Abstract
The conversion of peptide and proteins from their soluble state into well-organized aggregates, together with the accompanied oxidation of methionine residue, presents a significant challenge to human health, to the manufacture of protein therapeutics, and to the synthesis of proteins and glycoproteins. Despite their fundamental importance, little is known about the molecular basis of these two side reactions and their control. Here, using chemical peptide synthesis, we further confirmed the importance of the balance between hydrophobic interactions and electrostatic repulsive forces in inducing and inhibiting aggregation and methionine oxidation. Most importantly, through extending the established principle, we are able to effectively stabilize the problematic peptide fragment through the attachment of cleavable arginine tags. Future applications of our approach are expected to facilitate the synthesis and study of difficult peptides, proteins, and glycoproteins and will provide more opportunities for the optimization of protein biopharmaceuticals and for the development of cell-permeable biomolecules.
Keywords: erythropoietin, liquid chromatography, mass spectrometry
Degradation of peptides and proteins by aggregation is a significant problem both from the standpoint of fundamental research and for biopharmaceutical production (1, 2). Aggregation can be simply described as the oligomerization and/or polymerization of partially unfolded peptides and proteins. It can proceed through three commonly observed mechanisms: noncovalent interactions, disulfide bond formation/exchange, and nonreducible cross-linking. Through self-association, peptides and proteins can form soluble or insoluble aggregates. Formation of such aggregate species has been suggested to be intimately associated with the progression of many fatal neurodegenerative diseases. Moreover, the safety and immunogenicity of therapeutic proteins may be compromised by aggregate formation (3, 4). Hence, the development of strategies to control aggregation would have important implications in the treatment of neurodegenerative diseases and in the minimization of side effects associated with recombinant therapeutic proteins (5).
Aggregation is apt to be accompanied by the oxidation of methionine residues in adjacent chains (6, 7). The methionine residues on the surface of the polypeptide or protein are thought to be more readily oxidized (8), thereby generating primarily methionine sulfoxide (Fig. 1). Methionine oxidation is a complex process, closely connected with aging and the pathology of various diseases states (9). Although levels of methionine oxidation can be reduced by various strategies, effective and reliable control of this problem has not been achieved (10).
Fig. 1.
Aggregation and methionine oxidation of hEPO (43–77). (A) The sequences of the synthetic hEPO (43–77) fragments. (B) Methionine oxidation.
Aggregation and oxidation of methionine residues also present a significant challenge to protein and glycoprotein chemical synthesis (11–13). Although a natively folded protein can exhibit a reduced tendency to self-association by burying its hydrophobic residues and aggregation-prone regions in the closely packed interior, synthetic fragments en route to proteins are often particularly prone to aggregation and oxidation, due to the exposure of the hydrophobic regions and the methionine residues to the surface (14). These problems can be particularly significant in the synthesis of glycosylated globular proteins. In addition to possessing the hydrophobic nuclei around which the globular proteins can fold, these proteins may also have hydrophobic regions on their surfaces (15). Although the association of these exterior hydrophobic surfaces can be inhibited by the attached glycans in the correctly folded glycoprotein, during chemical synthesis, the loss of native conformation in the synthetic fragments can easily result in problematic aggregation and methionine oxidation (16).
Not unexpectedly, during the course of our synthetic studies toward the homogeneously glycosylated human erythropoietin (hEPO) (29–77), we have also encountered the problem with aggregation and methionine oxidation (17, 18). These side reactions further complicated the already challenging task of synthesizing the homogeneously glycosylated hEPO. In order to optimize and simplify the synthesis, we launched a program to improve the handling properties of the synthetic fragments. Toward this end, we first identified the peptide fragments that are responsible for the undesired reactions. From there, we showed that introduction of guanidino groups could enhance the stability of hEPO peptides. Finally, we were able to develop two cleavable arginine tags to improve the chemical and physical properties of the problematic fragment, partially protected hEPO (43–77) (19, 20).
