Abstract
Purpose
We have shown that incomplete blockade of the Human Epidermal Growth Factor (HER) pathway is a mechanism of resistance to treatment with trastuzumab (T) in HER2-overexpressing tumor xenografts. We now investigate whether the addition of lapatinib (L), a dual HER1/2 kinase inhibitor, to T results in more potent inhibition of the pathway and therefore inhibition of tumor growth, and whether reduced dose and intermittent treatment with the combination is equally effective.
Experimental Design
Nude mice bearing HER2-overexpressing MCF7/HER2-18 or BT474 xenograft tumors were treated with L, T, alone or in various combinations with other HER inhibitors. L+T for short duration (14, 42 days), intermittent administration (14 days on/off), and reduced dosing (1/2 dose) was also investigated. Inhibition of tumor growth, downstream signaling, proliferation, and induction of apoptosis were assessed. All statistical tests were two-sided.
Results
L+T was the most effective regimen in both MCF7/HER2-18 and BT474 xenografts with complete tumor regression (CR) observed in all mice. Intermittent and reduced dose treatment (½ dose) resulted in high rates of CR and low rates of tumor recurrence that were comparable to full dose continuous treatment. L+T resulted in significantly reduced downstream signaling and proliferation, and increased apoptosis.
Conclusions
L+T is a potent and effective combination even when given in reduced dose or intermittent schedule potentially resulting in lower toxicity and reduced cost if translated to patients. These findings warrant timely clinical testing.
BACKGROUND
Human epidermal growth factor receptor 2 (c-ErbB2, HER2/neu or HER2) is a clinically important therapeutic target in patients with HER2-overexpressing breast cancers. It is a component of a robust and complex network comprised of four tyrosine kinase receptors, HER1-4, which can be activated by multiple ligands which induce homo and heterodimerization. HER2 does not have a ligand and, therefore, is activated by partnering with itself or another family member (1–7). The pathway can also be activated by alterations downstream of the HER receptor layer including loss of the tumor suppressor gene PTEN or activating mutations in PI3K that may cause resistance to trastuzumab (8–10).
Trastuzumab, a humanized monoclonal antibody directed at the HER2 extracellular domain, inhibits this pathway. Its use resulted in significant reductions in recurrence and mortality in patients with HER2-positive breast cancer (11, 12). However, de novo and acquired drug resistance remain a clinical problem (13, 14).
Lapatinib, a dual HER1 and HER2 tyrosine kinase inhibitor, is approved for treatment of metastatic HER2 positive breast cancer and is being investigated in various clinical settings. It would be expected to effectively block the receptor layer by inhibiting signals generated by multiple dimer pairs (15–17). Based on our early report and data from other groups, lapatinib combined with trastuzumab is now being studied in the clinical setting (16–20).
We investigated the effect of lapatinib alone or in combination with other anti-HER agents in two xenograft models and identified lapatinib plus trastuzumab as the most potent combination. Given concerns about the toxicity and cost of long-term treatment with these expensive agents, we further investigated reduced dosing and intermittent scheduling of this potent combination.
METHODS
Reagents, hormones, and antibodies
0.36 mg, 60-day release, 17β-estradiol pellets (E2) were purchased from Innovative Research, Sarasota FL, and tamoxifen citrate (Tam; 500 µg subcutaneously in peanut oil, 5 days/week) was purchased from Sigma (St. Louis, Missouri). Lapatinib (L; 100mg/Kg free base active ingredient via gavage in 1% Tween once a day, 5 days/week) was provided by GlaxoSmithKline (Research Triangle, NC). Gefitinib (G; 100mg/kg via gavage in 1% Tween 80 5days/week) was provided by AstraZeneca (Macclesfield, United Kingdom). Trastuzumab (T; 10mg/kg intraperitoneally in sterile H2O twice a week) and pertuzumab (P; 12mg/kg intraperitoneally the first week and then 6mg/kg intraperitoneally in 1% sterile PBS weekly) were provided by Genentech (San Francisco, CA). Antibodies used for immunoblotting were to phosphorylated (p)-Tyr1248-HER2 (Millipore, Billerica, MA); total HER2, total and phosphorylated forms of AKT (Thr308), ERK1,2 MAPK (Thr202/Tyr204) and β-actin (Cell Signaling Technology, Beverly, CA.)
Immunohistochemistry (IHC)
Tumor tissue was fixed in 4% neutral-buffered formalin overnight before processing and paraffin embedding. IHC was performed on 4-micron sections from randomly arrayed in 4-mm core tissue arrays. BrDU labeling of tumor cell nuclei was visualized by staining with BrDU antibody (Biogenic, San Ramon, CA). Additional sections were used to stain for apoptotic cells using the cleaved caspase 3/7 antibody (Cell Signaling Technology, Beverly, Massachusetts) and for activated MAPK using the p-MAPK antibody (Cell Signaling Technology, Beverly, Massachusetts) as previously described (21, 22).
Tumors were scored by % of positive cells for BrDU and cleaved caspase 3/7 staining, and by Allred score for the activated MAPK staining (21, 23).
Tumor extracts and immunoblots
Frozen tumors from the different treatment groups were homogenized in lysis buffer containing 1% Triton X100, 50mM Hepes, pH7.4, 150mM NaCl, 1.5mM MgCl2, 1mM EGTA, 100mM NaF, 10mM NaPPi, 10% glycerol, 1mM PMSF, 1mM Na3VO4, 10µg/ml aprotinin, and 1× protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN). Tumor lysates were microcentrifuged at 14,000g for 10 minutes at 4°C. Cell supernatants were aliquoted and stored at −70°C. Protein concentration was measured by the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA). Equivalent amounts of protein (25 µg) from each sample were separated by electrophoresis on 8%–16% polyacrylamide gels containing sodium dodecyl sulfate (SDS-PAGE), and transferred by electroblotting onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH) followed by immunobloting with the specific antibodies as previously described (21). For all antibodies the reaction was in Odyssey Blocking Buffer (LI-COR Biosciences, NE) plus 0.05% Tween-20 overnight at 4°C. The blots were washed three times in PBS with tween-20 (PBST) and then incubated for 1 hour at room temperature with fluorescently labeled secondary antibody in Odyssey Blocking Buffer plus 0.05% Tween-20. The blots were then washed in PBST, after which the labeled protein was quantified by Odyssey Infrared Imaging System. Gels were reproduced at least twice. In each tumor sample, protein levels were corrected with β-actin (protein levels/actin levels × 102). Means of corrected expression levels were calculated and fold changes compared to the endocrine treatment alone group (without anti-HER therapy) are presented for each treatment group. Representative blots are presented.
