Abstract
Experiments tested the hypothesis that P2 receptor reactivity is impaired in Angiotensin II hypertensive rats fed an 8% NaCl diet (AngII+HS). Juxtamedullary afferent arteriolar autoregulatory behavior was determined over a pressure range of 65 to 200mmHg. Arteriolar responsiveness to P2X1 (β, γ-methylene ATP), or P2Y2 receptor (uridine triphosphate) activation were determined, in vitro. Systolic blood pressure averaged 126±3 mmHg and 225±4 mmHg in control and AngII+HS rats, respectively (P<0.05). In control kidneys, β, γ-methylene ATP (10−8 to 10−4 mol/L) reduced arteriolar diameter by 8±3, 13±5, 19±5, 22±6 and 24±9%, respectively, whereas uridine triphosphate reduced diameter by 2±1, 2±2, 9±3, 37±7 and 58±7%. Autoregulation was markedly blunted in AngII+HS kidneys, with arteriolar diameter remaining essentially unchanged when perfusion pressure increased to 200mmHg, compared to a 40±2% decline in diameter observed in normal kidneys over the same pressure range (P<0.05). P2X1 receptor-mediated vasoconstriction was significantly attenuated in AngII+HS kidneys. β, γ-methylene ATP reduced arteriolar diameter by 1±1, 3±2, 6±1, 9±3 and 7±1%, respectively (P<0.05) vs. control rats. Similar patterns were noted when hypertensive perfusion pressures were used. Uridine triphosphate-mediated responses were unchanged in AngII+HS rats compared to control, indicating preservation of P2Y2 receptor function. AngII+HS blunted P2X1-mediated increases in intracellular Ca2+ concentration in preglomerular smooth muscle cells. Therefore, AngII+HS rats exhibit attenuated afferent arteriolar responses to P2X1 receptor stimulation. These data support the hypothesis that P2X1 receptors are important for pressure-mediated autoregulatory responses. Impairment of P2X1 receptor function may explain the hypertension-induced decline in renal autoregulatory capability.
Keywords: P2 receptors, Ang II hypertension, Ca2+ signaling, autoregulation, UTP, ATP, adenosine
INTRODUCTION
Previous work indicates that P2X1 receptors contribute importantly to pressure-mediated autoregulatory vasoconstriction of afferent arterioles.1, 2 P2X1 receptors are ligand-gated channels that stimulate afferent arteriolar vasoconstriction through calcium (Ca2+) influx-dependent mechanisms.3–5 P2Y2 receptors also stimulate vasoconstriction but do so mainly through release of Ca2+ from intracellular stores.3, 4 Inactivation of P2X1 receptors blocks pressure-mediated autoregulatory vasoconstriction.1, 2 suggesting that increases in transmural pressure lead to autoregulatory vasoconstriction by ATP-dependent P2X1 receptor activation.
Renal perfusion is primarily controlled by agonist-induced and pressure-mediated adjustments in preglomerular resistance.6 Regulation of preglomerular resistance is central to maintenance of glomerular capillary pressure. Afferent arterioles are the main vascular elements responsible for pressure-mediated autoregulatory adjustments in preglomerular resistance to regulate renal blood flow and glomerular filtration rate.6 Autoregulatory reactivity is attenuated in several forms of hypertension, including the angiotensin II–infused (Ang II-infused) model 7–13; however the mechanisms responsible for blunted autoregulatory behavior are unclear. Along with a reduced response to pressure, afferent arterioles from hypertensive rats also show blunted vasoconstrictor responses to P2X1 receptor activation.11, 12 Therefore, reduction of P2X1 receptor reactivity strongly correlates with blunted autoregulatory efficiency.
This study was performed to extend the previous report.11 to determine if afferent arteriolar autoregulatory behavior and P2X1 receptor-mediated vasoconstriction could be eliminated in a model of salt-sensitive hypertension that includes increased dietary salt. For these studies, we combined chronic sub-pressor infusion of Ang II with a high salt diet. We examined autoregulatory behavior, afferent arteriolar P1 and P2 receptor reactivity and preglomerular smooth muscle cell Ca2+ signaling. Results indicate blunted but not abolished (passive vascular behavior) autoregulatory responsiveness, coincident with reduced P2X1 receptor reactivity and Ca2+ signaling.
METHODS
Please see http://hyper.ahajournals.org for an expanded description of the methods.
Animals
Experiments were performed on male Sprague-Dawley rats weighing 225–250 g. Some rats received chronic Ang II infusion (60 ng/min; 14 days) by osmotic minipump, as described.11, 12 Ang II infused rats received an 8% salt diet. Systolic blood pressure (SBP) and body weight was measured every 3–4 days. Animals were treated according to the NIH Guide for the Care and Use of Laboratory Animals using procedures approved by the Medical College of Georgia Institutional Animal Care and Use Committee.
Afferent Arteriole Responses
Videomicroscopy experiments were conducted in vitro using the blood-perfused juxtamedullary nephron technique, as described.12, 14 Sustained afferent arteriolar diameter was calculated from the average of measurements made during the final 2 minutes of each treatment period. For the autoregulatory portion, arteriolar diameters were measured during step (15 mmHg) increases in renal perfusion pressure from 65 to 170 mmHg in successive 5-minute periods 11 followed by a 10 min recovery period (100 mmHg). A separate group of kidneys underwent an autoregulatory profile that ranged from 95 to 200 mmHg to reflect the hypertensive pressures. Then arteriolar responses to P1 or P2 agonists were determined. Afferent arterioles were exposed to increasing concentrations of ATP β, γ,–methylene ATP, UTP or adenosine (10−8 to 10−4 mol/L), and diameter was measured.