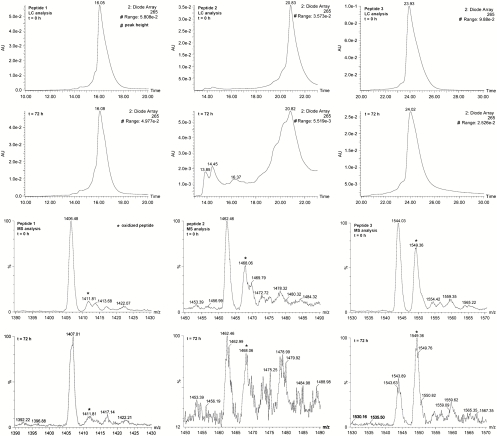
We commenced with an examination of the properties of the synthetic fragments directed to hEPO. Although several methods can be used to analyze peptide aggregation and methionine oxidation, we chose to employ high-performance liquid chromatography and mass spectrometry (LC-MS) techniques because of their convenience and suitability for the task. When analyzed by LC-MS, seriously aggregated peptides provide lower UV peak heights (1, 21). Similarly, inhibition of the irreversible oxidation of methionine residues in a given peptide would lead to a decrease of the +16 Da mass signal. Therefore, the extent of aggregation and methionine oxidation can be rapidly estimated based upon the UV absorption and the MS signal intensity (22–24).
Using Fmoc-chemistry-based solid-phase peptide synthesis (SPPS), we prepared three different variants of both hEPO (29–57) and (43–77), including one with free lysine residues and two variants, with allyl-oxycarbonyl (Alloc) and 1-(4,4-dimethyl-2,6-dioxo-cyclohexylidene)-3-methyl-butyl (ivDde) protected lysines, respectively (Fig. 1). As previously described in our work, lysine protection was necessary for the controlled synthesis of hEPO glycopeptide building blocks (18, 25, 26). The N terminus of each peptide was capped with an Fmoc protecting group to improve the UV detection. After purification by HPLC, the synthetic peptides were dissolved in a solution of H2O∶MeCN = 1∶1 (5% vol/vol AcOH). The aggregation propensity of each peptide was estimated based on the height of the peptide peaks after standing in air for 3 d. The extent of methionine oxidation was determined based on the signal intensity of the oxidized peptide peak.
The UV and MS analyses of the three hEPO (29–57) fragments demonstrated that this region is not central to aggregation. We then turned our attention to hEPO (43–77). The analytical results are shown in Fig. 2. After purification and reconstitution, unprotected peptide 1 was homogeneous and was eluted as a single peak. The MS peak corresponding to the oxidized species is estimated to be about 10% of the total peptide fraction. After standing in air at room temperature for 3 d, the UV peak height of peptide 1 did not change significantly. The height of the MS signal corresponding to the oxidized methionine also remained nearly unchanged. At time zero, the Alloc-protected peptide 2 is also a sharp peak and the oxidized peptide is about one-third of the total fraction. After 3 d, however, a large reduction in the height of the UV peak was observed. Meanwhile, there was a roughly 20% increase in the level of methionine oxidation. Similar changes were observed in the analysis of ivDde-protected peptide 3.
Fig. 2.
LC-MS analysis of synthetic peptide fragments 1, 2, and 3.
These results indicate that this region of hEPO (43–77) is likely to be responsible for the observed oxidative and degradative phenomena. These analyses revealed that the extent of the side reactions is strongly influenced by the properties of the peptide side chains. Although various possibilities can account for the decreased stability of the protected peptides, findings from previous studies suggested that the aggregation propensity of the synthetic fragments might be closely related to the inter- and intramolecular hydrophobic associations and electrostatic interactions between the positively charged peptide side chains. This view is supported by the results of an analysis of multiple aggregation-prone sequences (20, 27, 28). Through the analysis, Dobson and co-workers proposed that the positively charged lysine and arginine residues play an important role in disrupting the association of the hydrophobic regions. They can act as “sequence breakers” to reduce or even to completely eliminate the aggregation propensity of peptides and proteins (28). Likewise, the peptides and proteins lacking the positively charged residues exhibit a high tendency to build up the sequential patterns that favor the formation of aggregates.