Xenograft studies
MCF/HER2-18 (HER2 transfected) and BT-474 (gene-amplified for HER2) cells were maintained as described (21, 24, 25). Animal care was in accordance with institutional guidelines. MCF7/HER2-18 and BT-474 xenografts were established in ovariectomized 5–6 w e e k-old athymic mice (Harlan Sprague Dawley, Madison, Wisconsin) supplemented with estrogen pellets by inoculating subcutaneously (5 × 106 cells) as described previously (21, 26). When tumors reached the size of 200–250 mm3 (2–4 weeks), mice bearing MCF/HER2-18 xenografts were randomly allocated to continued estrogen (E2), estrogen deprivation alone (ED) by removal of the estrogen pellets, or ED plus tamoxifen citrate (Tam) (26). Mice treated with ED plus Tam were then randomized to receive L, or T alone, L+T, double dose L (2L), L+G, or L+P. Mice treated with E2 and mice treated by ED were randomly allocated to vehicle, L, T, or L+T. Another group was treated with continued E2, Tam, and L+T. Each treatment group contained a minimum of 12 mice. Groups of animals destined for molecular studies contained a minimum of 8 additional mice.
Animals bearing BT474 xenografts were randomly allocated to E2, to ED alone, ED+L, ED+T, ED+L+T, E2+L+T. Additional mice were treated with L+T for short duration (14, 42 days), intermittent administration (14 days on/14 days off), and dose reduction (1/2L + 1/2T). Each treatment group contained a minimum of 10 mice. Groups of animals destined for molecular studies contained a minimum of 6 additional mice.
Tumor volumes were measured weekly as described previously (21, 26). Mice were sacrificed and tumors were harvested after short-term treatment (3 days) for analysis of associated biomarkers, when they reached the size of 1000 mm3, or at the end of the experiments. Each tumor analyzed was from a different mouse; tumor tissues were removed from each individual mouse and kept at −190 °C or formalin-fixed and paraffin embedded for later analyses.
Statistical analysis
Tumor growth curves were constructed using the mean tumor volume at each time point with error bars representing the standard error of the mean. Animals that died of other causes prior to the first animal developing a resistant tumor were not included in the calculation of tumor growth curves but were included in all other analyses. Time to treatment resistance (TTR), defined as time in days to developing tumors that are 2.5 times the volume at baseline, was calculated for each mouse. In the experiments with intermittent scheduling and reduced dosing, and to detect small but significant differences, the rate of tumor progression was measured at day 315 (TP315). A tumor was considered to have progressed if two consecutive 10% or greater increases in tumor size were detected starting from a tumor size >0.
The median TTR along with 95% CI were estimated using the Kaplan–Meier method and compared by generalized Wilcoxon test. P-values for the xenograft studies were adjusted for multiple comparisons using the Hommel method to control for type I error when appropriate. Complete regression (CR) was defined as complete tumor disappearance for at least 3 consecutive measurements. CR rates were calculated based on the total number of animals treated in each group. Pairwise comparisons of tumor proliferation and survival pathways based on IHC assessments were made using nonparametric Wilcoxon rank sum test. All statistical tests were two-sided.
RESULTS
Lapatinib plus trastuzumab combination in MCF-7/HER2-18 tumors
As we have previously shown, in MCF7/HER2-18 tumor xenografts concurrent targeting of the HER pathway and the estrogen receptor (ER) greatly enhances therapeutic efficacy (21). Although treatment with L alone, T alone, or L+T significantly delayed estrogen stimulated growth and prolonged TTR in this model (figure 1A and table 1), the benefit was short-lived and tumor growth resumed.
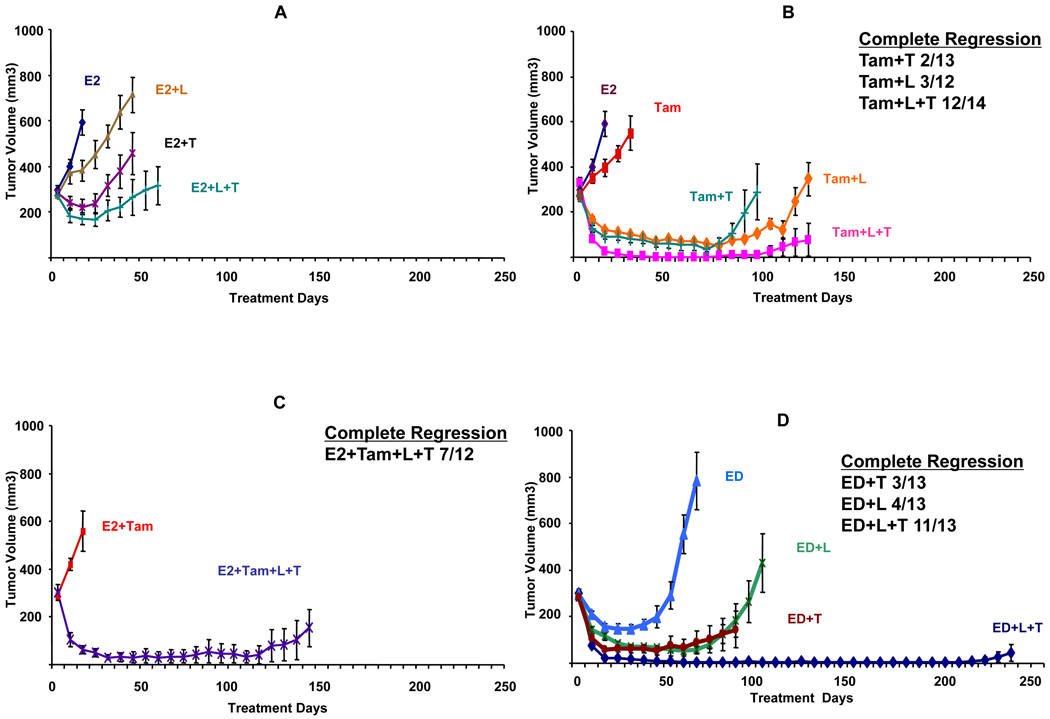
Figure 1.