Renal Microvascular Smooth Muscle Cell Isolation
Preglomerular microvessels were isolated from separate rats as described. 11 Dispersed cells were collected and loaded with the calcium-sensitive fluorescent probe, fura 2-acetoxymethyl ester (fura 2-AM; 10.0 mol/L) for study. Measurement of intracellular calcium concentration ([Ca2+]i) in single preglomerular smooth muscle cells was performed as described.5, 11 Cells were exposed to PSS containing ATP, β, γ-methylene ATP or UTP (10 μmol/L), or to adenosine (1 and 100 μmol/L). Ca2+ responses were evaluated by averaging the magnitude of the peak [Ca2+]i achieved during 200 seconds of agonist exposure.
Western Blot Analysis of P2X1 Receptor Expression in Kidney
Homogenates of renal cortex, medulla and preglomerular microvessels were prepared using kidneys from separate control or AngII+HS rats, as described.11, 15
RESULTS
AngII+HS treatment increased SBP (225±4 mmHg; P<0.05) significantly compared to normotensive control rats (126±3 mmHg; Table 1; Figure S1). SBP increased similarly in all groups of AngII+HS rats prepared for different agonist protocols (Table 1). Body weights were similar across all groups and averaged approximately 230 g prior to initiation of Ang II infusion and HS diet. AngII+HS rats gained weight during the 14-day treatment period but not as rapidly as normotensive controls. By day 14, body weights averaged 292±7 g in AngII+HS rats compared to 357±15 g in controls (P<0.05).
Table 1.
Mean Systolic Blood Pressure Data on Days 0, 7 and 14.
| Treatment Group (n=) | Systolic Pressure (mmHg) | ||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| Control (24) | 128 ± 4 | 125 ± 8 | 126 ± 3 |
| ATP (7) | 118 ± 4 | 206 ± 16 | 234 ± 6 |
| β, γ-methylene ATP (7) | 115 ± 5 | 186 ± 24 | 232 ± 8 |
| UTP (8) | 123 ± 3 | 215 ± 3 | 213 ± 10 |
| Adenosine (7) | 123 ± 4 | 206 ± 4 | 227 ± 7 |
Autoregulatory studies
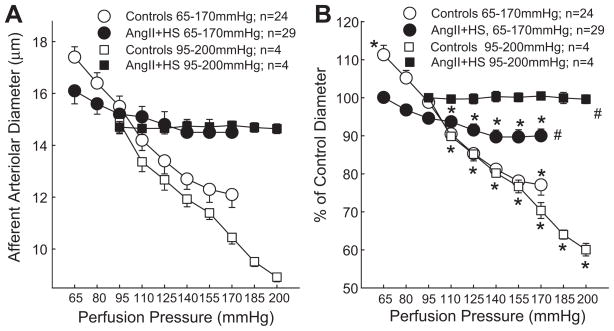
Afferent arteriolar diameter averaged 15.7±0.5 μm in control rats at 100 mmHg (n=24), and decreased by 23±3% (P<0.05 vs. control) when renal perfusion pressure increased from 65 to 170 mmHg (Figure 1). In contrast, increasing perfusion pressure to 170 mmHg in AngII+HS rats reduced afferent diameter by just 9±2% (P<0.05 vs. control group) from a control of 16.0±0.4 μm (Figure 1). The magnitude of the pressure-mediated vasoconstrictor response declined significantly in hypertensive AngII+HS kidneys compared to normotensive controls.
Figure 1.
Afferent arteriolar response to increasing renal perfusion pressure in kidneys of normotensive (Open Symbols) and AngII+HS (Closed Symbols) rats. Autoregulatory profiles ranging from 65 to 170mmHg are depicted by circular symbols whereas profiles generated from 95 to 200mmHg are depicted by square symbols. Panel A represents the actual diameter responses expressed in microns (μm). Panel B expresses the same data normalized as a percent of the starting diameter for each group (% of control). Data points represent the means±SE. * represents a significant difference (P<0.05) from the baseline diameter at 100 mmHg; # represents a significant difference (P<0.05) from the response of normotensive controls; n = the number of afferent arterioles studied.
In separate rats, autoregulatory behavior was determined between 95 and 200 mmHg. Baseline arteriolar diameter averaged 14.9±0.4 μm in control rats, at 95 mmHg (n=4), and decreased by 40±2% (P<0.05 vs. control) when renal perfusion pressure increased to 200 mmHg (Figure 1). In contrast, increasing perfusion pressure from 95 to 200 mmHg in AngII+HS (n=4) rats had no significant effect on afferent diameter. The pressure-mediated vasoconstrictor response was significantly blunted in hypertensive AngII+HS kidneys compared to normotensive controls.
Afferent arteriolar responses to ATP
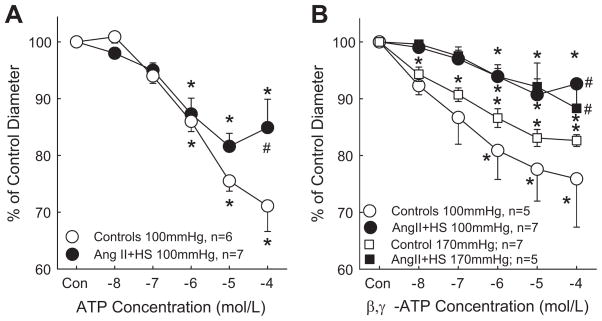
Responses to ATP are presented in Figure 2A. Baseline diameters were similar between the control and AngII+HS groups, averaging 15.7±0.9 μm (n=6) and 14.9±0.5 μm (n=7), respectively. In normotensive rats, superfusion with ATP changed arteriolar diameter to 101±1, 94±1, 86±2, 76±2 and 71±5% of control diameter for ATP concentrations of 10−8 to 10−4 mol/L. AngII+HS blunted vasoconstrictor responses to ATP as diameter decreased only to 98±1, 95±1, 87±3, 81±4 and 85±5% of control over the same concentration range. These data demonstrate that arteriolar responses to ATP are attenuated in AngII+HS kidneys.