Hydrophobicity profiles and hydrophobic cluster analyses for peptides 1, 2, and 3 clearly support such a hypothesis (29). As shown in Fig. 1, protection of the lysine side chains with hydrophobic groups, i.e., Alloc and ivDde, results in the formation of two adjacent clusters of hydrophobic residues, which are highlighted with yellow lines. Structurally, this feature is very similar to that of amyloid β-peptide (1–42), which contains two hydrophobic regions and three positively charged residues (30). Because of the inter- and intramolecular association of these two regions, Aβ-peptide (1–42) can self-assemble spontaneously into aggregates. Analogously, we propose that the association of the hydrophobic regions may also promote the aggregation of peptide 2 and 3, which in turn leads to the oxidation of Met 54 to methionine sulfoxide (31). Normally, methionine thioether is a rather poor electron donor for molecular oxygen, presumably reflecting the oxidation and reduction potentials of Met and oxygen (32). However, the conformational changes induced by aggregation may have major effects on the capacity of methionine to act as a reducing agent. The protection of the lysine residues with the Alloc and ivDde groups may considerably change the structural features of hEPO (43–77), thereby resulting in placement of the sulfur atom of Met 54 in proximity to an amide oxygen (7, 33). Analogous to the oxidation of Aβ-peptide (1–42), such a close association could facilitate the formation of a sulfur radical cation amide oxygen bond, thereby accelerating the conversion of the thioether group to the corresponding sulfoxide (32, 34).
Following the above analysis, it seemed reasonable to assume that if the association of the two hydrophobic regions in peptides 2 and 3 can be inhibited, then both aggregation and methionine oxidation can be prevented. Among all the possible ways to achieve this goal, the most reliable strategy would be to change the net charges of the target molecules (35). According to previous studies, aggregation can be controlled by ensuring a balance between hydrophobic attractions and electrostatic charge–charge repulsions (28, 36). Presumably, the effects of charged residues are mediated through electrostatic repulsions occurring intramolecularly as well as intermolecularly. Apparently, incorporation of more positive charges into the peptide can cause more repulsions and lead to the shifting of the balance toward inhibition of aggregate formation. This idea was suggested by previous studies. For example, Dobson and co-workers have shown that the presence of evenly distributed lysine and arginine residues strongly stabilizes peptides against self-oligomerization (28). They have also used arginine as a sequence breaker to inhibit the self-assembly of peptides with high propensity to aggregate (20). In a similar study, Liu and co-workers observed a stabilization of GFP by supercharging with multiple arginine residues (37). However, because only the guanidino group on the side chain of arginine is compatible with the reaction conditions employed for our synthesis of hEPO glycopeptide fragments, lysine could not be used to improve the handling properties of the synthetic fragments (38).
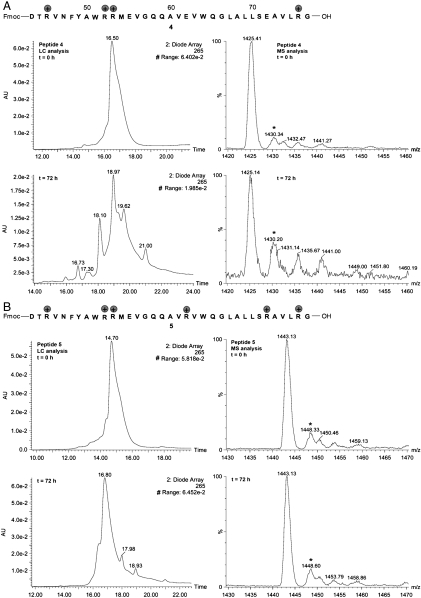
With this in mind, we set out to investigate the relationship between the number of guanido groups and the aggregation propensity of the synthetic peptide fragment. Using SPPS, we produced peptide 4 by replacing the lysine residues at positions 45 and 52 with arginines. The LC-MS analysis of this variant is shown in Fig. 3A. Interestingly, although arginine is believed to have lower intrinsic aggregation propensity, in our study, its antiaggregation effects for hEPO (43–77) peptide are less pronounced than lysine (28). In light of these findings, another peptide variant was designed by replacing two additional glutamic acids at positions 62 and 72 by arginines (Fig. 3B). Happily, as judged by LC-MS analysis, the supercharged version of hEPO (43–77) effectively maintained not only its structural stability, but its chemical stability as well. The success of this study indicates that the charge–charge interactions mediated by four guanidino groups are sufficient for inhibiting the aggregation of hEPO (43–77).