Growth of MCF7/HER2-18 xenograft tumors in athymic female mice treated with variable anti-HER single agents and combinations, with or without ER targeted therapy. A. E2 treatment alone or with lapatinib (E2+L), trastuzumab (E2+T), or their combination (E2+L+T). B. Tamoxifen treatment alone or lapatinib (Tam+L), trastuzumab (Tam+T), or their combination (Tam+L+T). C. Tamoxifen treatment in the presence of estrogen with the combination of lapatinib and (E2+Tam+L+T). D. Estrogen deprivation (ED) alone or along with lapatinib (ED+L), trastuzumab (ED+T), or their combination (ED+L+T). Results are presented as the mean tumor volume; error bars represent the standard error. In panels B, C, D, and E, for each group, the number of mice with complete tumor regression and the total number of mice are shown. E. Tamoxifen treatment with alternative combinations of HER family inhibitors—lapatinib and gefitinib (Tam+L+G), double dose lapatinib 200mg/kg/day (Tam+2L), and tamoxifen with lapatinib and pertuzumab (Tam+L+P). Complete regression was defined as complete tumor regression documented on 3 consecutive weekly measurements.
Table 1.
Complete tumor response and time to treatment resistance with variable treatment combinations in MCF7/HER2-18 tumor xenografts.
| Treatment+ | Complete Tumor Response % |
Time to Treatment Resistance in Days (95% CI) |
|---|---|---|
| E2 | 0% | 23 (14–43) |
| Tam | 0% | 49 (24–60) |
| E2+L | 0% | 44 (30–56) |
| E2+T | 15% | 53 (39–68) |
| E2+L+T | 0% | 103 (42–136) |
| Tam+T | 15% | 115 (90–146) |
| Tam+L | 25% | 143 (122–174) |
| Tam+2L | 0%§ | 150 (122- NA*) |
| Tam+L+G | 8% | 147 (109-NA*) |
| Tam+L+P | 14% | 178 (138-NA*) |
| Tam+L+T | 86% | 229 (170–342) |
| E2+Tam+L+T | 58% | 182 (143–185) |
Tam: tamoxifen, E2: estrogen T: trastuzumab, L: lapatinib, G: gefitinib, P: pertuzumab.
NA: 95% CI upper limit not achieved.
Several animals had tiny nodules that did not satisfy the definition of complete response.
Figure 1B shows the growth curves in tumors treated with tamoxifen alone or along with HER inhibitors, and table 1 shows the CR rate and the median TTR in these tumors. As previously shown, this xenograft model is tamoxifen stimulated due to, at least in part, cross-talk between non-nuclear ER and the HER pathway (24, 27–29). When tamoxifen-treated mice were treated with single anti-HER agents (Tam+L, Tam+T), CR occurred in some mice and TTR was significantly prolonged (table 1). However, in mice treated with the combination (Tam+L+T), CR was observed in 86% of mice (12/14) (figure 1B). The median TTR was significantly prolonged with this combination to 229 days (95% CI: 170–342) although resistance to this combination eventually emerged. This combination was effective even in the presence of estrogen (E2+Tam+L+T), (figure 1C and table 1).
Additionally, ED was used to target ER to mimic aromatase inhibitor therapy in postmenopausal patients. Figure 1D shows that the addition of L or T to ED improved the CR rate and the TTR. However, the most effective regimen was ED combined with L+T. CR was observed in 85% (11/13) of tumors and after 231 days, only 2 mice had resistant tumors (p<0.0001, 0.039, and 0.035 compared to ED, ED+L, and ED+T, respectively; generalized Wilcoxon test with Hommel adjustment).
After 231 days, (L+T) treatment was stopped in 9 mice with no evidence of tumor and they were randomized to retreatment with estrogen (4 mice) or continued ED (5 mice) and followed for 70 additional days. Two of the four mice retreated with estrogen had tumor regrowth, while none of the mice continued on ED showed regrowth of tumors.
Other inhibitors of the HER pathway in MCF-7/HER2-18 tumors
To better understand the mechanism of action and potency of L+T, we evaluated other HER targeting regimens (figure 1E). To exclude the possibility that L+T was more effective than L alone because of suboptimal dosing of L, and based on published data suggesting that higher dose of lapatinib may be more effective (30, 31), one group of mice was given a double dose of L (200 mg/Kg, 5 day/week; Tam+2L). Since lapatinib is a less potent inhibitor of HER1 than HER2, another group was treated with L and gefitinib (G) for more potent EGFR inhibition (Tam+L+G). Lastly, L was combined with pertuzumab (P) (2C4, Genentech), a monoclonal antibody that inhibits HER2 dimerization, to determine if this potent inhibitor could replace T in the combination (Tam+L+P) (Table 1).
