Abstract
We report the design and synthesis of an indolyne that displays a reversal in regioselectivity, in both nucleophilic addition and cycloaddition reactions, compared to typical 4,5-indolynes. Our approach utilizes simple computations to predict regioselectivity in reactions of unsymmetrical arynes. With this methodology, novel benzenoid-substituted indoles can be accessed with significant regiocontrol. Furthermore, the technology provides an unconventional tactic for the synthesis of C4-substituted indole alkaloids, as demonstrated by a synthesis of indolactam V.
The past decades have witnessed a resurgence in the chemistry of arynes. Whereas classical methods for aryne generation are typically plagued by low yields, modern methodologies have overcome these limitations; arynes can now be employed efficiently in a variety of synthetic applications.1 These advances have been accompanied by an interest in exploiting unsymmetrical arynes as synthetic intermediates, although such studies have been largely constrained by a lack of regiocontrol.2
In collaboration with Houk and coworkers, we recently proposed that distortion in unsymmetrical arynes controls regioselectivity, and simple computations may be used to make selectivity predictions.3 To test this distortion model and its predictive powers, we sought to control regioselectivity in nucleophilic addition to indolynes,3,4,5 which are unsymmetrical arynes that our laboratory has examined for their synthetic versatility. A method for overturning indolyne regioselectivity has not been previously established, but would provide an invaluable tool for synthesizing both natural and unnatural derivatives of the medicinally privileged indole scaffold.6,7
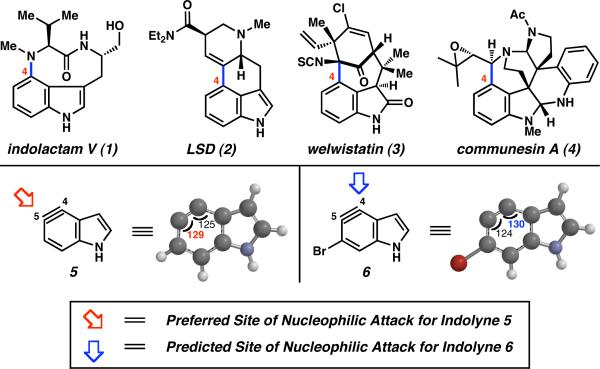
In this communication, we demonstrate that regioselectivity in the nucleophilic addition to indolynes can be readily manipulated using the predictive capabilities of the distortion model. The studies provide access to unique benzenoid-substituted indoles and offer a strategically distinct approach to C4-substituted indole alkaloids.8 The latter notion is exemplified by a concise synthesis of indolactam V (1, Figure 1).9
Figure 1.
C4-substituted alkaloids 1–4 and indolynes 5 and 6.
As highlighted in Figure 1, we focused our efforts on 4,5-indolynes, which could potentially be used to access 4-substituted indole derivatives, such as 1–4.8 Whereas 4,5-indolyne 5 exhibits a preference for nucleophilic attack at C5,3b we hypothesized that brominated derivative 6 may be prone to undergo attack at C4.10,11 Although the relative influence of the bromide substituent and ring fusion on aryne distortion were not obvious,12 simple calculations were used to validate our hypothesis. Specifically, geometry optimization of bromoindolyne 6 showed that C4 possesses a larger internal angle compared to C5 (i.e., θ4 = 130° and θ5 = 124°).13 Following our distortion model,3 the flatter, more electropositive carbon (C4) was predicted to be the preferred site of attack by nucleophiles.14
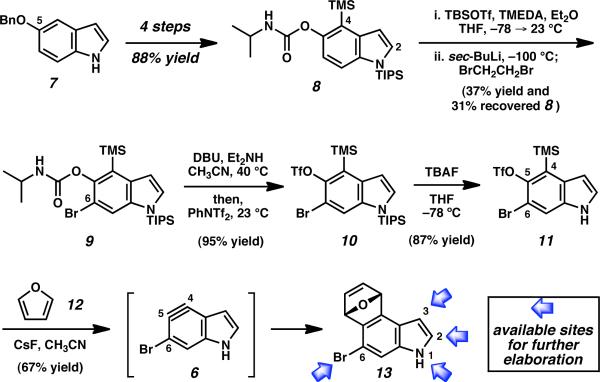
We envisioned accessing bromoindolyne 6 from a silyltriflate precursor. Although halobenzynes have not previously been generated by the Kobayashi method,15 we were able to synthesize the targeted silyltriflate 11 using the route shown in Scheme 1. Commercially available 5-benzyloxyindole (7) was elaborated to silylcarbamate 8 using our previously reported, high-yielding sequence.3b Subsequent C6 bromination was achieved using the general lithiation/quenching protocol developed by Snieckus and Hoppe to afford intermediate 9.16,17 Installation of the triflate (9→10), followed by removal of the N-TIPS group, furnished the desired indolyne precursor 11. The sequence is robust and can be used to prepare gram quantities of 11. To validate that indolyne 6 would be accessible, silyltriflate 11 was treated with CsF in the presence of furan to generate oxabicycle 13. Of note, compound 13 possesses sites for further functionalization on both the pyrrolo7,18 and benzenoid ring.19
Scheme 1.
