Abstract
Background
It remains debated whether to include resting electrocardiogram (ECG) in the routine care of patients infected with Human immunodeficiency virus (HIV). This is largely because data are limited regarding the prevalence and prognostic significance of ECG abnormalities in HIV-infected patients.
Methods
This analysis included 4518 HIV-infected patients (28% females and 29% blacks) from The Strategies for Management of Antiretroviral Therapy (SMART) study, a clinical trial aimed to compare two HIV treatment strategies. ECG abnormalities were classified using the Minnesota Code. Multivariable adjusted Cox proportional hazards analysis was used to examine the association between baseline ECG abnormalities and incident cardiovascular disease.
Results
More than half of the participants (N=2325, 51.5%) had either minor or major ECG abnormalities. Minor ECG abnormalities (48.6%) were more common than major ECG abnormalities (7.7%). During a median follow-up of 28.7 months, 155 (3.4%) participants developed incident cardiovascular disease. After adjusting for the study treatment arms, the presence of major, minor, and either minor or major ECG abnormalities were significantly predictive of incident cardiovascular disease [Hazard ratio (95% Confidence Interval): 2.76 (1.74, 4.39), p<0.001; 1.58 (1.14, 2.20), p=0.006; 1.57 (1.14, 2.18), p=0.006, respectively]. However, after adjusting for demographics, common cardiovascular risk factors and HIV characteristics (full model), presence of major ECG abnormalities was still significantly predictive of cardiovascular disease [1.83 (1.12, 2.97), p=0.015)], but not minor or minor or major abnormalities taken together [1.26 (0.89, 1.79), p=0.18; 1.25 (0.89, 1.76), p=0.20, respectively]. Individual ECG abnormalities that significantly predicted cardiovascular disease in the fully adjusted model included major isolated ST/T abnormalities, major prolongation of QT interval, minor isolated ST/T and minor isolated Q/QS abnormalities.
Conclusion
Nearly one in two of the HIV-infected patients in SMART study had ECG abnormalities; one in thirteen had major ECG abnormalities. Presence of ECG abnormalities, especially major ECG abnormalities was independently predictive of incident cardiovascular disease. These results suggest that the ECG could provide a convenient risk screening tool in HIV-infected patients.
Keywords: HIV/AIDS, ECG, Cardiovascular Disease, SMART Study
INTRODUCTION
Individuals infected with Human Immunodeficiency Virus (HIV) are at increased risk of cardiovascular disease, especially coronary artery disease (1-7). The pathophysiological basis for such a higher risk is complex; some could be related to the HIV/AIDS disease process and others could be related to treatment with antiretroviral drugs (8). The identification of simple prognostic markers for risk stratification of cardiovascular disease in this high risk population is important. The electrocardiogram (ECG) is the least-expensive and most available clinical tool for evaluation of cardiovascular disease. In the general population, it is well established that ECG abnormalities are predictive of incident cardiovascular events, independent of traditional risk factors (9-14). Although the prevalence of ST/T abnormalities and QT prolongation has been previously reported in HIV-infected population (15, 16, 17), data are sparse regarding the prevalence and prognostic significance of other ECG abnormalities. Filling this gap in the current knowledge will expand our understanding of the epidemiology of cardiovascular disease in HIV infected patients, and may help in developing appropriate risk stratification tools in this high risk group. In this analysis, we sought to examine the prevalence and prognostic significance of ECG abnormalities in HIV-infected patients enrolled in The Strategies for Management of Antiretroviral Therapy (SMART) study. We hypothesized that ECG abnormalities would be common among SMART participants and presence of these abnormalities would increase cardiovascular disease risk.
METHODS
SMART study
The SMART study was an open-label randomized trial comparing two antiretroviral treatment strategies (18, 19). The viral suppression strategy, which was the control arm, was defined to be consistent with the guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents (20) i.e. available antiretroviral regimens were to be used in an uninterrupted manner with the goal of maximal and continuous suppression of HIV replication. The experimental drug conservation strategy entailed the episodic use of antiretroviral therapy according to CD4+ count thresholds i.e. the use of antiretroviral therapy was deferred until the CD4+ count decreased to less than 250 cells per cubic millimeter, at which time antiretroviral therapy was to be initiated (or reinitiated) and continued until the CD4+ count increased to more than 350 cells per cubic millimeter. On January 10, 2006, the data and safety monitoring board recommended stopping enrollment in the SMART trial because of a safety risk in the drug conservation group. Following this change in protocol all patients were advised to receive continuous antiretroviral therapy and were followed for another 1.5 years (19). This analysis describes findings through July 11, 2007.