Figure 2.
Afferent arteriolar response to increasing concentrations of ATP (Panel A) or β, γ-methylene ATP (Panel B). Results from kidneys perfused at 100mmHg are depicted by circular symbols whereas data from kidneys perfused at 170mmHg are depicted by square symbols. Agonists were applied in the superfusate in successive log increases in concentration from 10−8 to 10−4 mol/L. Data points represent means±SE. * represents a significant difference (P<0.05) from the starting diameter; # represents a significant difference (P<0.05) from normotensive controls; n = the number of afferent arterioles studied.
Afferent arteriolar responses to P2X1 receptor activation
Autoregulatory behavior is linked to P2X1 receptor activation.1, 2 Blunted reactivity to ATP could reflect reduced P2X1 receptor reactivity. Arteriolar responses to the P2X1 agonist, β, γ-methylene ATP, are presented in Figure 2B. Baseline diameters were similar between control and AngII+HS groups at a perfusion pressure of 100 mmHg, averaging 15.8±0.2 μm (n=5) and 16.7±0.3 μm (n=7), respectively. In control rats, β, γ-methylene ATP decreased arteriolar diameter to 92±2, 87±5, 81±6, 78±6 and 76±9% of control diameter for concentrations of 10−8 to 10−4 mol/L. AngII+HS markedly blunted the vasoconstrictor responses. β, γ-methylene ATP decreased diameter to just 99±0.4, 97±2, 94±1, 91±3 and 93±1% of control over the same concentration range. These data indicate that afferent arteriolar responses to P2X1 receptor stimulation are greatly attenuated in kidneys from AngII+HS-treated hypertensive rats.
We also examined P2X1 receptor reactivity in separate kidneys perfused at the hypertensive pressure (170mmHg; Figure 2B). Consistent with intact autoregulatory behavior, baseline diameters were significantly lower in the control kidneys 12.5±0.2 μm (n=7) compared to AngII+HS kidneys, 14.9±0.3 μm (P<0.05; n=5). In control rats at 170mmHg, β, γ-methylene ATP decreased arteriolar diameter to 95±1, 92±1, 88±2, 85±2 and 83±1% of control diameter for concentrations of 10−8 to 10−4 mol/L. AngII+HS still blunted the vasoconstrictor responses such that β, γ-methylene ATP decreased diameter to just 99±1, 98±2, 94±2, 92±4 and 88±5% of control over the same concentration range. Collectively, these data indicate that afferent arteriolar responses to P2X1 receptor stimulation are greatly attenuated in kidneys from AngII+HS-treated hypertensive rats perfused at normotensive or hypertensive pressures.
Afferent arteriolar responses to P2Y receptor activation
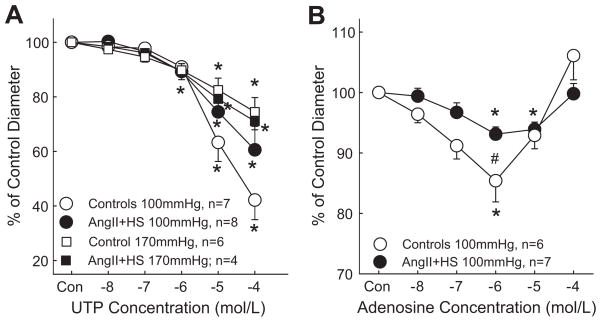
Afferent arterioles exhibit significant vasoconstrictor responses to the P2Y2 agonists UTP and ATP-γ-S.1, 3, 6 Therefore, studies were performed to determine the effect of AngII+HS hypertension on arteriolar responses to UTP (Figure 3A). Baseline diameters at 100mmHg were similar between control and AngII+HS groups, averaging 15.5±0.4 μm (n=7) and 15.2±0.8 μm (n=8), respectively. In control rats, UTP decreased arteriolar diameter to 98±1, 98±2, 91±3, 63±7 and 42±7% of control for concentrations of 10−8 to 10−4 mol/L. In AngII+HS kidneys, arteriolar diameter declined to 100±2, 97±3, 89±3, 75±7 and 61±9% of control over the same concentration range. The magnitudes of these changes are similar to responses in control kidneys. These data indicate that afferent arteriolar responses to P2Y2 receptor stimulation are unchanged in AngII+HS hypertension.
Figure 3.
Afferent arteriolar response to increasing concentrations of UTP (Panel A) or adenosine (Panel B). Results from kidneys perfused at 100mmHg are depicted by circular symbols whereas data from kidneys perfused at 170mmHg are depicted by square symbols. Agonists were applied in the superfusate in successive log increases in concentration from 10−8 to 10−4 mol/L. Data points represent the means ± SE. * represents a significant difference (P<0.05) from the starting diameter; # represents a significant difference (P<0.05) from normotensive controls; n = the number of afferent arterioles studied.
We also examined the responses to UTP in kidneys perfused at hypertensive pressure (Figure 3A). Baseline diameters were significantly smaller in control kidneys compared to AngII+HS groups, averaging 13.0±0.2 μm (n=6) and 15.5±0.1 μm (n=4), respectively, consistent with intact autoregulation. UTP reduced diameter similarly between the control and AngII+HS groups at 170mmHg but overall the maximum responses were smaller at hypertensive pressures than at normotensive pressures. UTP decreased arteriolar diameter to 97±1, 95±2, 90±3, 83±4 and 75±5% of control for concentrations of 10−8 to 10−4 mol/L in control kidneys. In AngII+HS kidneys, arteriolar diameter averaged 98±1, 96±2, 89±3, 79±2 and 71±3% of control over the same concentration range. These data indicate that afferent arteriolar responses to P2Y2 receptor stimulation are unchanged in AngII+HS hypertension compared to normotensive kidneys.