Fig. 3.
Handling properties of different hEPO (43–77) fragments. (A) The sequence and LC-MS analysis of peptide 4. (B) The sequence and LC-MS analysis of peptide 5.
Through this work, we have successfully identified a hEPO (43–77) variant with increased stability in solution and side-chain functionalities that are compatible with the reaction conditions. However, it is important to note that such engineering actually changed the native sequence of hEPO (43–77). Because the amino acids in this region were proposed to play direct roles in the high affinity interaction between hEPO and its receptor, modifications of the residues must face the risk of compromising the biological activity of hEPO (39). Our goal, at least presently, is to conserve the primary structure of hEPO in the fully synthetic product.
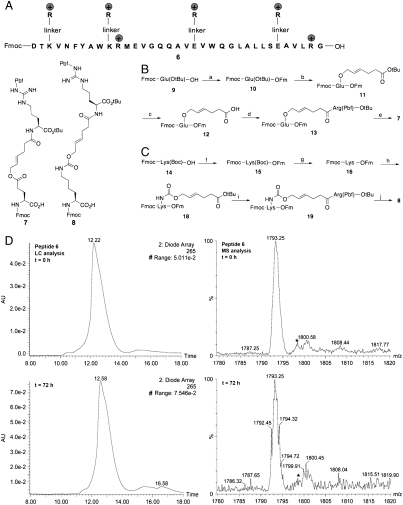
To overcome the problem, we sought to develop a specifically designed hEPO (43–77) fragment that, through the incorporation of four guanidine groups, would exhibit the desired stability, fragment-coupling selectivity, and functional activity. Inspired by the work on “traceless linkers” (40, 41), we theorized that guanidino groups can be attached to the lysine and glutamic acid residues using cleavable linkers (Fig. 4A) (41). These linkers must be able to survive the basic and acidic conditions required for SPPS and must subsequently be easily cleaved from the peptide side chains under mild conditions. On the basis of previous studies, we found the allylic linkers to offer significant advantages for the proposed studies (42, 43). The allylic ester and allylic carbamate linkers can serve as side-chain protection for lysine and glutamic acid during SPPS. The arginine moiety attached at the other end of the linker can promote the stability of the synthetic fragment. In addition, it has been shown that the allylic linkers are stable under SPPS conditions and can be effectively removed by palladium(0)-catalyzed deprotection in the presence of nucleophiles.
Fig. 4.
Preparation and characterization of peptide 6. (A) The sequence of peptide 6. (B) The synthesis of building block 7. Pbf = 2,2,4,6,7-Pentamethyldihydrobenzofuran-5-sulfonyl. Key: (a) Fm-OH, DCC, DMAP, CH2Cl2, 20 min, 99%; (b) TFA∶CH2Cl2 (1∶1), 30 min; BrCH2CH = CHCH2CH2CO2tBu, Bu4NBr, NaHCO3, H2O, CH2Cl2, THF, 7.5 h, 60%; (c) TFA∶CH2Cl2 (1∶1), 35 min, 86%; (d) H-Arg(Pbf)-OtBu, DCC, DMAP, CH2Cl2, 30 min, 74%; (e) Piperidine, CH2Cl2, 20 min; Fmoc-OSu, NaHCO3, H2O, THF, 2.5 h, 29%. (C) The synthesis of building block 8. Key: (f) Fm-OH, DCC, DMAP, CH2Cl2, 20 min, 28%; (g) 4M HCl in dioxane, 1 h, 50%; (h) SuOC( = O)OCH2CH = CHCH2CH2CO2tBu 17, NaHCO3, H2O, CH3CN, 1 h, 40%; (i) TFA∶CH2Cl2 (1∶1), 40 min; H-Arg(Pbf)-OtBu, DCC, DMAP, CH2Cl2, 10 min, 50%; (j) Piperidine, CH2Cl2, 10 min; Fmoc-OSu, NaHCO3, H2O, THF, 2 h, 94%. (D) LC-MS analysis of synthetic peptide 6.