Study population
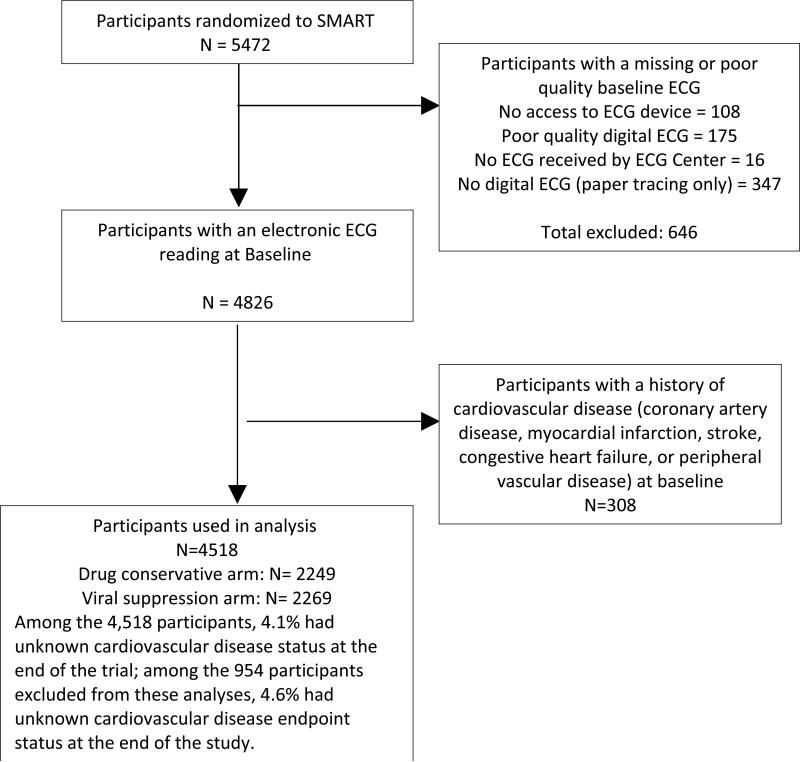
Individuals infected with HIV who were older than 13 years and were not pregnant or breast-feeding were eligible for the SMART study if their CD4+ count exceeded 350 cells per cubic millimeter and they were willing to participate. All participants in the trial were considered eligible for the present analysis, except those with a missing or poor quality ECG (n=646) or with known cardiovascular disease (n=308), leaving 4518 participants who were included in this analysis (Figure 1).
Figure 1.
Study flow diagram
Data Collection and Follow-up
Before randomization, participants’ antiretroviral therapy history and medical history were obtained, as were the nadir CD4+ count; the highest recorded plasma HIV RNA level; the CD4+ count, CD4+ percentage, and HIV RNA level at baseline; and the three most recent CD4+ counts, CD4+ percentages, and HIV RNA levels before baseline. Follow-up visits were scheduled monthly for the first 2 months, every 2 months thereafter for the first year, and every 4 months in the second and subsequent years. At each visit, a history was taken and an examination conducted, and the CD4+ count and HIV RNA level were measured. At the baseline visit and at each annual visit, a 12-lead ECG was obtained.
Ascertainment of ECG abnormalities
Identical electrocardiographs (GE MAC 1200 models, GE Milwaukee, WI) were used in almost all SMART clinics and standard 12-lead ECGs were recorded in all participants by strictly standardized procedures. ECG technicians were trained to reduce chest electrode placement errors, thereby reducing inter-individual variability. A 10-second segment of simultaneous ECG leads was sampled at a rate of 500 samples per second per lead. The electronic ECGs stored in the electrocardiographs were transmitted regularly over analogue phone lines to the SMART central ECG Reading Center, EPICARE (Epidemiological Cardiology Research Center), located at Wake Forest University, Winston-Salem, NC for analysis. ECGs were visually inspected for technical errors and inadequate quality. The ECGs were processed with the 2001 version of the GE Marquette 12-SL program (GE, Milwaukee, WI). ECG abnormalities were classified using the Minnesota ECG Code classification system (21). Individual ECG abnormalities were further grouped into minor or major abnormalities based on the same Minnesota Code ECG classification.