Afferent arteriolar responses to adenosine receptor activation
Adenosine influences afferent arteriolar diameter through activation of A1 receptors and is postulated to mediate tubuloglomerular feedback responses.16 Experiments were performed to compare arteriolar responses to adenosine in control and AngII+HS rats. Baseline diameters were similar between the control (15.1±0.9 μm; n=6) and AngII+HS (15.1±0.8 μm; n=7) groups (Figure 3B). In control rats, adenosine changed arteriolar diameter to 96±1, 91±2, 85±4, 93±2 and 106±4% of baseline for concentrations of 10−8 to 10−4 mol/L. AngII+HS had only slight effects on the responses to adenosine. In these kidneys, the same concentrations of adenosine changed arteriolar diameter to 99±1, 97±2, 93±1, 94±1 and 100±2% of control. Only the response at 10−6 mol/L in AngII+HS was statistically reduced (P=0.045) compared to control arterioles.
The lack of response to P2X1 receptor stimulation cannot be attributed to complete loss of contractile ability by afferent arterioles because 55mM KCl treatment evoked similar vasoconstrictor responses in control (20±6%) and AngII+HS (22±4%) kidneys. This response is only slightly smaller than typical responses of 33±2% that we have previously published.3 In addition, UTP and adenosine still produced vasoconstriction similar to normotensive controls.
Ca2+ responses to β, γ-methylene ATP and ATP
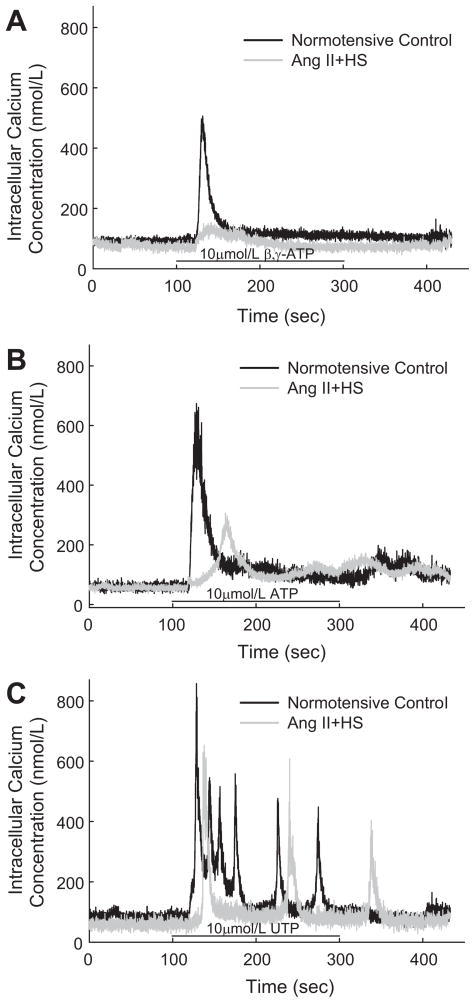
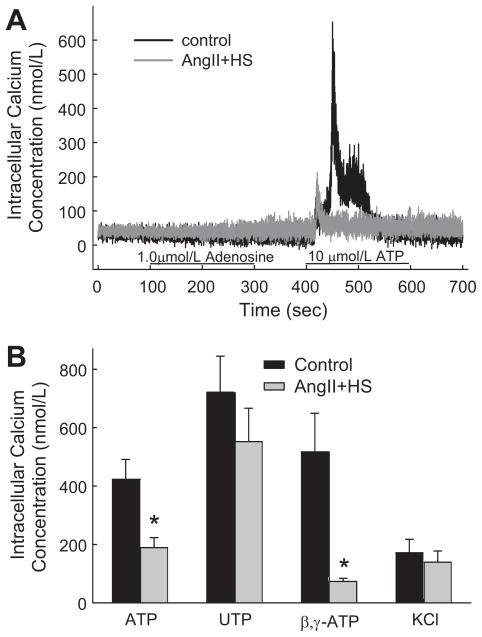
P2 receptors utilize [Ca2+]i as a major signaling pathway mediating vasoconstriction.1, 3, 6, 17, 18 Experiments were performed to compare the Ca2+ signaling responses of preglomerular smooth muscle cells isolated from control and AngII+HS rats (Figures 4 and 5). As shown by the typical traces in Figure 4A, the P2X1 agonist, β, γ-methylene ATP (10 μmol/L) evoked a rapid, biphasic increase in [Ca2+]i in cells from normotensive rats and that response was markedly blunted in cells from AngII+HS rats. Peak [Ca2+]i responses increased by 517±132 nmol/L in control cells (12 cells/4 rats) compared to 73±11 nmol/L in AngII+HS cells (18 cells/7 rats; Figure 5B). Similarly, [Ca2+]i responses evoked by the dual P2X/P2Y agonist, ATP (10 μmol/L) were significantly reduced in AngII+HS cells compared to controls (Figure 4B). ATP stimulated a muted increase in [Ca2+]i in AngII+HS cells averaging 189±34 nmol/L (34 cells/11 rats) compared to 428±68 nmol/L in control cells (68 cells/10 rats; Figure 5B).
Figure 4.