As shown in Table 1 and figure 1E, doubling the L dose (Tam+2L) did not improve efficacy and although several animals had only small residual tumors, none achieved CR. The median TTR was 150 days (95% CI: 122-Not achieved (NA)). Adding G to L (Tam+L+G) also did not improve the CR rate (8%) or the median TTR (147 days, 95% CI: 109-NA). In comparison with Tam+L+T, the substitution of P for T (Tam+L+P) led to inferior results with lower tumor regression rate (14%) and a shorter median TTR (178 days, 95% CI: 138-NA). Although the p-value adjusted for multiple comparisons did not achieve statistical significance when comparing TTR in the Tam+L+P group to the Tam+L+T group, the notable difference in CR (Tam+L+P, 14%; Tam+L+T, 86%) suggests that Tam+L+T is the superior regimen.
Lapatinib plus trastuzumab combination in BT474 tumors
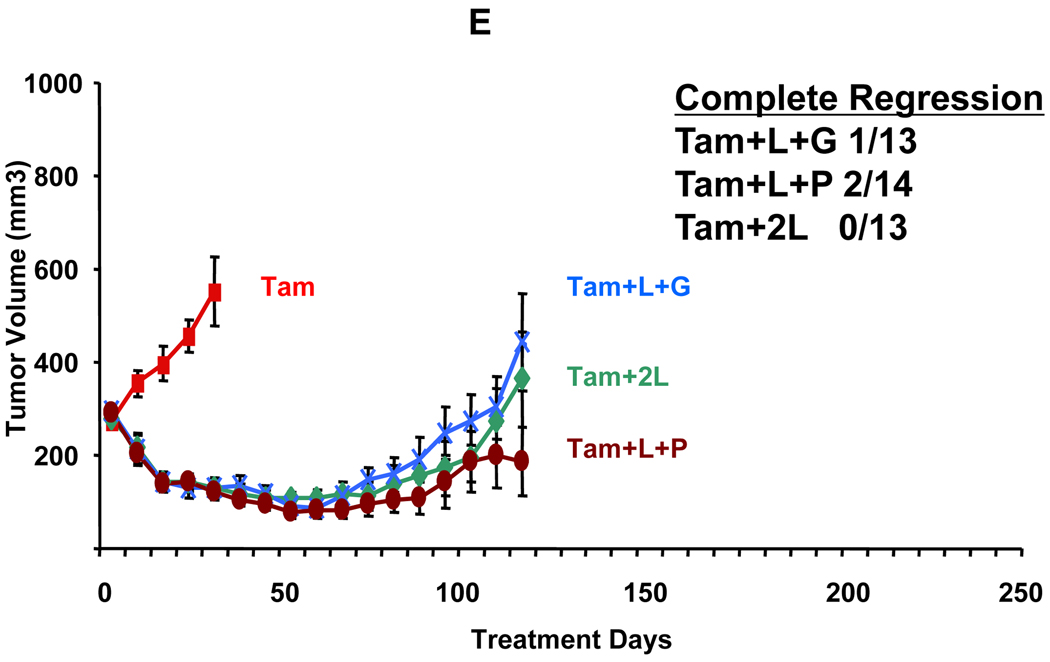
The BT474 cell line is an ER-positive human breast cancer cell line that is naturally amplified for HER2 (25). As we have shown before, established BT474 xenograft tumors are estrogen independent despite expressing ER and grow similarly with and without estrogen (figure 2A) (21). L and T alone led to complete tumor regression in 45% (5/11) and 91% (10/11) of ED-treated mice, respectively. However, BT474 tumors were exquisitely sensitive to L+T. All tumors completely regressed in the presence of estrogen (13/13) and 92% completely regressed in its absence (11/12) (figures 2B and 2A, respectively). By day 231 of treatment, four out of the original eleven mice on the L alone arm and two out of eleven mice on T alone arm had developed resistant tumors. In contrast, none of the animals treated with L+T developed any tumors at any point during the experiment. This again indicates the superiority of this combination in BT474 as well as in MCF7/HER2-18 tumors.
Figure 2.
Growth of BT474 xenograft tumors in athymic mice treated with estrogen supplementation (E2) or estrogen deprivation (ED) alone or with HER blocking agents. A. Estrogen deprivation (ED) alone or along with lapatinib (ED+L), trastuzumab (ED+T), or their combination (ED+L+T). B. Continued Estrogen supplementation alone or with the combination of lapatinib and trastuzumab (E2+L+T). Results are presented as the mean tumor volume error bars represent the standard error. For each group, the number of mice with complete tumor regression and the total number of mice are shown. Complete regression was defined as complete disappearance of the tumor for 3 consecutive weeks.
Lapatinib plus trastuzumab intermittent and reduced dose regimen in BT474 tumors
We next asked whether alternative treatment regimens of (L+T) with reduced dosing or changes in scheduling were as effective as full continuous dosing.