Ascertainment of cardiovascular events
Using pre-established criteria, an independent end-point review committee reviewed major clinical events, including cardiovascular events (18). The end-point review committee classified the underlying cause of death using the Coding of Death in HIV project system (22). A composite cardiovascular disease outcome (incident myocardial infarction, coronary artery disease, congestive heart failure, peripheral vascular disease, stroke, sudden death, and/or cardiovascular death) was used in this analysis
Statistical analysis
Frequency distributions of all variables were first inspected to identify anomalies and outliers possibly caused by measurement artifacts. Descriptive statistics are presented as means with standard deviations (SD). The prevalence of individual ECG abnormalities as well as grouped abnormalities (as minor or major) was determined. Cox proportional hazards models were used to assess the association between baseline ECG abnormalities, separately, with incident cardiovascular disease. For each abnormality, participants were classified into three groups: (a) abnormality present, (b) abnormality absent, other abnormality present, and (c) no ECG abnormalities (reference group). All models considered these 3 groups. Hazard ratios for abnormality present compared to no ECG abnormalities are shown. Three models were created: Model 1 adjusted for the trial treatment groups; model 2 adjusted for model 1 plus demographic characteristics [age, sex, and ethnicity]; model 3 adjusted for model 2 plus clinical variables [smoking status, total/high density lipoprotein (HDL) cholesterol ratio, body mass index, diabetes, and blood pressure and lipid lowering drugs], and HIV characteristics [baseline duration of HIV infection, baseline CD4 count, HIV-RNA and antiretroviral treatment status]. The interactions between treatment assignment and the presence of ECG abnormalities were examined. Follow-up time was measured from baseline to first cardiovascular event, non-cardiovascular disease death, lost to follow-up, or end of study (July 11, 2007). An expanded model which included an interaction with log failure time was used to assess the proportional hazards assumption. All reported p-values are two-sided and P<0.05 was considered statistically significant. SAS, version 9.1 (SAS Institute, Inc., Cary, North Carolina) was used in all analyses.
RESULTS
The average age of the study population (N=4518) at baseline was 43.5 ± 9.3 years, 29% were blacks, and 28% were females. The baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline characteristics of the study population
| Number | 4518 |
| Age (years) | 43.5 ± 9.3 |
| Females (%) | 1279 (28.3%) |
| Blacks (%) | 1311 (29.0%) |
| Smoking (%) | |
| Current | 1799 (39.8%) |
| Ever | 2892 (64.0%) |
| Total cholesterol (mg/dL) | 195.7 ± 47.8 |
| LDL cholesterol (mg/dL) | 114.4 ± 35.3 |
| HDL cholesterol (mg/dL) | 43.3 ± 14.7 |
| Triglycerides (mg/dL) | 217.7 ± 217.6 |
| Total cholesterol/HDL | 5.0 ± 2.4 |
| Body mass index (kg/m2) | 25.9 ± 5.4 |
| Diabetes mellitus (%) | 287 (6.4%) |
| Blood pressure-lowering drugs (%) | 723 (16.0%) |
| Lipid lowering drugs (%) | 615 (13.6%) |
| Baseline CD4 count (cells/mm3) | 657.4 ± 255.4 |
| HIV RNA (% ≤ 400 copies/mL) | 3226 (71.6%) |
| Antiretroviral use at baseline (%) | |
| Antiretroviral naïve | 210 (4.6%) |
| No prior use of antiretroviral drugs, | 516 (11.4%) |
| Protease inhibitors | 1736 (38.4%) |
| Non-nucleoside Reverse Transcriptase Inhibitor | 1623 (35.9%) |
| Others | 433 (9.6%) |
Values are expressed as mean± SD or N (%)
Approximately 51% of the participants had either minor or major ECG abnormalities, 8% had major ECG abnormalities, and 49% had minor abnormalities. Major ventricular conduction defects, major Q/QS abnormalities and major isolated ST/T abnormalities were the most common major abnormalities (1.3%, 1.8% and 4.7%, respectively). Minor isolated ST/T abnormalities, high R waves, and incomplete bundle branch block were the most common minor ECG abnormalities (11.4%, 12.6% and 17.4%, respectively) (Table 2).