To test our proposition, we first prepared two arginine-modified building blocks, 7 and 8. For the synthesis of 7, the commercially available Glu derivative, Fmoc-Glu(OtBu)-OH 9, was used as the starting material. This was converted to the 9-fluorenylmethyl ester 10 using N,N′-dicyclohexylcarbodiimide (DCC) as the coupling agent. Following purification, the ester was deprotected by TFA, to afford the free acid. The synthesis of the adduct 11 was achieved by reacting the side-chain carboxyl group with the allylic bromide under phase transfer conditions (44). Removal of the t-butyl group from 11, followed by amide formation between 12 and H-Arg(Pbf)-OtBu, furnished the compound 13. Treatment of amino acid 13 with excess piperidine followed by Fmoc protection of the α-amine group, afforded the desired molecule 7. The synthetic procedures for building block 8 are very similar to those of 7, except that the third step was replaced by the formation of an allylic carbamate.
Peptide 6, which contains four removable allylic type tags at positions 45, 52, 62, and 72, was prepared by solid-phase synthesis using building blocks 7 and 8 on a Novasyn® TGT resin (Novabiochem). The peptide was cleaved from the resin with TFA/triisopropyl silane/H2O (95∶2.5∶2.5). All the acid-labile side-chain protecting groups, including the two Pbf protecting groups in 7 and 8, were removed by this treatment. The resulting crude peptide was purified by HPLC. The LC-MS analysis of this peptide is shown in Fig. 4D. From the analysis, a significant reduction in the rate of aggregation and methionine oxidation was observed when compared with the Alloc and ivDde-protected synthetic fragments. Specifically, we found that the UV peak of peptide 6 did not change with time. After 3 d, the intensity of the electrospray ionization-MS peak for the oxidized peptide remains very low. Additionally, when stirred in the DMSO with hydroxy-3,4-dihydro-4-oxo-1,2,3-benzotriazine and N,N-diisopropylethylamine under air at room temperature, the typical reaction conditions for direct-aminolysis reaction, no significant decrease of peptide 6 in the reaction solution was observed. Most importantly, we were able to efficiently cleave the allylic ester and the allylic carbamate from peptide 6 by using tetrakis(triphenylphosphine)palladium(0) in DMSO in the presence of excess triphenylsilane (42–45).
In summary, we have developed a strategy to improve the physical and chemical properties of the synthetic peptide fragments, especially those with multiple hydrophobic regions. Although the detailed mechanism of action by which the cleavable Arg tags exert their antideterioration effect is not established, on the basis of previous investigation, we surmise that their antiaggregation and antioxidation properties could be attributed to the increased number of guanidino groups in the synthetic fragment (46). Collectively, our data would seem to indicate that the arginines on the periphery of the two hydrophobic patches are able to distort detrimental associations in solution. The suppression of hydrophobic interactions seems to carry with it a corresponding decrease in aggregation and methionine oxidation rates. Such an understanding should be of particular significance in analyzing the principles governing the processes of peptide and protein aggregation and oxidation and could well facilitate the general design of stable polypeptides for protein and glycoprotein chemical synthesis. By varying the linkers that are used to anchor the arginine residues, the approach described here could also be extremely useful in the optimization of biopharmaceuticals and in the development of cell-permeable molecules (20, 47).