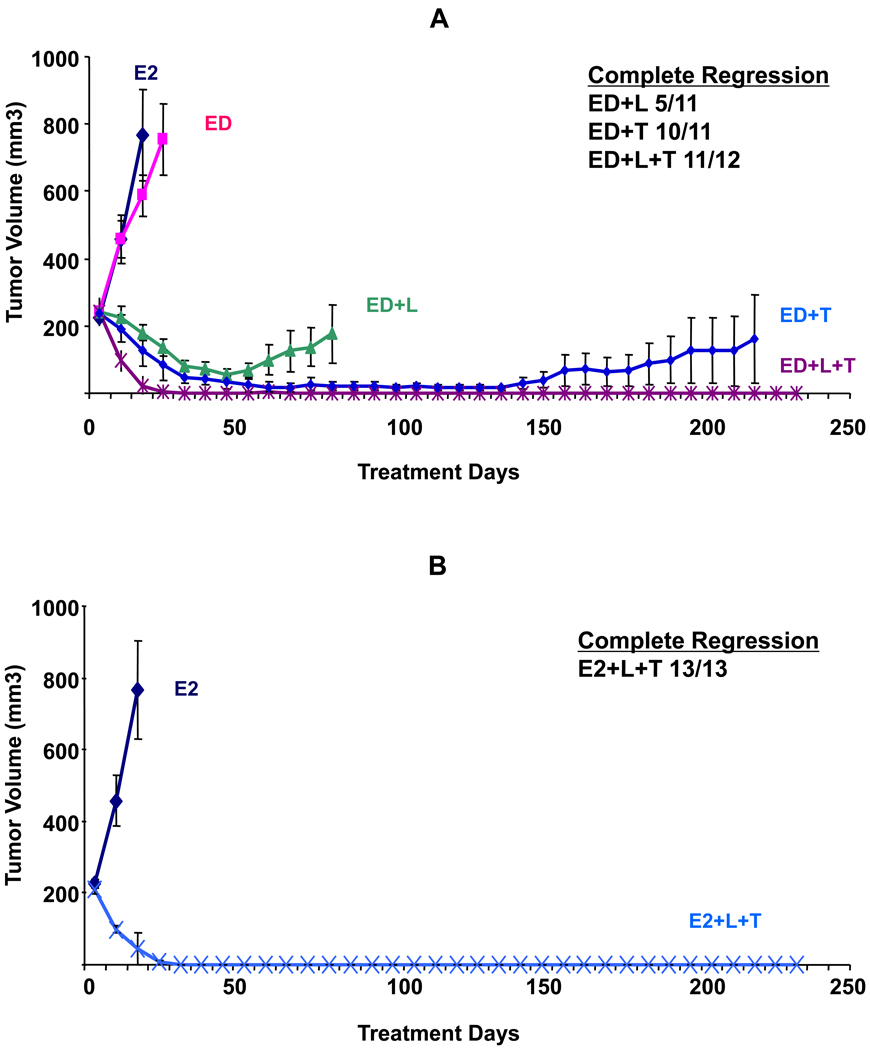
As shown in figure 3A and table 2, several control treatment groups from prior experiments were repeated to ensure reproducibility of our results (E2, ED, L, T arms, and L+T),) and all yielded similar results.
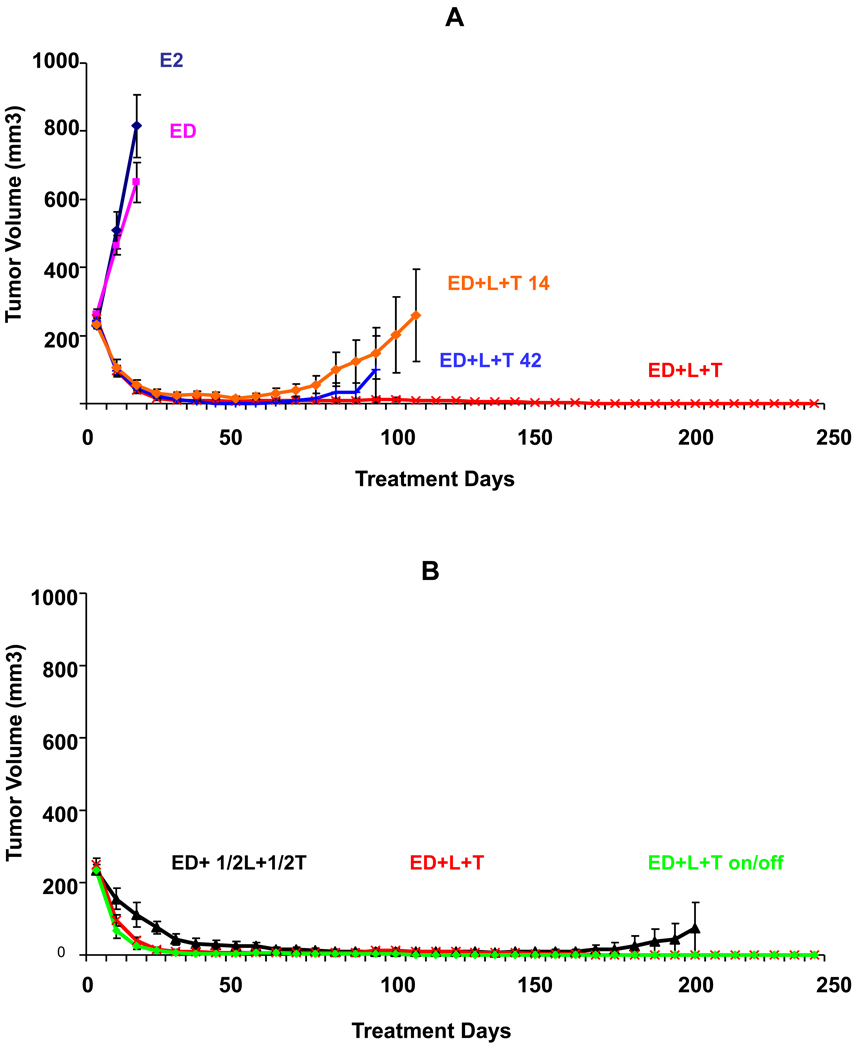
Figure 3.
Growth of BT474 xenograft tumors in athymic mice treated with estrogen supplementation (E2), or estrogen deprivation (ED) alone and with Lapatinib (L), trastuzumab (T), or their combination (L+T) in various doses and schedules. A. Growth of tumors in animals treated with ED plus short durations of (L+T) for 14 days (ED+L+T 14) and 42 days (ED+L+T 42) compared to control groups treated with E2, ED, ED+L, and ED+T. B. Growth of tumors treated with ED with full continuous (L+T), reduced dose therapy (1/2L+1/2T), or intermittent therapy (ED+L+T 14 on/off). For each group, the number of mice with complete tumor regression and the total number of mice are shown. Complete regression was defined as complete disappearance of the tumor for 3 consecutive weeks.
Table 2.