Table 2.
Prevalence of ECG abnormalities at baseline (N=4518)*
| MAJOR ABNORMALITIES ** | 349 (7.7%) |
| Ventricular conduction defect | 58 (1.3%) |
| Major Q/QS waves abnormalities | 80 (1.8%) |
| Minor Q/QS with ST/T abnormalities | 13 (0.3%) |
| Major isolated ST/T abnormalities | 211 (4.7%) |
| Left ventricular hypertrophy | 56 (1.2%) |
| Atrial fibrillation/flutter | 3 (0.1%) |
| Major AV conduction abnormalities | 4 (0.1%) |
| Major QT prolongation index (QTI ≥ 116%) | 14 (0.3%) |
| MINOR ABNORMALITIES ** | 2194 (48.6%) |
| Minor isolated Q/QS | 194 (4.3%) |
| Minor isolated ST/T | 516 (11.4%) |
| High R-waves | 570 (12.6%) |
| ST elevation | 399 (8.8%) |
| Incomplete bundle branch block (BBB) | 785 (17.4%) |
| Incomplete right BBB | 162 (3.6%) |
| Incomplete left BBB | 623 (13.8%) |
| Minor QT prolongation index (QTI ≥ 112%) | 35 (0.8%) |
| Short PR interval | 92 (2.0%) |
| Left axis deviation | 132 (2.9%) |
| Right axis deviation | 2 (0.0%) |
| Frequent ventricular premature beats (VPBs) | 29 (0.6%) |
| Other minor arrhythmias | 313 (6.9%) |
| ANY MAJOR OR MINOR ABNORMALITY ** | 2325 (51.5%) |
Values expressed as N (%).
The sum of individual ECG abnormalities under each category of major, minor or major/minor ECG abnormalities is larger than the total number of major, minor and major/minor abnormalities respectively. This is because some patients have more than one abnormality (overlap). However, the reported “over all” minor, major or minor/major abnormalities represent the proportion of participants with at least one ECG abnormality (with no overlap).
During the follow-up period, 155 (3.4%) participants developed incident cardiovascular events which were distributed as follows: 56 coronary artery disease, 17 myocardial infarction, 21 congestive heart failure, 25 peripheral vascular disease, 11 stroke, 12 cardiovascular death, and 13 sudden death. Among participants with major ECG abnormalities, minor but not major abnormalities, and neither major nor minor ECG abnormalities, the percents of participants with unknown cardiovascular disease endpoint status at the end of the study were 2.0%, 4.2%, and 4.4%, respectively. Table 3 shows the results of the multivariable adjusted Cox Proportional Hazards models for the association between major ECG abnormalities and incident cardiovascular disease events. As shown, presence of any major ECG abnormalities was significantly associated with an increased risk of incident cardiovascular disease in all models [Hazard ratio (95% Confidence Interval): 2.76 (1.74, 4.39), P<0.001; 1.91 (1.18, 3.08), p=0.008; 1.84 (1.13, 2.98), p=0.014; 1.83 (1.12, 2.97), p=0.015 in models 1-4, respectively]. Among the major ECG abnormalities, presence of major isolated ST/T abnormalities, and major prolongation of QT interval were the most significant predictors of incident cardiovascular disease.
Table 3.