Materials and Methods
All commercial reagents and solvents were used without further purification. All reactions were performed under an atmosphere of argon or nitrogen. Automated peptide synthesis was performed on an Applied Biosystems Pioneer continuous flow peptide synthesizer using commercially available and synthetic building blocks. The deblock solution was a mixture of 100/5/5 of dimethylformamide/piperidine/1,8-Diazabicyclo[5.4.0]undec-7-ene. The peptide cleavage solution was a mixture of 95/2.5/2.5 of TFA/TIS/H2O. The purification of the peptides was achieved using a Ranin HPLC solvent delivery system equipped with a Rainin UV-1 detector and Varian Dynamax using Varian Microsorb 300-5, C4 250 × 21.4 mm columns at a flow rate of 16.0 mL/ min. The analysis of the peptide was performed using a Waters 2695 Separations Module and a Waters 996 Photodiode Array Detector equipped with Varian Microsorb 300-5, C4 250 × 2.0 mm columns at a flow rate of 0.2 mL/ min. The mobile phase for HPLC analysis and separations was a mixture of 0.05% TFA (vol/vol) in water (solvent A)/0.04% TFA in acetonitrile (solvent B). Each purified synthetic peptide variant was dissolved in MeCN/H2O/AcOH (47.5∶47.5∶5) to make a 0.12-mM solution. At time zero, 10 μL of the peptide solution were injected into the LC-MS system operated as described above. The analyte was eluted using a linear gradient method: 0 min /30% solvent B and 30 min /95% solvent B. After standing in air for 3 d, each analyte was characterized again using the same conditions. A detailed description of materials and methods is given in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Rebecca Lambert for valuable discussions. We also thank Dr. George Sukenick, Hui Fang, and Sylvi Rusli of Sloan–Kettering Institute’s NMR core facility for mass spectral and NMR assistance and Laura Wilson for assistance with the preparation of the manuscript. Support for this research was provided by National Institute of Health Grant CA28824 (to S.J.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100195108/-/DCSupplemental.
References
- 1.Morris AM, Watzky MA, Finke RG. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim Biophys Acta. 2009;1794:375–397. doi: 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Weiss WF, 4th, Young TM, Roberts CJ. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J Pharm Sci. 2009;98:1246–1277. doi: 10.1002/jps.21521. [DOI] [PubMed] [Google Scholar]
- 3.Di Carlo M. Beta amyloid peptide: From different aggregation forms to the activation of different biochemical pathways. Eur Biophys J. 2010;39:877–888. doi: 10.1007/s00249-009-0439-8. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg AS. Effects of protein aggregates: An immunologic perspective. AAPS J. 2006;8:E501–507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrada LD, Soto C. Inhibition of protein misfolding and aggregation by small rationally-designed peptides. Curr Pharm Design. 2006;12:2557–2567. doi: 10.2174/138161206777698792. [DOI] [PubMed] [Google Scholar]
- 6.Glaser CB, Yamin G, Uversky VN, Fink AL. Methionine oxidation, alpha-synuclein and Parkinson’s disease. Biochim Biophys Acta. 2005;1703:157–169. doi: 10.1016/j.bbapap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield DA, Kanski J. Methionine residue 35 is critical for the oxidative stress and neurotoxic properties of Alzheimer’s amyloid beta-peptide 1–42. Peptides. 2002;23:1299–1309. doi: 10.1016/s0196-9781(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 8.Levine RL, Berlett BS, Moskovitz J, Mosoni L, Stadtman ER. Methionine residues may protect proteins from critical oxidative damage. Mech Ageing Dev. 1999;107:323–332. doi: 10.1016/s0047-6374(98)00152-3. [DOI] [PubMed] [Google Scholar]
- 9.Stadtman ER. Protein oxidation and aging. Free Radical Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 10.Yamin G, Glaser CB, Uversky VN, Fink AL. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. J Biol Chem. 2003;278:27630–27635. doi: 10.1074/jbc.M303302200. [DOI] [PubMed] [Google Scholar]