Typical traces of the [Ca2+]i response evoked by β, γ-methylene ATP (Panel A), ATP (Panel B), and UTP (Panel C) in control (Black trace) and AngII+HS (Gray trace) rats. Panel A: representative response of a microvascular smooth muscle cell to 10 μmol/L β, γ-methylene ATP in control and AngII+HS rats. Panel B: representative response to 10 μmol/L ATP in control and AngII+HS rats. Panel C: representative response to 10 μmol/L UTP in control and AngII+HS rats. Agonist application is indicated by the black bar along the X-axis.
Figure 5.
Typical and mean [Ca2+]i responses evoked by P1 and P2 receptor stimulation. Panel A presents typical traces evoked by adenosine and ATP in preglomerular smooth muscle cells from control (Black trace) and AngII+HS (Gray trace) rats. Panel B illustrates the mean peak [Ca2+]i responses to P2 agonists and KCl in control (Black bars) and AngII+HS (Gray bars) rats. *P<0.05, significant decrease in the peak [Ca2+]i response compared with normotensive controls.
Ca2+ responses to UTP, adenosine and KCl
Renal microvessels express P2X1, P2Y2 receptors and adenosine A1 and A2 receptors.1,19 Experiments were performed to determine the impact of AngII+HS hypertension on [Ca2+]i induced by UTP (P2Y2 agonist), adenosine and KCl in preglomerular smooth muscle cells. In controls, UTP stimulated a sharp, biphasic increase in [Ca2+]i (Figures 4C and 5B. This response peaked rapidly averaging 722±123 nmol/L (22 cells/8 rats) then declined to a lower plateau. Spontaneous Ca2+ oscillations were frequently observed during the plateau phase. In AngII+HS cells, [Ca2+]i responses were not significantly altered and averaged 552±114 nmol/L (30 cells/9 rats).
In contrast, adenosine (1 μmol/L) responses were not detectable (Figure 5A). In control cells, [Ca2+]i averaged 48±6 nmol/L (12 cells/3 rats) during control conditions and 49±6 nmol/L during exposure to adenosine. Similarly, in cells from AngII+HS kidneys, [Ca2+]i averaged 40±4 nmol/L (12 cells/3 rats) during control conditions and 40±4 nmol/L during adenosine treatment. As a positive control to verify reactivity in adenosine treated cells, a second agonist exposure period was imposed using 10 μmol/L ATP. The same cells that failed to show adenosine-mediated Ca2+ responses exhibited marked increases in [Ca2+]i. These positive control ATP responses produced average increases of 452±130 nmol/L compared to 134±37 nmol/L cells from control and AngII+HS cells, respectively (Figure 5A; P<0.05). Similarly, responses to a 100-fold higher concentration of adenosine (100 μmol/L), were also not detectable. In control cells, [Ca2+]i averaged 70±4 nmol/L (16 cells/4 rats) during control conditions and 102±6 nmol/L during exposure to adenosine. Similarly, in cells from AngII+HS kidneys, [Ca2+]i averaged 60±4 nmol/L (18 cells/3 rats) during control conditions and 124±16 nmol/L during adenosine treatment. Positive controls using a 10-fold lower concentration of ATP (10 μmol/L ATP) than adenosine produced average increases of 469±131 nmol/L compared to 283±55 nmol/L cells from control and AngII+HS cells, respectively (P<0.05). As a second positive control, we also exposed separate cells to 90 mmol/L KCl to assess Ca2+ responses to non-receptor-dependent stimuli. KCl responses averaged 171±46 nmol/L (23 cells/11 rats) in control cells and 140±38 nmol/L (24 cells/8 rats) in AngII+HS cells (Figure 5B).
Western analysis for changes in P2X1 receptor expression
Preglomerular microvessels from both normotensive control and AngII+HS rats exhibited strong P2X1 receptor protein expression (Figure S2). Densitometric analysis revealed that protein expression was similar in preglomerular microvessels from control rats (40±2 arbitrary densitometry units; n=6) versus microvessels from AngII+HS rats (37±4 arbitrary density units; n=6). P2X1 receptor protein expression was undetectable in renal cortex and renal medulla in both normotensive and hypertensive settings.
DISCUSSION
Autoregulatory behavior is essential for maintaining stable renal blood flow, glomerular capillary pressure and glomerular filtration rate. Autoregulatory impairment is potentially harmful to the kidney by limiting the microvasculature’s ability to protect glomeruli from harmful hypertensive pressures.8, 9, 20 Autoregulatory efficiency is reduced in models of reduced renal mass 21, Goldblatt hypertension 9, Dahl salt-sensitive hypertension 10, L-NAME hypertension 7 and Ang II hypertension 11–13 and high dietary salt.22 While impairment of autoregulation is recognized, the mechanism(s) responsible remain unclear. The study examines afferent arteriolar reactivity to postulated autoregulatory messenger molecules and receptors to link known autoregulatory signaling pathways with functional impairment in AngII+HS hypertension.
Autoregulation represents the combined influences of myogenic and tubuloglomerular feedback mechanisms.6, 16, 20 ATP and adenosine are extracellular messenger molecules postulated to mediate autoregulatory adjustments in preglomerular resistance. ATP and adenosine vasoconstrict afferent arterioles by activating P2 and P1 (A1) receptors, respectively.1, 16, 19 The P2 receptor family is divided into two structurally and functionally distinct subfamilies classified P2X and P2Y.17, 18 P2X receptors are ligand-gated channels that function as nonselective cation channels.17–19 P2Y receptors are metabotropic, G-protein coupled receptors that signal largely through the release of calcium from intracellular stores.18, 19 In contrast, P1 receptors are activated by extracellular adenosine and are divided into three families classified as A1, A2 and A3 receptors.18, 19 A1 and A2 receptors are also G-protein coupled receptors that signal largely by inhibiting or stimulating adenylyl cyclase activity, respectively.18, 19 Consequently, A1 receptor activation inhibits cAMP production and evokes vasoconstriction whereas A2 receptor activation stimulates cAMP accumulation and evokes vasorelaxation. A3 receptors appear to signal through modulation of [Ca2+]i. Accordingly, we examined the impact of AngII+HS on afferent arteriolar autoregulatory behavior and reactivity to P2 and P1 receptor activation.