Associations between baseline major ECG abnormalities and incident cardiovascular events
| Model 1: Adjusted for treatment group | Model 2: Adjusted for Model 1 plus age, gender and race | Model 3: Adjusted for Model 2 plus smoking status, total /HDL cholesterol ratio, body mass index, diabetes and lipid lowering drugs, baseline duration of HIV infection, baseline CD4, HIV-RNA and ART status | ||||
|---|---|---|---|---|---|---|
| ECG abnormalities* | HR [95% CI] | P | HR [95% CI] | P | HR [95% CI] | P |
| Any major ECG abnormalities | 2.76 (1.74, 4.39) | <0.001 | 1.91 (1.18, 3.08) | 0.008 | 1.83 (1.12, 2.97) | 0.015 |
| Ventricular conduction defect | 1.40 (0.34, 5.75) | 0.64 | 0.73 (0.18, 3.01) | 0.66 | 0.74 (0.18, 3.06) | 0.67 |
| Major Q-Waves abnormalities | 2.28 (0.92, 5.69) | 0.08 | 1.73 (0.69, 4.33) | 0.24 | 1.55 (0.61, 3.92) | 0.36 |
| Major isolated ST/T abnormalities | 2.71 (1.56, 4.72) | <0.001 | 1.91 (1.08, 3.40) | 0.027 | 1.82 (1.02, 3.26) | 0.043 |
| Left ventricular hypertrophy | 2.28 (0.83, 6.27) | 0.11 | 1.37 (0.49, 3.83) | 0.55 | 1.14 (0.40, 3.24) | 0.80 |
| Major QT prolongation | 9.44 (2.30, 38.76) | 0.002 | 7.94 (1.93, 32.71) | 0.004 | 6.18 (1.46, 26.21) | 0.013 |
Referent value in the Cox proportional hazards models is absence of ECG abnormalities
** Hazards ratios for less frequent major ECG abnormalities (based on table 2) are not shown. However, the reported hazards ratios associated with any major ECG abnormalities take into account these infrequent ECG abnormalities.
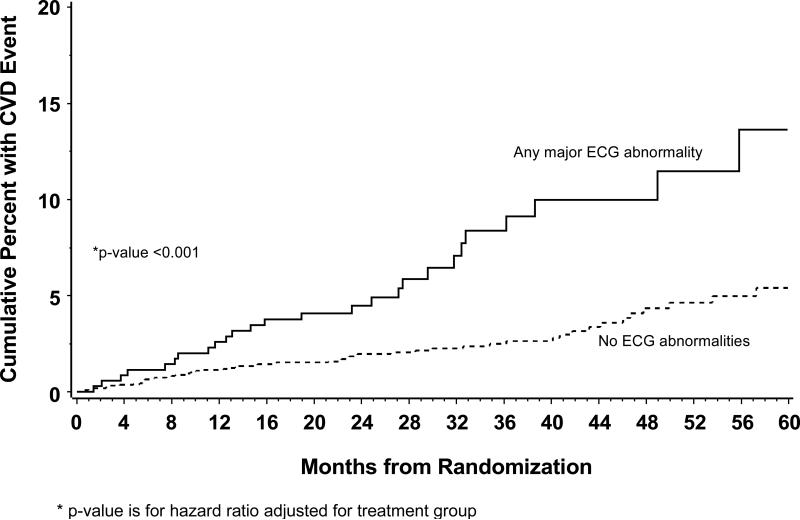
Figure 2 shows Kaplan-Meier estimates of the cumulative risk of a cardiovascular disease event in those with major ECG abnormalities compared to those without. The risk of cardiovascular disease associated with major ECG abnormalities persists over the follow-up period (p>0.99 for proportional hazards). As shown in Table 4, diabetes, smoking, use of blood pressure lowering drugs (denoting hypertension), higher total/HDL cholesterol, male gender, age, and drug conservation treatment group were associated with an increased risk of cardiovascular disease.
Figure 2.
Kaplan-Meier estimates of the cumulative risk of a cardiovascular disease event according to presence of major ECG abnormalities
Table 4.