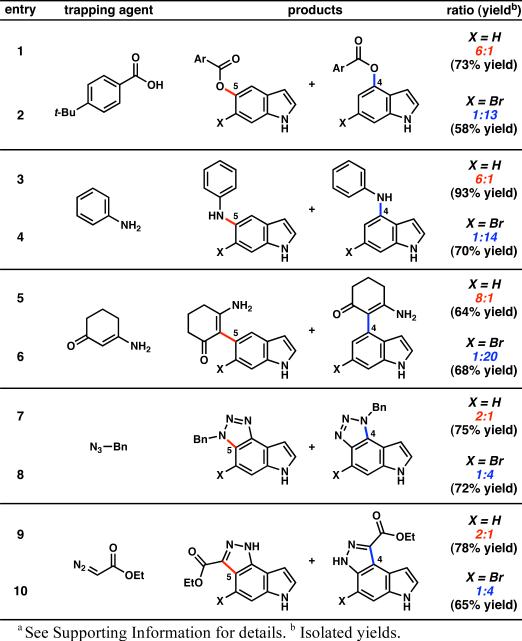
With access to bromoindolyne 6 and parent indolyne 5,3b each from a silyltriflate precursor, a comparative regioselectivity study was carried out with a range of trapping agents (Table 1). In each case, the indolyne precursors were treated with CsF in the presence of the appropriate trapping agent. When 4-t-Bu-benzoic acid was used to trap indolyne 5,20 the reaction occurred with significant regioselectivity favoring attack at C5 (entry 1). In contrast, the corresponding reaction of bromoindolyne 6 displayed a preference for attack at C4 (regioselectivity = 1:13 for attack at C5:C4). Similar trends were observed in reactions involving aniline20 (entries 3 and 4) and an enamine derivative21 (entries 5 and 6). In the latter case, selectivity was 1:20 favoring attack at C4 on indolyne 6. Cycloadditions were also examined and similar reversals in regioselectivity were observed. For example, cycloaddition of indolyne 5 with benzyl azide22 gave a mixture of products, favoring attack at C5 over C4 in a 2:1 ratio (entry 7). However, the corresponding reaction with indolyne 6 led to a 1:4 mixture of products favoring attack at C4 (entry 8). Analogous results were obtained in reactions with diazoesters2a,23 to furnish unique indolylpyrazoles (entries 9 and 10). These results clearly indicate that bromoindolyne 6 displays a reversal in regioselectivity compared to its non-brominated counterpart 5 and validate the distortion model for predicting regioselectivities in additions to unsymmetrical arynes.
Table 1.