Previous work supports the hypothesis that P2X1 receptor activation is essential for transducing increases in perfusion pressure with afferent arteriolar vasoconstriction to produce autoregulatory resistance adjustments.1, 2 Pharmacological blockade of P2 receptors with nonselective blockers and with P2X1 receptor selective antagonists uniformly inhibit autoregulatory behavior.1, 2, 13, 23 Global deletion of P2X1 receptors inhibits autoregulation in P2X1 knockout mice compared to wild-type littermates.2 Accordingly, decreased afferent arteriolar reactivity to P2 receptor activation was implicated in the functional impairment of autoregulation in AngII+HS rats. ATP is postulated as the endogenous ligand for renal microvascular P2 receptors, so we determined the arteriolar response to P2 receptor stimulation with ATP. Results indicate that arteriolar responsiveness to P2 receptor activation by ATP is blunted in AngII+HS rats compared to normotensive rats similarly to Ang II hypertension alone.11 This impairment is consistent with impaired autoregulation but doesn’t differentiate which P2 receptor subtype(s) are involved. Interestingly, this impairment is not exacerbated by addition of high salt to the hypertensive setting.
Experiments were conducted to assess the impact of AngII+HS on afferent arteriolar reactivity to P2X1 receptor activation. We selected β, γ-methylene ATP as an agonist with high selectivity for P2X1 receptors and some ability to bind to P2X3 receptors.17 Current and previous work clearly indicate strong P2X1 receptor expression by the preglomerular microvasculature 11, 24–26, but P2X3 receptor expression by afferent arterioles is not established. In preliminary experiments, we could not detect P2X3 receptor protein by western blot in preglomerular microvessels of normotensive animals (data not shown). Lewis and Evans report “barely detectable” P2X3 immunostaining of small intrarenal arteries but did not report on afferent arterioles.26 Turner et. al. did not detect P2X3 immunostaining in renal arterioles.25 Finally, Harhun et al detects PCR product for P2X1 and P2X4 while not detecting gene expression for P2X2, P2X3, P2X5 or P2X7 receptors.27 These data suggest that P2X1 receptors are the primary mechanism by which β, γ-methylene ATP vasoconstricts afferent arterioles. In the current report, arteriolar vasoconstriction to β, γ-methylene ATP was blunted in kidneys from AngII+HS rats compared to normotensive controls. Blunted reactivity was observed regardless of whether the kidneys were perfused at normotensive or hypertensive pressures, suggesting an inherent decline in P2X1 receptor signaling. These same kidneys also exhibited impaired autoregulatory behavior, supporting the hypothesis that P2X1 receptors mediate pressure-dependent autoregulation. These data further suggest that impaired autoregulation in AngII+HS rats reflects impaired P2X1 receptor signaling.
Afferent arterioles also respond to the P2Y2 receptor agonist, UTP and to the P1 receptor agonist, adenosine. Arteriolar responses to these agonists were essentially unchanged in AngII+HS rats compared to normotensive controls despite impaired autoregulation. Interestingly, P2Y2-mediated vasoconstriction was uniformly blunted in control and AngII+HS kidneys when perfused at hypertensive pressures. The mechanism for this remains unclear but because the decline in function was similar in both the normotensive and hypertensive groups, it cannot explain the decline in autoregulatory efficiency. Compared to a previous study with Ang II hypertensive rats on a normal salt diet 11, the response to adenosine was attenuated at the 1.0 μmol/L concentration. The importance of this is unclear but reduced adenosine reactivity cannot explain hypertension-induced impairment of autoregulatory efficiency because adenosine-reactivity was normal in AngII hypertension alone where autoregulation was clearly impaired. Addition of salt to the hypertensive sequence may have a direct effect on adenosine receptor expression/function independent of blood pressure. Overall, these data support the postulate that autoregulation is mediated primarily by P2X1 receptors with P2Y2 receptors or P1 receptors not making major contributions to this pressure-dependent response.
Exactly how hypertension impairs autoregulatory behavior is unclear. Chronic P2 receptor blockade in vivo prevents mesangial cell proliferation and vascular thickening of afferent arterioles of Ang II hypertensive rats, despite having no detectable effect on blood pressure, renin or renal Ang II content.28 Recent studies suggest that local inflammation may influence arteriolar function and inhibit autoregulatory efficiency.12 In those studies, simultaneous treatment with the broad-spectrum anti-inflammatory agent, pentosan polysulfate, provided protection against autoregulatory impairment in Ang II hypertensive rats without changing the progression or magnitude of the hypertension.12 Pentosan polysulfate also preserved arteriolar reactivity to P2X1 stimulation with β, γ-methylene ATP. In other studies, pentosan polysufate is renoprotective in reduced renal mass hypertension and it restored glomerular hemodynamics, independent of arterial hypertension.29–31 Transient exposure to chronic Ang II-infusion confers greater salt-sensitivity and interstitial inflammation 32 suggesting that induction of Ang II hypertension elicits inflammation in the kidney that negatively impacts microvascular function. They also suggest that inflammation is linked to renal injury imposed by adding salt to a hypertensive setting. Collectively, it is possible that initiation of intrarenal inflammation is directly linked to impaired P2X1 receptor function.