Association of baseline characteristics (major ECG abnormalities, demographics, and clinical characteristics) with incident cardiovascular events
| Hazard ratio (95% Confidence Interval) | P | |
|---|---|---|
| Major ECG abnormality | ||
| Abnormality present | 1.83 (1.12, 2.97) | 0.015 |
| Abnormality absent, other abnormality present | 1.13 (0.78, 1.62) | 0.51 |
| No abnormalities | Ref. | - |
| Treatment Group (drug conservative vs. viral suppression) | 1.43 (1.03, 1.99) | 0.032 |
| Age (year) | 1.05 (1.03, 1.07) | <0.001 |
| Gender (female vs. male) | 0.60 (0.38, 0.96) | 0.032 |
| Race | ||
| Black | 1.03 (0.70, 1.50) | 0.90 |
| Asian | 0.45 (0.06, 3.29) | 0.43 |
| Other | 0.79 (0.43, 1.45) | 0.45 |
| White | Ref | - |
| Smoking Status | ||
| Current | 1.82 (1.19, 2.77) | 0.006 |
| Past | 1.17 (0.73, 1.87) | 0.51 |
| Never | Ref | - |
| Total/HDL Cholesterol Ratio | 1.04 (1.00, 1.09) | 0.034 |
| Baseline body mass index | 1.00 (0.97, 1.04) | 0.84 |
| Diabetes (yes vs. no) | 1.83 (1.16, 2.89) | 0.010 |
| Use of blood pressure-lowering drugs | 2.00 (1.39, 2.88) | <0.001 |
| Use of lipid lowering drugs | 1.04 (0.69, 1.58) | 0.84 |
| Years since HIV diagnosis | 1.00 (0.97, 1.04) | 0.91 |
| Baseline CD4 (per 100) | 1.03 (0.97, 1.10) | 0.35 |
| Baseline HIV-RNA and antiretroviral status | ||
| Off Antiretroviral therapy | 1.29 (0.76, 2.21) | 0.35 |
| On Antiretroviral therapy, HIV-RNA ≤ 400 | 0.99 (0.64, 1.53) | 0.95 |
| On Antiretroviral therapy, HIV-RNA > 400 | Ref. | - |
Presence of any minor ECG abnormalities was also significantly associated with incident cardiovascular events in model 1 [1.58 (1.14, 2.20), p=0.006], an association that was attenuated in the demographic and full multivariable models [1.31 (0.94, 1.83), p=0.11 and 1.26 (0.89, 1.79), p=0.18, respectively]. Among the minor ECG abnormalities, presence of minor isolated ST/T abnormalities and minor isolated Q/QS were the most significant predictors of incident CVD across all models (results not shown). Similarly, presence of any ECG abnormalities (major or minor) was significantly associated with incident cardiovascular events in model 1 [1.57 (1.14, 2.18), p<0.01], an association that was attenuated in the demographic and full models. 1.29 (0.93, 1.80), p-value=0.13 and 1.25 (0.89, 1.76), p=0.02), respectively
There was no statistically significant interaction between the study treatment groups with major ECG abnormalities, minor ECG abnormalities and major/minor ECG abnormalities [p for interaction =0.74, 0.16, 0.14, respectively].
DISCUSSION
This study examined the prevalence and prognostic significance of different ECG abnormalities as defined by the Minnesota Code ECG classification system in a well-defined multi-racial population infected with HIV who were free of cardiovascular disease at the study baseline. Two key findings can be derived from this analysis. First, ECG abnormalities are very common in patients infected with HIV; nearly one in two of our study population had an ECG abnormality. Second, presence of ECG abnormalities in HIV-infected patients carries a poor prognosis. These data suggest that important predictive information can be derived from such a widely available non-invasive tool: the ECG. It may be useful to introduce the ECG as a part of the routine care of HIV infected patients. However, the cost-effectiveness of this strategy needs to be evaluated in future studies. While abnormalities considered major were associated with an increased risk of cardiovascular disease, the more commonly occurring minor abnormalities had limited prognostic importance.
While almost 52% of our study population had at least one minor or major ECG abnormality, the reported prevalence of similar abnormalities in the general population with similar characteristics (middle-aged, biracial and free of cardiovascular disease) using the same ECG classification system ranged from 16-32% (14, 23-26). Thus, prevalence in the HIV population may be 2-3 times greater than in non-infected populations. Although it is difficult to precisely compare the results of our study with results from previous studies that have been conducted years ago, the similarities in basic demographics and the ECG methodology make it reasonable to conclude the high prevalence of ECG abnormalities in HIV-infected patients is high relative to the general population. Except for the reported prevalence of QT and ST abnormalities (15, 16, 17), we could not find a comprehensive description of ECG abnormalities in other HIV-infected populations with which to compare our results.
The remarkably high prevalence of ECG abnormalities in HIV-infected patients is concordant with the many reports of higher risk of cardiovascular disease in this population (1-6). It is not clear, however, whether HIV infection per se, exposure to antiretroviral therapy, or other HIV-specific risk factors is the cause of this increased risk (8). Since our analysis was not positioned to answer this question, this issue should be investigated in another study.