Nucleophilic Additions to Indolyne 5 and 6-Bromoindolyne 6a
|
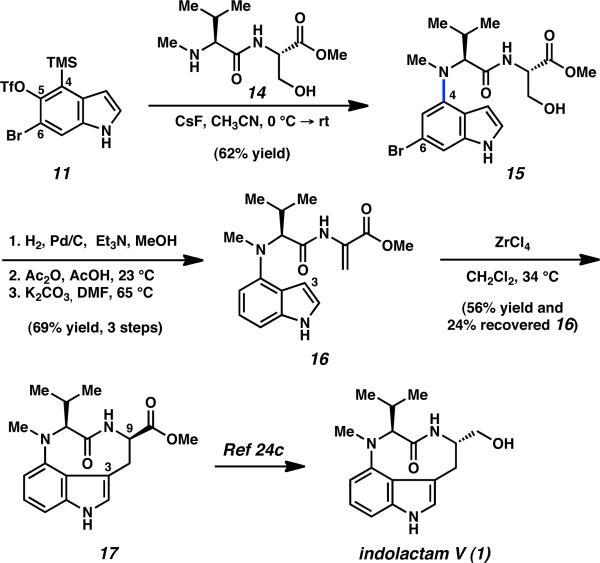
To probe the utility of our findings in a complex setting, we undertook a total synthesis of indolactam V (1), one of many biologically active C4-substituted indoles.8,9 In nearly all previous syntheses of 1,24 the nine-membered ring is fashioned by late-stage amide bond formation. We envisioned a strategically distinct approach to 1, which involved initial C4 functionalization using bromoindolyne 6, followed by ring closure at C3.25 As shown in Scheme 2, treatment of silyltriflate 11 with peptide 14 in the presence of CsF furnished C4-aminated product 15 in 62% yield, even though 14 possesses multiple nucleophilic sites. As expected based on our distortion model and experimental studies (see Table 1), the transformation proceeded with high regioselectivity.26 Subsequent debromination, followed by dehydration,27 provided unsaturated ester 16 without event. We next examined the critical cyclization at C3. After extensive experimentation, it was found that exposure of 16 to ZrCl4 in CH2Cl2 facilitated the desired annulation to give tricycle 17,28 thus completing a formal synthesis of 1.24c,29 Interestingly, the stereochemical configuration at C9 of 17 was set with complete diastereoselectivity, albeit in the undesired sense. Nonetheless, C9 epimerization and reduction using Nakatsuka's protocol24c furnished indolactam V (1). This is the first total synthesis in which an indolyne has been exploited for its electrophilic character.30 We expect that our approach to 1, using an umpolung of typical indole reactivity, will be suitable for the synthesis of other indole alkaloids.
Scheme 2.
In summary, we have designed and synthesized an indolyne (i.e., 6) that displays a reversal in regioselectivity, in both nucleophilic addition and cycloaddition reactions, compared to the parent 4,5-indolyne 5. Our approach validates the aryne distortion model,3 which in turn, utilizes simple computations13 to predict regioselectivity in reactions of unsymmetrical arynes. With this methodology, novel benzenoid-substituted indoles can be accessed with significant regiocontrol. Moreover, the technology provides an unconventional tactic for the synthesis of C4-substituted indole alkaloids, as demonstrated by our synthesis of indolactam V (1). Further studies aimed at probing the aryne distortion model in complex molecule synthesis are currently underway in our laboratory.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to the NIH-NIGMS (R01 GM090007), Boehringer Ingelheim, DuPont, Eli Lilly, the Foote Fellowship (S.M.B.), and the University of California, Los Angeles, for financial support. We thank the Garcia-Garibay laboratory (UCLA) for access to instrumentation, Dr. Robert Paton and Professor Ken Houk (UCLA) for helpful discussions, Dr. John Greaves (UC Irvine) for mass spectra, and Kyle Quasdorf (UCLA), Joshua Melamed (UCLA), and Jordan Cisneros (UCLA) for experimental assistance.
Footnotes
Supporting Information Available: Detailed experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.For reviews, see: Pellissier H, Santelli M. Tetrahedron. 2003;59:701–730.Wenk HH, Winkler M, Sander W. Angew. Chem. Int. Ed. 2003;42:502–528. doi: 10.1002/anie.200390151.Sanz R. Org. Prep. Proced. Int. 2008;40:217–291.Chen Y, Larock RC. Arylation Reactions Involving the Formation of Arynes. In: Ackermann L, editor. Modern Arylation Methods. Wiley–VCH; Weinheim: 2009. pp. 401–473.