We previously reported that P2X1 receptor expression appears normal in kidneys from Ang II hypertensive rats.11 The current report confirms and extends that observation by indicating that P2X1 receptor expression was also unchanged when salt was combined with Ang II-infused hypertension. The explanation for impaired P2X1 receptor reactivity in the face of relatively normal P2X1 receptor expression is unclear. Vial and coworkers reported that P2X1 receptors are influenced by their association with lipid rafts.33, 34 Disruption of lipid rafts renders P2X1 receptors dysfunctional. It is conceivable that hypertension, with or without excess salt, could alter the microvascular lipid raft environment and lead to reduced P2X1 receptor functionality despite normal levels of P2X1 receptor protein expression. Similarly, it is possible that perivascular inflammation could negatively impact P2X1 receptor association with lipid rafts, and reduce P2X1 receptor activity. Alternatively, P2X1 receptor trafficking could be disrupted in hypertension and lead to reduced P2X1 receptor expression in the sarcolemma. A fourth possibility is that signaling downstream of P2X1 receptor activation is compromised. Preglomerular microvascular P2X1 receptors signal through calcium and rho-kinase activity.3–5, 11, 14 Rho-kinase inhibition blocks autoregulation and P2X1 receptor-mediated vasoconstriction while having a more modest effect on P2Y2 receptor-mediated vasoconstriction.14 More extensive studies are required to help resolve these possibilities.
P2X1 receptor-dependent Ca2+ signaling is impaired in Ang II hypertensive rats so alterations in downstream signaling pathways/events are potential explanations for the blunted autoregulatory and P2X1 receptor mediated events.11 Consistent with previous findings, Ca2+ signaling responses for ATP and P2X1 receptor activation are markedly impaired, but the degree of impairment is not notably different from that seen with Ang II-infusion alone.11 Importantly, blunted Ca2+ signaling responses are unique to the P2X1 receptor as responses to UTP-dependent P2Y2 receptor activation and KCl depolarization were unchanged. In addition, the response to 10 μmol/L ATP, which activates P2X and P2Y receptors, was also significantly blunted. This may reflect signaling loss of the P2X Ca2+ component and retention of the P2Y portion of the overall ATP-dependent response. These data support a key role for P2X1 receptor activation, and ligand-gated Ca2+ signaling in mediating autoregulatory vasoconstriction. Importantly, 1 and 100 μmol/L adenosine did not increase [Ca2+]i in cells that yielded robust [Ca2+]i responses to ATP. Given that afferent arteriolar autoregulation is Ca2+-dependent 6, lack of a Ca2+ response to adenosine calls into question the suitability of adenosine as a major signaling molecule mediating overall autoregulatory control.
This study demonstrates that AngII+HS hypertension significantly impairs autoregulation of rat juxtamedullary afferent arterioles. Coincident with this impairment is a decline in afferent arteriolar reactivity to ATP and to P2X1 receptor activation while reactivity to the P2Y2 agonist, UTP or to the P1 receptor agonist, adenosine are essentially unchanged. Importantly, autoregulatory impairment was manifested at perfusion pressures as high as 200mmHg eliminating the possibility that these hypertensive rats merely operate with a right-shifted pressure-flow relationship. In addition, refractory arteriolar reactivity to P2X1 receptor stimulation was apparent at both normotensive and hypertensive pressures while KCl-mediated vasoconstriction was clearly evident. Decreased reactivity to P2X1 receptor activation cannot be attributed to decreased P2X1 receptor expression as western blot analysis reveals no detectable difference in P2X1 receptor protein levels in preglomerular microvascular tissue but Ca2+ signaling responses to P2X1 activation are markedly blunted. This study extends prior reports of impaired autoregulatory behavior in hypertensive models by implicating decreased afferent arteriolar reactivity to P2X1 receptor activation as a causal link.
PERSPECTVES
The impact of hypertension on renal microvascular function is an important variable in the progression to hypertensive renal injury. Efficient autoregulatory control protects healthy kidneys from barotrauma that comes from acute elevations in renal perfusion pressure. Hypertension, however, brings chronic elevations in blood pressure that impairs microvascular autoregulatory function, microvascular reactivity to some vasoactive agents and leads to glomerular hypertrophy and injury. Resolving the mechanisms that underlie this loss of function will facilitate our ability to design therapeutic interventions that will prevent renal decline early in the hypertensive progression, and may permit reversal of existing impairment. This would indeed provide an improved quality of life and prolong normal renal function in afflicted patients.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health (DK 44628, HL 074167; HL095499).
Footnotes
DISCLOSURES
None of the authors have conflicts of interest to disclose.