- 11.Chen SY, et al. Synthetic erythropoietic proteins: Tuning biological performance by site-specific polymer attachment. Chem Biol. 2005;12:371–383. doi: 10.1016/j.chembiol.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins N. Modifications of therapeutic proteins: Challenges and prospects. Cytotechnology. 2007;53:121–125. doi: 10.1007/s10616-007-9075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson EC, Kent SB. Towards the total chemical synthesis of integral membrane proteins: A general method for the synthesis of hydrophobic peptide-thioester building blocks. Tetrahedron Lett. 2007;48:1795–1799. doi: 10.1016/j.tetlet.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiti F, et al. Kinetic partitioning of protein folding and aggregation. Nat Struct Biol. 2002;9:137–143. doi: 10.1038/nsb752. [DOI] [PubMed] [Google Scholar]
- 15.Karpusas M, Whitty A, Runkel L, Hochman P. The structure of human interferon-β: Implications for activity. Cell Mol Life Sci. 1998;54:1203–1216. doi: 10.1007/s000180050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoiberg-Nielsen R, Westh P, Arleth L. The effect of glycosylation on interparticle interactions and dimensions of native and denatured phytase. Biophys J. 2009;96:153–161. doi: 10.1529/biophysj.108.136408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoda T, Arakawa T, Yamaguchi H. N-glycans stabilize human erythropoietin through hydrophobic interactions with the hydrophobic protein surface: Studies by surface plasmon resonance analysis. J Biochem. 2002;131:511–515. doi: 10.1093/oxfordjournals.jbchem.a003128. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Y, et al. Toward homogeneous erythropoietin: Fine tuning of the C-terminal acyl donor in the chemical synthesis of the Cys(29)-Gly(77) glycopeptide domain. J Am Chem Soc. 2009;131:5432–5437. doi: 10.1021/ja808705v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filpula D, Zhao H. Releasable PEGylation of proteins with customized linkers. Adv Drug Deliver Rev. 2008;60:29–49. doi: 10.1016/j.addr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Fowler SB, et al. Rational design of aggregation-resistant bioactive peptides: Reengineering human calcitonin. Proc Natl Acad Sci USA. 2005;102:10105–10110. doi: 10.1073/pnas.0501215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaraman M, Kodali R, Wetzel R. The impact of ataxin-1-like histidine insertions on polyglutamine aggregation. Protein Eng Des Sel. 2009;22:469–478. doi: 10.1093/protein/gzp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackland CE, et al. Monitoring of protein conformation by high-performance size-exclusion liquid chromatography and scanning diode array second-derivative UV absorption spectroscopy. J Chromatogr. 1991;540:187–198. doi: 10.1016/s0021-9673(01)88808-7. [DOI] [PubMed] [Google Scholar]
- 23.Galeva NA, Esch SW, Williams TD, Markille LM, Squier TC. Rapid method for quantifying the extent of methionine oxidation in intact calmodulin. J Am Soc Mass Spectr. 2005;16:1470–1480. doi: 10.1016/j.jasms.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Hattan SJ, Parker KC. Methodology utilizing MS signal intensity and LC retention time for quantitative analysis and precursor ion selection in proteomic LC-MALDI analyses. Anal Chem. 2006;78:7986–7896. doi: 10.1021/ac0610513. [DOI] [PubMed] [Google Scholar]
- 25.Tan Z, Shang S, Halkina T, Yuan Y, Danishefsky SJ. Toward homogeneous erythropoietin: Non-NCL-based chemical synthesis of the Gln(78)-Arg(166) glycopeptide domain. J Am Chem Soc. 2009;131:5424–5431. doi: 10.1021/ja808704m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kan C, et al. Toward homogeneous erythropoietin: Chemical synthesis of the Ala(1)-Gly(28) glycopeptide domain by “alanine” ligation. J Am Chem Soc. 2009;131:5438–5443. doi: 10.1021/ja808707w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–808. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 28.Pawar AP, et al. Prediction of “aggregation-prone” and “aggregation-susceptible” regions in proteins associated with neurodegenerative diseases. J Mol Biol. 2005;350:379–392. doi: 10.1016/j.jmb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins MR, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- 30.Dasilva KA, Shaw JE, McLaurin J. Amyloid-beta fibrillogenesis: structural insight and therapeutic intervention. Exp Neurol. 2010;223:311–321. doi: 10.1016/j.expneurol.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Johansson AS, et al. Attenuated amyloid-beta aggregation and neurotoxicity owing to methionine oxidation. Neuroreport. 2007;18:559–563. doi: 10.1097/WNR.0b013e3280b07c21. [DOI] [PubMed] [Google Scholar]