Our results in the HIV-infected population extend the previously reported findings in the general population that presence of major ECG abnormalities carries a poor prognosis (9-14). According to our results and similar to those in the general population, it seems that different ECG abnormalities have different levels of prognostic significance in patients infected with HIV. Although not all of the associations between ECG abnormalities with incident cardiovascular disease reached statistical significance, the presence of any major ECG abnormalities, minor or major ST/T abnormalities, minor isolated Q/QS and major QT prolongation were significantly associated with incident cardiovascular disease even after extensive adjustment for demographic, clinical and HIV characteristics of the participants. Presence of major ECG abnormalities, diabetes, use of blood pressure medications (denoting hypertension) and current smoking were the strongest predictors of incident cardiovascular disease ; each was associated with almost double the risk of incident cardiovascular disease . Notably, the hazard ratio associated with major abnormalities is similar to that of diabetes. These findings are important if risk prediction equations for incident cardiovascular disease are to be developed for HIV-infected patients.
The significant association between most Q/ST/T abnormalities with incident cardiovascular disease could be explained by the notion that these ECG variables denote underlying myocardial ischemia/infarction which is a major component of our composite outcome. However, it is unclear why other ECG abnormalities that also denote underlying myocardial ischemia were not significant predictors as well. A possible explanation could be related to the relatively few cardiovascular disease events in our study which might have resulted in lack of enough power to detect significant associations with low prevalence ECG abnormalities. The relatively small number of events could also explain the wide confidence intervals of the risk estimates (hazards ratio) associated with some ECG abnormalities (such as major prolongation of QT) that showed significant associations with cardiovascular disease.
In addition to the statistical power concern noted above, our analysis should be read in the context of other limitations. Using a composite outcome in our study was a necessity, given the limited number of events which is common among similar HIV-related studies (8). A common concern about using composite outcomes is that different components of the outcome might not have the same pathophysiological basis. However, the components of our composite outcome share a common risk factor which is “atherosclerosis”. Noteworthy, ECG abnormalities have been repeatedly reported to be associated with atherosclerosis (27, 28). Another possible limitation is that this analysis was conducted on patients who chose to enter a clinical trial of HIV treatment. Finally, over 600 participants with missing, poor quality or non-digital ECGs were excluded. These exclusions reduced power and might have biased some associations. However, with consideration of the reasons for the missing ECGs as detailed in Figure 1, the data are likely missing at random.
Despite these limitations, our study has a number of strengths that warrant highlighting. This analysis was conducted on a well-defined population of HIV-infected patients free of cardiovascular disease. The key exposure variables (ECG abnormalities) and the outcome (incident cardiovascular disease ) were carefully ascertained i.e. ECG recordings were obtained by trained technicians using a standardized protocol and were read centrally using a standard ECG classification (21), and the events were adjudicated by an independent adjudication committee.
In conclusion, ECG abnormalities are very common in HIV-infected patients and they were predictive of incident cardiovascular disease. This makes the ECG a potentially useful non-invasive, low-budget test for early detection of cardiovascular disease in HIV-infected patients who are receiving antiretroviral therapy. However, its value in risk stratification should be tested in other HIV-infected populations.
Acknowledgments
We would like to thank the SMART study participants for their contribution.