Complete response (CR), time to complete response (TCR), and tumor progression rate at 315 days (TP 315) in treatment arms with alternate dosing/scheduling of lapatinib/trastuzumab in BT474 tumor xenografts.
| Full L+T § |
T | L | 1/2L+1/2T | L+T 42 days |
L+T 14days |
L+T 14 days on/off |
|
|---|---|---|---|---|---|---|---|
| N (mice) | 13 | 19 | 13 | 15 | 15 | 15 | 14 |
| Median TCR | 35 | 63 | 70 | 49 | 42 | 35 | 35 |
| P* | Ref | 0.008 | 0.0003 | 0.03 | 0.58 | 0.72 | 0.46 |
| CR (%) | 100 | 89 | 77 | 93 | 100 | 80 | 100 |
| TP 315 (%) | 0 | 16 | 46 | 13 | 28 | 60 | 14 |
| P* | Ref | 0.13 | 0.009 | 0.18 | 0.05 | 0.001 | 0.17 |
P value of generalized Wilcoxon test comparing other treatment groups to reference group (Ref).
T: trastuzumab, L: lapatinib
Short treatment duration for just 14 days led to CR in 80% of mice and median time to CR was similar to continuous (L+T) (35 days in both groups, p=0.72, generalized Wilcoxon test). This indicates that the treatment effect carried on beyond its brief duration, as tumors continued to shrink after treatment was stopped. Although the rate of eventual tumor progression was clearly higher in this group (60% vs. 0%, p=0.001, generalized Wilcoxon test), still this brief duration of treatment resulted in prolonged tumor control with no tumor progression in 6 out of 15 mice. Similarly, when treatment lasted just 42 days, CR rate and median time to CR were similar to full (L+T) but the rate of tumor progression at 315 days was higher than with continuous L+T (27% vs. 0% tumor progression, p=0.05, generalized Wilcoxon test).