- 2.With the exception of 3-alkoxybenzynes, most unsymmetrical arynes show modest or variable regioselectivity. For pertinent reviews, see ref 1. For recent examples involving naphthalynes, see: Jin T, Yamamoto Y. Angew. Chem. Int. Ed. 2007;46:3323–3325. doi: 10.1002/anie.200700101.Rémond E, Tessier A, Leroux FR, Bayardon J, Jugé S. Org. Lett. 2010;12:1568–1571. doi: 10.1021/ol100304c. For recent examples involving 3-alkylbenzynes, see: Spiter C, Keeling S, Moses JE. Org. Lett. 2010;12:3368–3371. doi: 10.1021/ol101150t.Crossley JA, Browne DL. Tetrahedron Lett. 2010;51:2271–2273.Hari Y, Sone R, Aoyama T. Org. Biomol. Chem. 2009;7:2804–2808. doi: 10.1039/b907796k.Akai S, Ikawa T, Takayanagi S.-i., Morikawa Y, Mohri S, Tsubakiyama M, Egi M, Wada Y, Kita Y. Angew. Chem. Int. Ed. 2008;47:7673–7676. doi: 10.1002/anie.200803011. For benzynocycloalkanes, see: Hamura T, Ibusuki Y, Sato K, Matsumoto T, Osamura Y, Suzuki K. Org. Lett. 2003;5:3551–3554. doi: 10.1021/ol034877p. Scattered reports involving 3-silylbenzynes have appeared, which can react with significant regioselectivities; see: Dai M, Wang Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6613–6616. doi: 10.1016/j.tetlet.2008.09.019.Matsumoto T, Sohma T, Hatazaki S, Suzuki K. Synlett. 1993:843–846.; see also ref 2f. For a recent example of alkoxybenzynes undergoing regioselective reactions, see: Tadross PM, Gilmore CD, Bugga P, Virgil SC, Stoltz BM. Org. Lett. 2010;12:1224–1227. doi: 10.1021/ol1000796. see also references therein.
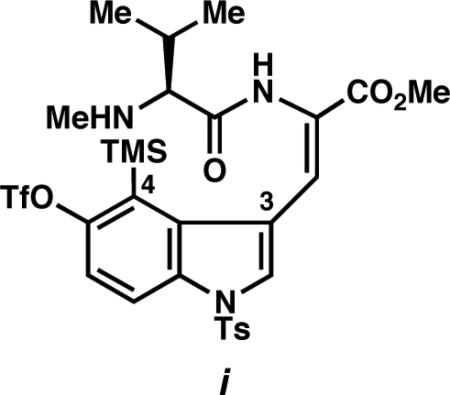
- 3.a Cheong PH-Y, Paton RS, Bronner SM, Im G-Y, Garg NK, Houk KN. J. Am. Chem. Soc. 2010;132:1267–1269. doi: 10.1021/ja9098643. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Im G-Y, Bronner SM, Goetz AE, Paton RS, Cheong PH-Y, Houk KN, Garg NK. J. Am. Chem. Soc. 2010;132:17933–17944. doi: 10.1021/ja1086485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For seminal indolyne studies, see: Julia M, Huang Y, Igolen J. C. R. Acad. Sci., Ser. C. 1967;265:110–112.Igolen J, Kolb A. C. R. Acad. Sci., Ser. C. 1969;269:54–56. For related studies, see: Julia M, Goffic FL, Igolen J, Baillarge M. C. R. Acad. Sci., Ser. C. 1967;264:118–120.Julia M, Igolen J, Kolb M. C. R. Acad. Sci., Ser. C. 1971;273:1776–1777.