Funding sources
The SMART study was sponsored by the National Institute of Allergy and Infectious Disease, National Institutes of Health (grants U01AI042170, U01AI46362, and U01AI068641).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
REFERENCES
- 1.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Møller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 3.Friis-Møller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 4.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–710. doi: 10.1056/NEJMoa022048. 9. [DOI] [PubMed] [Google Scholar]
- 6.Iloeje UH, Yuan Y, L'Italien G, et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med. 2005;6:37–44. doi: 10.1111/j.1468-1293.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 7.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 8.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. l 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crow RS, Prineas RJ, Hannan PJ, Grandits G, Blackburn H. Prognostic associations of Minnesota Code serial electrocardiographic change classification with coronary heart disease mortality in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1997;80:138–144. doi: 10.1016/s0002-9149(97)00307-x. [DOI] [PubMed] [Google Scholar]
- 10.Daviglus ML, Liao Y, Greenland P, et al. Association of nonspecific minor ST-T abnormalities with cardiovascular mortality: the Chicago Western Electric Study. JAMA. 1999;281:530–536. doi: 10.1001/jama.281.6.530. [DOI] [PubMed] [Google Scholar]
- 11.De Bacquer D, Martins Pereira LS, De Backer G, De Henauw S, Kornitzer M. The predictive value of electrocardiographic abnormalities for total and cardiovascular disease mortality in men and women. Eur Heart J. 1994;15:1604–1610. doi: 10.1093/oxfordjournals.eurheartj.a060441. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, Xie X, Liu K, et al. Impact of minor electrocardiographic ST-segment and/or T-wave abnormalities on cardiovascular mortality during long-term follow-up. Am J Cardiol. 2003;91:1068–1074. doi: 10.1016/s0002-9149(03)00150-4. [DOI] [PubMed] [Google Scholar]
- 13.Liao YL, Liu KA, Dyer A, et al. Major and minor electrocardiographic abnormalities and risk of death from coronary heart disease, cardiovascular diseases and all causes in men and women. J Am Coll Cardiol. 1988;12:1494–1500. doi: 10.1016/s0735-1097(88)80016-0. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland SE, Gazes PC, Keil JE, Gilbert GE, Knapp RG. Electrocardiographic abnormalities and 30-year mortality among white and black men of the Charleston Heart Study. Circulation. 1993;88:2685–2692. doi: 10.1161/01.cir.88.6.2685. [DOI] [PubMed] [Google Scholar]
- 15.Carr A, Grund B, Neuhaus J, El-Sadr WM, Grandits, et al. Asymptomatic myocardial ischaemia in HIV-infected adults. AIDS. 2008;22(2):257–67. doi: 10.1097/QAD.0b013e3282f20a77. [DOI] [PubMed] [Google Scholar]
- 16.Charbit B, Rosier A, Bollens D, Boccara, et al. Relationship between HIV protease inhibitors and QTc interval duration in HIV-infected patients. Br J Clin Pharmacol. 2009;67(1):76–82. doi: 10.1111/j.1365-2125.2008.03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinsch N, Buhr C, Krings P, et al. Prevalence and risk factors of prolonged QTc interval in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2009;10(4):261–8. doi: 10.1310/hct1004-261. [DOI] [PubMed] [Google Scholar]
- 18.The SMART Study Group CD4+ count-guided interruption of antiretroviral therapy. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 19.SMART Study Group Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy, a randomized trial. Ann Int Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 20. [February 2, 2010];Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. December 2009. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 21.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code manual of electrocardiographic findings. John Wright–PSG; Littleton, MA: 1982. [Google Scholar]
- 22.Lifson AR, Belloso WH, Carey C, et al. Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials. 2008;9:177–85. doi: 10.1310/hct0903-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De-Bacquer D, De-Backer G, Kornitzer M. Prevalence of ECG findings in large population based samples of men and women. Heart. 2000;84:625–633. doi: 10.1136/heart.84.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strogatz DS, Tyroler HA, Watkins LO, Hames CG. Electrocardiographic abnormalities and mortality among middle-aged black men and white men of Evans County, Georgia. J Chron Dis. 1987;40:149–155. doi: 10.1016/0021-9681(87)90066-x. [DOI] [PubMed] [Google Scholar]
- 25.De-Bacquer D, Pereira M, De-Backer G, De-Henauw S, Kornitzer M. Prevalence and correlate of ECG abnormalites in the adult Belgian population. J Electrocardiol. 1995;28(1):1–11. doi: 10.1016/s0022-0736(05)80002-0. [DOI] [PubMed] [Google Scholar]
- 26.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR. Electrocardiographic findings in a healthy biracial population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Cardiol. 1998;81:453–459. doi: 10.1016/s0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 27.Möhlenkamp S, Schmermund A, Lehmann N, et al. Subclinical coronary atherosclerosis and resting ECG abnormalities in an unselected general population 2008. Atherosclerosis. 2008;196(2):786–794. doi: 10.1016/j.atherosclerosis.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, Walsh JA, Prineas RJ, et al. Association of electrocardiographic abnormalities with coronary artery calcium and carotid artery intima-media thickness in individuals without clinical coronary heart disease (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2009;104(8):1086–1091. doi: 10.1016/j.amjcard.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]