Figure 3B shows two alternate dosing methods, reduced or intermittent dosing. Treatment with reduced dosing, (½ L + ½ T) led to CR in 93% of mice. Although, median time to CR was longer compared to continuous full dose L+T, (49 days vs. 35 days, respectively, p= 0.03, generalized Wilcoxon test), the rate of tumor progression at 315 days was not significantly different (13% vs. 0%, respectively, p=0.18, generalized Wilcoxon test). On the other hand, intermittent treatment with L+T (14 days on treatment and 14 days off) led to CR and median time to CR similar to continuous full dose (L+T) (100% and 35 days in both groups). The difference in rate of tumor progression between intermittent vs. full L+T groups was not statistically significant (14% vs. 0% respectively, p=0.17, generalized Wilcoxon test).
Effect of Lapatinib plus trastuzumab on tumor cell proliferation and apoptosis
We next examined the effects of these treatments on tumor cell proliferation, apoptosis, and key downstream signaling intermediaries in the HER pathway.
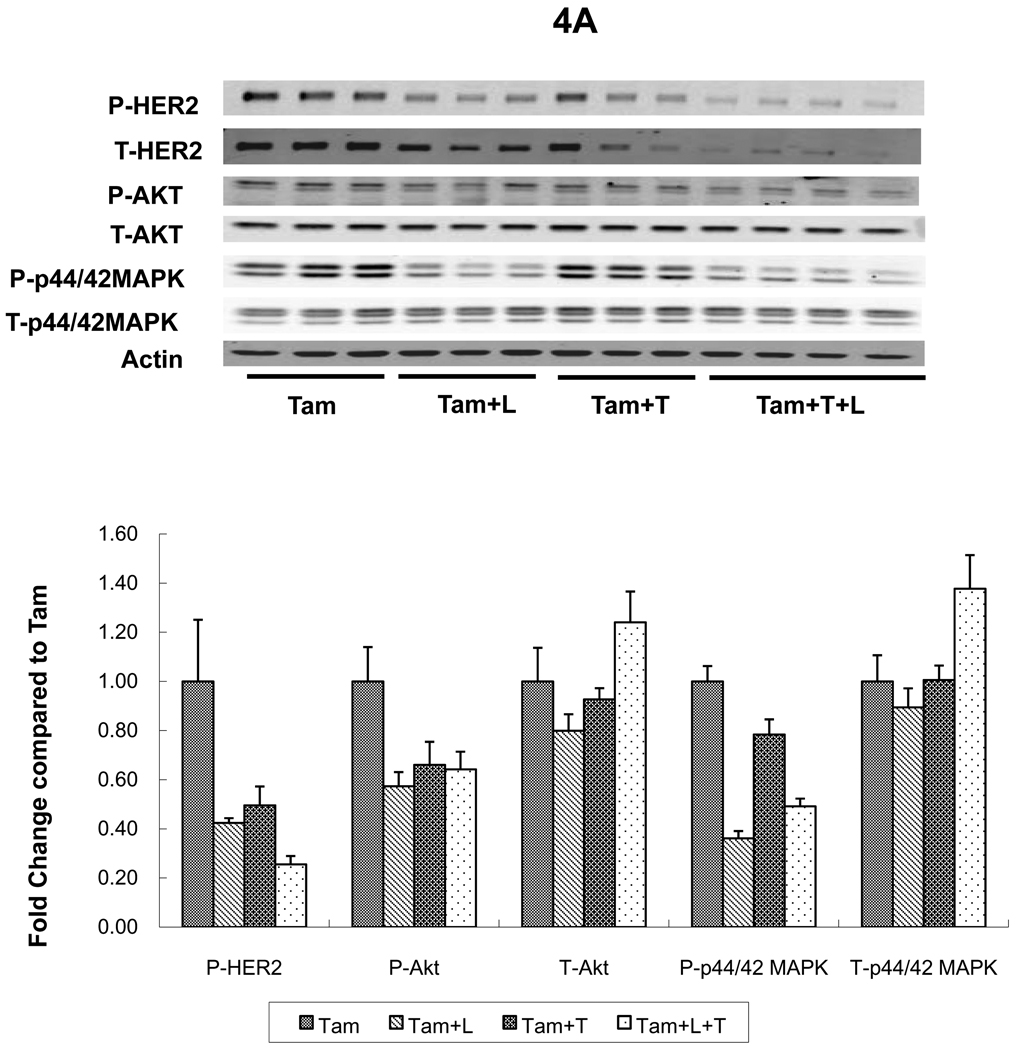
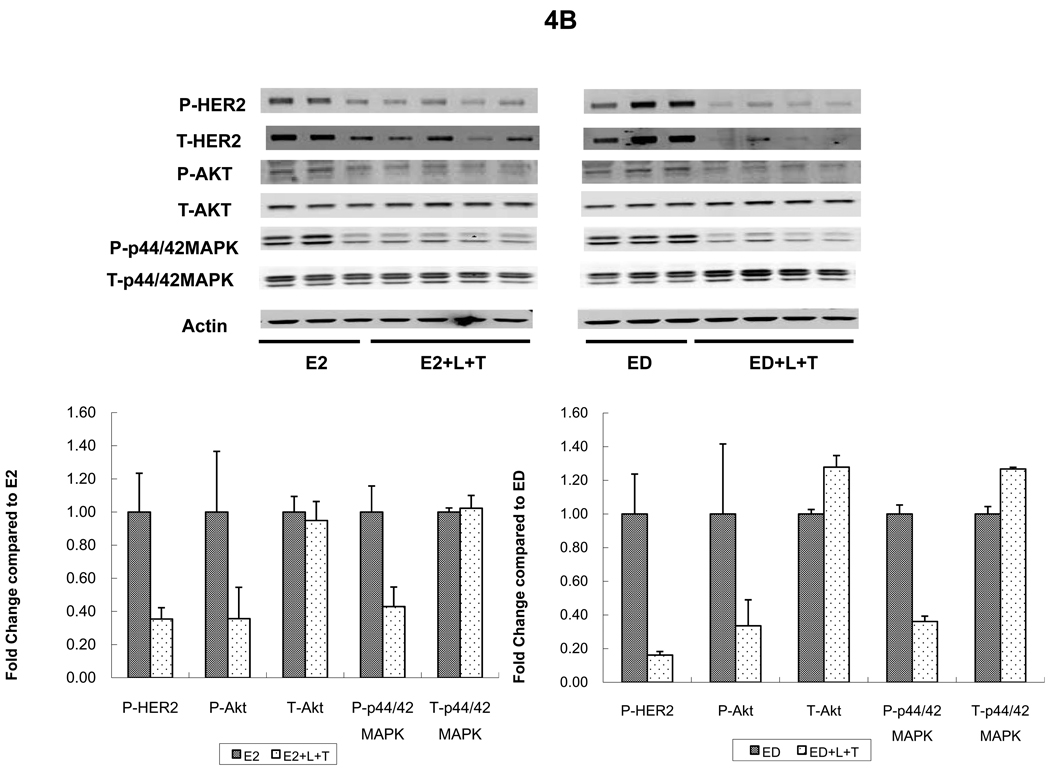
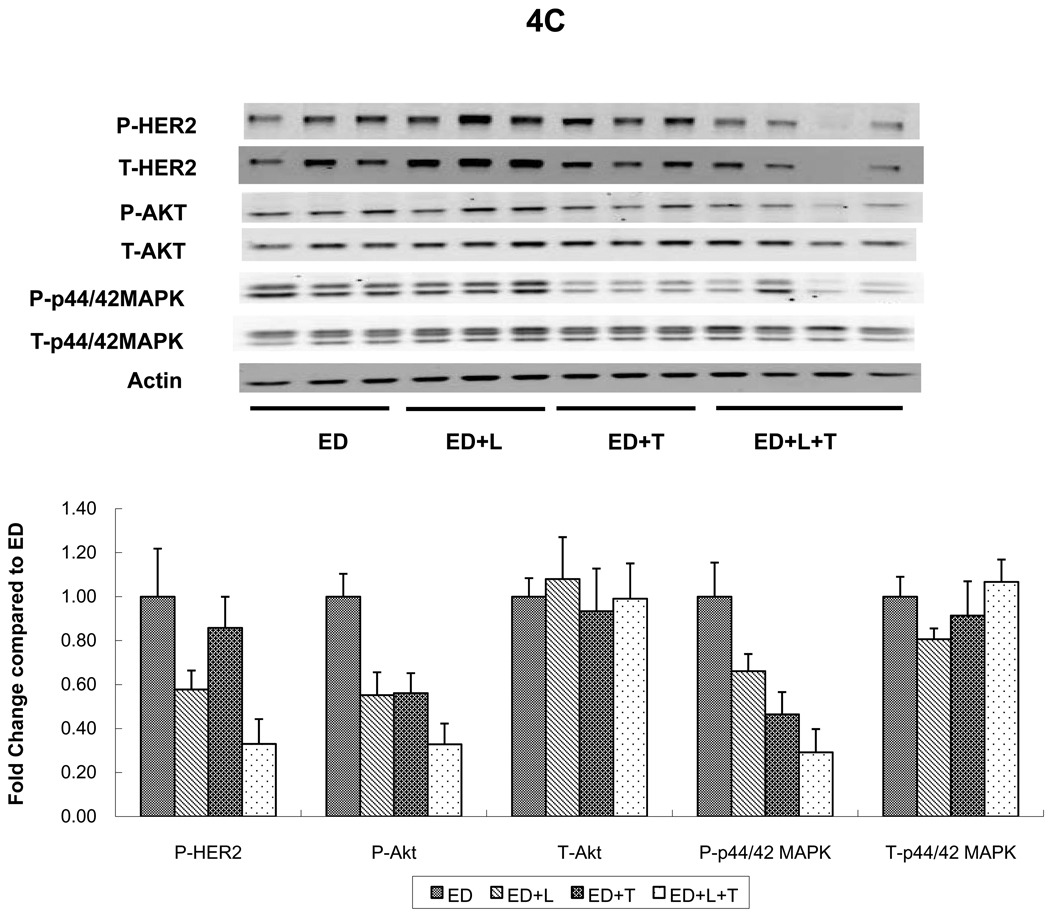
Levels of p-HER2 were suppressed in MCF7/HER2-18 tumors from mice treated with L or T (figures 4A and 4B). However, the combination (L+T) was more potent in inhibiting p-HER2 levels regardless of whether mice received continued estrogen, estrogen deprivation, or Tam (figure 4A). The same trends were noted in BT474 tumors where L and T each inhibited p-HER2 individually but were more potent in combination figure 4C). Although total HER2 appears reduced in both tumor models in mice treated with the L+T combination, this is likely related in part to tumor shrinkage and tumor cell death. When corrected to pancytokeratin, total HER2 was not significantly reduced in animals treated with L+T (data not shown).