- 32.Schoneich C. Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer’s disease. Biochim Biophys Acta. 2005;1703:111–119. doi: 10.1016/j.bbapap.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Kanski J, Aksenova M, Schoneich C, Butterfield DA. Substitution of isoleucine-31 by helical-breaking proline abolishes oxidative stress and neurotoxic properties of Alzheimer’s amyloid beta-peptide. Free Radical Bio Med. 2002;32:1205–1211. doi: 10.1016/s0891-5849(02)00821-3. [DOI] [PubMed] [Google Scholar]
- 34.Schoneich C. Redox processes of methionine relevant to beta-amyloid oxidation and Alzheimer’s disease. Arch Biochem Biophys. 2002;397:370–376. doi: 10.1006/abbi.2001.2621. [DOI] [PubMed] [Google Scholar]
- 35.Arakawa T, Tsumoto K, Kita Y, Chang B, Ejima D. Biotechnology applications of amino acids in protein purification and formulations. Amino Acids. 2007;33:587–605. doi: 10.1007/s00726-007-0506-3. [DOI] [PubMed] [Google Scholar]
- 36.Calamai M, Taddei N, Stefani M, Ramponi G, Chiti F. Relative influence of hydrophobicity and net charge in the aggregation of two homologous proteins. Biochemistry. 2003;42:15078–15083. doi: 10.1021/bi030135s. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence MS, Phillips KJ, Liu DR. Supercharging proteins can impart unusual resilience. J Am Chem Soc. 2007;129(33):10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aimoto S. Contemporary methods for peptide and protein synthesis. Curr Org Chem. 2001;5:45–87. [Google Scholar]
- 39.Cheetham JC, et al. NMR structure of human erythropoietin and a comparison with its receptor bound conformation. Nat Struct Biol. 1998;5:861–866. doi: 10.1038/2302. [DOI] [PubMed] [Google Scholar]
- 40.Guillier F, Orain D, Bradley M. Linkers and cleavage strategies in solid-phase organic synthesis and combinatorial chemistry. Chem Rev. 2000;100:2091–2158. doi: 10.1021/cr980040+. [DOI] [PubMed] [Google Scholar]
- 41.Gartner ZJ, Kanan MW, Liu DR. Multistep small-molecule synthesis programmed by DNA templates. J Am Chem Soc. 2002;124:10304–10306. doi: 10.1021/ja027307d. [DOI] [PubMed] [Google Scholar]
- 42.Seitz O, Kunz H. A novel allylic anchor for solid-phase synthesis—Synthesis of protected and unprotected O-glycosylated mucin-type glycopeptides. Angew Chem Int Edit. 1995;34:803–805. [Google Scholar]
- 43.Seitz O, Kunz H. HYCRON, an allylic anchor for high-efficiency solid phase synthesis of protected peptides and glycopeptides. J Org Chem. 1997;62:813–826. [Google Scholar]
- 44.Nakahara Y, Ando S, Ito Y, Hojo H. New allyl ester linker and solid-phase block synthesis of the serglycin core region. Biosci Biotechnol Biochem. 2001;65:1358–1368. doi: 10.1271/bbb.65.1358. [DOI] [PubMed] [Google Scholar]
- 45.Seitz O, Wong CH. Chemoenzymatic solution- and solid-phase synthesis of O-glycopeptides of the mucin domain of MAdCAM-1. A general route to O-LacNAc, O-sialyl-LacNAc, and O-sialyl-Lewis-X peptides. J Am Chem Soc. 1997;119:8766–8776. [Google Scholar]
- 46.Javor S, Natalello A, Doglia SM, Reymond J-L. α-Helix stabilization within a peptide dendrimer. J Am Chem Soc. 2008;130:17248–17249. doi: 10.1021/ja8076236. [DOI] [PubMed] [Google Scholar]
- 47.Sakai N, Takeuchi T, Futaki S, Matile S. Direct observation of anion-mediated translocation of fluorescent oligoarginine carriers into and across bulk liquid and anionic bilayer membranes. ChemBioChem. 2005;6:114–122. doi: 10.1002/cbic.200400256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.