- 5.For recent indolyne studies, see: Bronner SM, Bahnck KB, Garg NK. Org. Lett. 2009;11:1007–1010. doi: 10.1021/ol802958a.Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. doi: 10.1021/ol9007684.Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org. Lett. 2007;9:4135–4137. doi: 10.1021/ol701595n.Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65. doi: 10.1016/j.tetlet.2008.10.086.Buszek KR, Brown N, Luo D. Org. Lett. 2009;11:201–204. doi: 10.1021/ol802425m.Brown N, Luo D, Decapo JA, Buszek KR. Tetrahedron Lett. 2009;50:7113–7115. doi: 10.1016/j.tetlet.2009.09.083.Garr AN, Luo D, Brown N, Cramer CJ, Buszek KR, VanderVelde D. Org. Lett. 2010;12:96–99. doi: 10.1021/ol902415s.
- 6.a Horton DA, Bourne GT, Smyth ML. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]; b Rodrigues de Sa Alves F, Barreiro EJ, Fraga CAM. Mini-Rev. Med. Chem. 2009;9:782–793. doi: 10.2174/138955709788452649. [DOI] [PubMed] [Google Scholar]
- 7.a Sundberg RJ. The Chemistry of Indoles. Academic Press; New York: 1970. [Google Scholar]; b Sundberg RJ. Pyrroles and Their Benzoderivatives: Synthesis and Applications. In: Katritzky AR, Rees CW, editors. Comprehensive Heterocyclic Chemistry. Vol. 4. Pergamon Press; Oxford: 1984. pp. 313–376. [Google Scholar]; c Sundberg RJ. Indoles (Best Synthetic Methods) Academic Press; New York: 1996. pp. 7–11. [Google Scholar]; d Joule JA. Indole and its Derivatives. In: Thomas EJ, editor. Science of Synthesis: Houben–Weyl Methods of Molecular Transformations. Vol. 10. George Thieme Verlag; Stuttgart: 2000. Category 2. Chapter 10.13. [Google Scholar]
- 8.A Beilstein/Crossfire search shows that more than 600 C4-substituted indole-containing natural products exist and nearly 10,000 bioactive C4-substituted indoles have been reported.
- 9.For a pertinent review on indolactam isolation, tumor-promoting activity, and derivatives, see: Irie K, Koshimizu K. Comments Agric. Food Chem. 1993;3:1–25. For a recent report on indolactam V's stem cell differentiating abilities, see: Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. Nat. Chem. Biol. 2009;5:258–265. doi: 10.1038/nchembio.154.
- 10.Using a bromide to control aryne regioselectivity is ideal, since a bromide can be easily removed or readily used for further arene functionalization; see also ref 19.
- 11.For examples where an adjacent halide influences benzyne regioselectivities; see: Biehl ER, Nieh E, Hsu KC. J. Org. Chem. 1969;34:3595–3599.Moreau-Hochu MF. Tetrahedron. 1977;33:955–959.Ghosh T, Hart H. J. Org. Chem. 1988;53:3555–3558.Hart H, Ghosh T. Tetrahedron Lett. 1988;29:881–884.Wickham PP, Reuter KH, Senanyake D, Guo H, Zalesky M, Scott WJ. Tetrahedron Lett. 1993;34:7521–7524.Gokhale A, Scheiss R. Helv. Chem. Acta. 1998;81:251–267.Gribble GW, Keavy DJ, Branz SE, Kelly WJ, Pals MA. Tetrahedron Lett. 1998;29:6227–6230.
- 12.To our knowledge, the relative influences of a polar substituent and ring fusion on aryne distortion has not been evaluated previously.
- 13.Geometry optimization of indolynes was performed using DFT methods (B3LYP/6-31G*) on MacSpartan software. Calculations typically require 3–5 min.
- 14.The influence of the bromide substituent on indolyne distortion is believed to be the result of inductive effects. The observed regioselectivities likely arise from a combination of indolyne distortion and steric effects caused by the bromide substituent.