References
- 1.Inscho EW. ATP, P2 receptors and the renal microcirculation. Purinergic Signalling. 2009;5:447–460. doi: 10.1007/s11302-009-9147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inscho EW, Cook AK. P2 receptor-mediated afferent arteriolar vasoconstriction during calcium channel blockade. Am J Physiol Renal Physiol. 2002;282:F245–F255. doi: 10.1152/ajprenal.0038.2001. [DOI] [PubMed] [Google Scholar]
- 4.Inscho EW, LeBlanc EA, Pham BT, White SM, Imig JD. Purinoceptor-mediated calcium signaling in preglomerular smooth muscle cells. Hypertension. 1999;33:195–200. doi: 10.1161/01.hyp.33.1.195. [DOI] [PubMed] [Google Scholar]
- 5.White SM, Imig JD, Inscho EW. Calcium signaling pathways utilized by P2X receptors in preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol. 2001;280:F1054–F1061. doi: 10.1152/ajprenal.2001.280.6.F1054. [DOI] [PubMed] [Google Scholar]
- 6.Navar LG, Arendshorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. The Renal Microcirculation. In: Tuma RF, Duran WN, Ley K, editors. Handbook of Physiology: Microcirculation. 2. San Diego: Elsevier; 2008. pp. 550–683. [Google Scholar]
- 7.Bouriquet N, Casellas D. Chronic L-NAME hypertension in rats and autoregulation of juxtamedullary preglomerular vessels. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;269:F190–F197. doi: 10.1152/ajprenal.1995.269.2.F190. [DOI] [PubMed] [Google Scholar]
- 8.Cupples WA. Interactions contributing to kidney blood flow autoregulation. Curr Opin Nephrol Hypertension. 2007;16:39–45. doi: 10.1097/MNH.0b013e3280117fc7. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi K, Epstein M, Saruta T. Altered myogenic responsiveness of the renal microvasculature in experimental hypertension. J Hypertens. 1996;14:1387–1401. doi: 10.1097/00004872-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal Injury in Dahl rats. Hypertension. 1997;30:975–983. doi: 10.1161/01.hyp.30.4.975. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, Pollock JS, Imig JD, Inscho EW. Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertension. 2005;46:562–568. doi: 10.1161/01.HYP.0000179584.39937.41. [DOI] [PubMed] [Google Scholar]
- 12.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in Ang II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol. 2010;298:F1276–F1284. doi: 10.1152/ajprenal.00743.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmond DA, Inscho EW. P2X1 receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol. 2010;298:F1360–F1368. doi: 10.1152/ajprenal.00016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inscho EW, Cook AK, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregullatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol. 2009;296:F590–F597. doi: 10.1152/ajprenal.90703.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider MP, Inscho EW, Pollock DM. Attenuated vasoconstrictor responses to endothelin in afferent arterioles during a high-salt diet. Am J Physiol Renal Physiol. 2007;292:F1208–F1214. doi: 10.1152/ajprenal.00280.2006. [DOI] [PubMed] [Google Scholar]
- 16.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: Adenosine, ATP and nitric oxide. Ann Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 17.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 18.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 19.Jackson EK. P1 and P2 receptors in the renal system. In: Abbracchio MP, Williams K, editors. Handbook of Experimental Pharmacology. New York: Springer-Verlag; 2001. pp. 33–71. [Google Scholar]
- 20.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: Effects of salt intake and reduced renal mass. Hypertension. 2010;55:983–989. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeed A, DiBona GF, Marcussen N, Guron G. High-NaCl intake impairs dynamic autoregulation of renal blood flow in ANG II-infused rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1142–R1149. doi: 10.1152/ajpregu.00326.2010. [DOI] [PubMed] [Google Scholar]
- 23.Takenaka T, Inoue T, Kanno Y, Okada H, Hill CE, Suzuki H. Connexins 37 and 40 transduce purinergic signals mediating renal autoregulation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1–R11. doi: 10.1152/ajpregu.00269.2007. [DOI] [PubMed] [Google Scholar]
- 24.Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford APDW, Townsend-Nicholson A, Burnstock G. Localization of the P2X1 purinoceptors by autoradiography and immunohistochemistry in the rat kidney. Am J Physiol Renal Physiol. 1998;274:F799–F804. doi: 10.1152/ajprenal.1998.274.4.F799. [DOI] [PubMed] [Google Scholar]
- 25.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs. 2003;175:105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 26.Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res. 2001;38:332–40. doi: 10.1159/000051064. [DOI] [PubMed] [Google Scholar]
- 27.Harhun MI, Povstyan OV, Gordienko DV. Purinoreceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol. 2010;160:987–997. doi: 10.1111/j.1476-5381.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2008;294:F161–F169. doi: 10.1152/ajprenal.00281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bobadilla NA, Tack I, Tapia E, Sanchez-Lozada LG, Santamaria J, Jimenez F, Striker LJ, Striker GE, Herrera-Acosta J. Pentosan polysulfate prevents glomerular hypertension and structural injury despite persisting hypertension in 5/6 nephrectomy rats. J Am Soc Nephrol. 2001;12:2080–2087. doi: 10.1681/ASN.V12102080. [DOI] [PubMed] [Google Scholar]
- 30.Herrera-Acosta J, Tapia E, Sanchez-Lozada LG, Franco M, Striker LJ, Striker GE, Rodriguez-Iturbe B. Restoration of glomerular haemodynamics and renal injury independent of arterial hypertension in rats with subtotal renal ablation. J Hypertens. 2002;20:S29–S35. [PubMed] [Google Scholar]
- 31.Sanchez-Lozada LG, Tapia E, Johnson RJ, Rodíguez-Iturbe B, Herrera-Acosta J. Glomerular hemodynamic changes associated with arteriolar lesions and tubulointerstitial inflammation. Kidney Int. 2003;64:S9–S14. doi: 10.1046/j.1523-1755.64.s86.3.x. [DOI] [PubMed] [Google Scholar]
- 32.Franco M, Martinez F, Rodriguez-Iturbe B, Johnson RJ, Santamaria J, Montoya A, Nepomuceno T, Bautista R, Tapia E, Herrera-Acosta J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2006;291:F1281–F1287. doi: 10.1152/ajprenal.00221.2006. [DOI] [PubMed] [Google Scholar]
- 33.Vial C, Fung CYE, Goodall AH, Mahaut-Smith MP, Evans RJ. Differential sensitivity of human platelet P2X1 and P2Y1 receptors to disruption of lipid rafts. Biochem Biophys Res Commun. 2006;343:415–419. doi: 10.1016/j.bbrc.2006.02.174. [DOI] [PubMed] [Google Scholar]
- 34.Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem. 2005;280:30705–30711. doi: 10.1074/jbc.M504256200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.