02386466. [DOI] [PubMed] [Google Scholar]
- 20.Kasting JF. Evolution of Earth’s atmosphere and hydrosphere: Hadean to recent. In: Engels MH, Macko SA, editors. Organic Geochemistry: Principles and Applications. New York: Plenum; 1993. pp. 611–622. [Google Scholar]
- 21.Ferris JP, Nicoden DE. Ammonia photolysis and ammonia in chemical evolution. Nature. 1972;238:268–269. [Google Scholar]
- 22.Summers DP, Chang S. Prebiotic ammonia from reduction of nitrite by iron (II) on the early Earth. Nature. 1993;365:630–633. doi: 10.1038/365630a0. [DOI] [PubMed] [Google Scholar]
- 23.Weber AL. Sugar-driven prebiotic synthesis of ammonia from nitrate. Origins Life Evol B. 2010;40:245–252. doi: 10.1007/s11084-010-9208-z. [DOI] [PubMed] [Google Scholar]
- 24.Williams LB, Hervig RL, Holloway JR, Hutcheon I. Boron isotope geochemistry during diagenesis: Part 1. Experimental determination of fractionation during illitization of smectite. Geochim Cosmochim Acta. 2001;65:1769–1782. [Google Scholar]
- 25.Pizzarello S, Cronin JR. Non racemic amino acids in the Murchison and Murray meteorites. Geochim Cosmochim Acta. 2000;64:329–338. doi: 10.1016/s0016-7037(99)00280-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.