- 15.Himeshima Y, Sonoda Y, Kobayashi H. Chem. Lett. 1983:1211–1214. [Google Scholar]
- 16.a Kauch M, Snieckus V, Hoppe D. J. Org. Chem. 2005;70:7149–7158. doi: 10.1021/jo0506938. [DOI] [PubMed] [Google Scholar]; b Kauch M, Hoppe D. Synthesis. 2006:1578–1589. [Google Scholar]
- 17.The conversion of 8 to 9 is challenging because of competitive anionic Fries rearrangement.
- 18.For a recent review, see: Bandini M, Eichholzer A. Angew. Chem. Int. Ed. 2009;48:9608–9644. doi: 10.1002/anie.200901843.
- 19.The C6 bromide provides a convenient handle for functionalization through metal-catalyzed cross-couplings, radical reactions, or halogen-metal exchange.
- 20.For the addition of nucleophiles to arynes generated from silyltriflates, see: Liu Z, Larock RC. J. Org. Chem. 2006;71:3198–3209. doi: 10.1021/jo0602221.
- 21.Ramtohul YK, Chartrand A. Org. Lett. 2007;9:1029–1032. doi: 10.1021/ol063057k. [DOI] [PubMed] [Google Scholar]
- 22.a Shi F, Waldo JP, Chen Y, Larock RC. Org. Lett. 2008;10:2409–2412. doi: 10.1021/ol800675u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Campbell-Verduyn L, Elsinga PH, Mirfeizi L, Dierckx RA, Feringa BL. Org. Biomol. Chem. 2008;6:3461–3463. doi: 10.1039/b812403e. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Shi F, Martinez PDG, Raminelli C, Larock RC. J. Org. Chem. 2008;73:219–226. doi: 10.1021/jo702062n. [DOI] [PubMed] [Google Scholar]
- 24.For synthesis of indolactam V, see: Endo Y, Shudo K, Itai A, Hasegawa M, Sakai S.-i. Tetrahedron. 1986;42:5905–5924.de Laszlo SE, Ley SV, Porter RA. J. Chem. Soc., Chem. Commun. 1986:344–346.Masuda T, Nakatsuka S.-i., Goto T. Agric. Biol. Chem. 1989;53:2257–2260.Mascal M, Moody CJ, Slawin AMZ, Williams DJ. J. Chem. Soc. Perkin Trans. 1. 1992:823–830.Semmelhack MF, Rhee H. Tetrahedron Lett. 1993;34:1395–1398.Kogan TP, Somers TC, Venuti MC. Tetrahedron. 1990;46:6623–6632.Meseguer B, Alonso-Díaz D, Griebenow N, Herget T, Waldmann H. Chem. Eur. J. 2000;6:3943–3957. doi: 10.1002/1521-3765(20001103)6:21<3943::aid-chem3943>3.3.co;2-k.Suárez AI, García MC, Compagnone RS. Synth. Commun. 2004;34:523–531.Xu Z, Zhang F, Zhang L, Jia Y. Org. Biomol. Chem. DOI 10.1039/C0OB01115K.
-
25.Prior to the development of our distortion model, we attempted to construct the C4–N bond of indolactam V by indolyne cyclization of substrate i (and derivatives thereof). These efforts led to substantial decomposition, without trace of cyclized products.
- 26.Less than 5% of the corresponding C5-substituted product was detected.
-
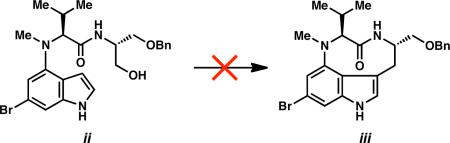
27.All attempts to activate the alcohol of 15 (or the corresponding des-bromo derivative) for indole displacement were accompanied by dehydration. Efforts to convert related indolyne adduct ii to cyclized product iii were also unsuccessful.
- 28.Angelini E, Balsamini C, Bartoccini F, Lucarini S, Piersanti G. J. Org. Chem. 2008;73:5654–5657. doi: 10.1021/jo800881u. [DOI] [PubMed] [Google Scholar]
- 29.Characterization data for 17 matched the data reported by Nakatsuka (see ref 24c and the Supporting Information)
- 30.For a total synthesis using an indolyne cycloaddition, see: ref 5e.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.