Figure 4.
HER2 and downstream signaling pathways were assessed in tumors from two cell lines grown as xenografts in athymic mice and treated for 3 days. A. MCF7/HER2-18 xenograft tumors treated with tamoxifen (Tam) alone or with lapatinib (Tam+L), trastuzumab (Tam+T), or their combination (Tam+L+T). B. MCF7/HER2-18 xenograft tumors grown with estrogen (E2) alone or with the combination of lapatinib and trastuzumab (E2+L+T) and estrogen deprivation (ED) alone or with the combination of lapatinib and trastuzumab (ED+L+T).
C. BT474 xenograft tumors treated with estrogen deprivation (ED) alone or with lapatinib (ED+L), trastuzumab (ED+T), or their combination (ED+L+T). Proteins from tumor lysates were separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis and subjected to immunoblot analysis with antibodies specific to total HER2, total Akt, total MAPK, phosphorylated HER2 (at Tyr1248); phosphorylated Akt (at Thr308) and phosphorylated p44/42 MAPK (at Thr202 and Tyr204); or β-actin. Protein expression and phosphorylation levels were quantified by Odyssey Infrared Imaging System. In each tumor, protein levels were normalized to the level of β-actin (the loading-control; by the formula, protein level/actin level *100). For each treatment arm, protein expression levels were compared with control group (Tam, E2, ED) as 1.00.
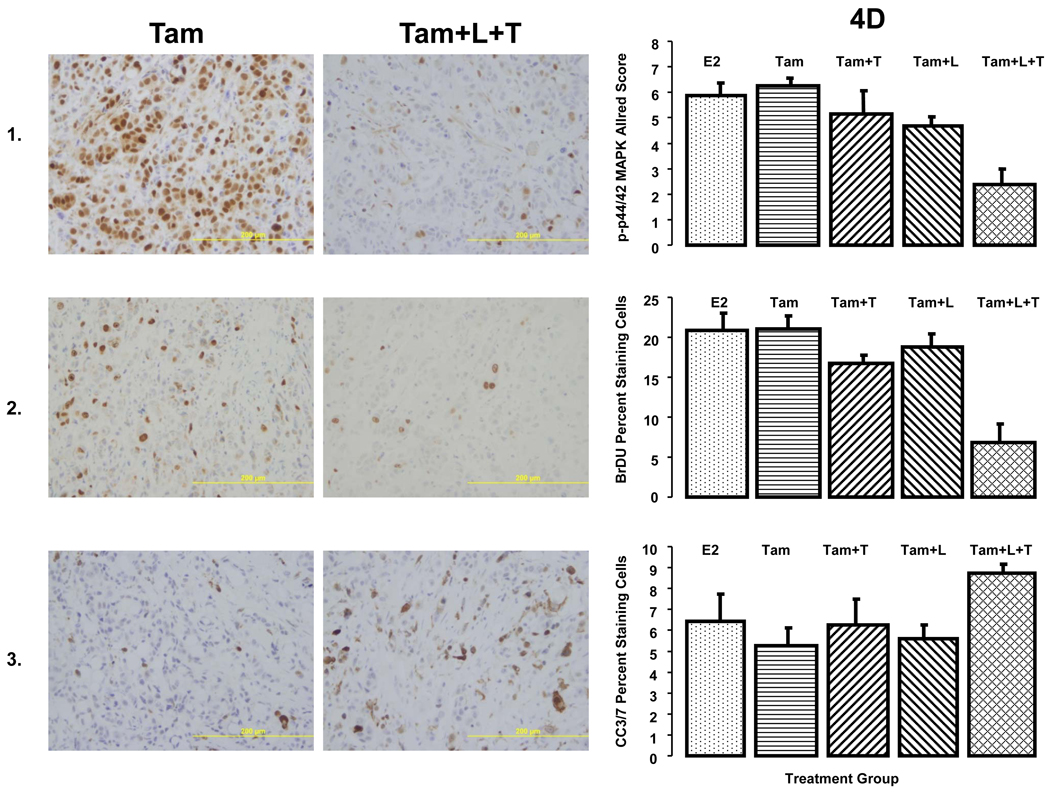
D. IHC studies on MCF7/HER2-18 xenografts: The pictures on the left are representative images of each biomarker staining. The panels on the left are quantitative representations of biomarker expression.
Panel 1. Expression of phosphorylated p44/42 MAPK (at Thr202 and Tyr204) in MCF7/HER2-18 xenograft tumors treated with estrogen (E2), tamoxifen (Tam) alone, or with lapatinib (Tam+L), trastuzumab (Tam+T), and the combination (Tam+L+T). The length of treatment was 3 days. Levels of phosphorylated MAPK were assessed by immunohistochemical staining and reported using the Allred Score. There were at least 8 tumors from each group. Panel 2. Cell proliferation: Bromodeoxyuridine staining was used to measure cell proliferation. Proliferation was measured in tumors, eight from each treatment group, after 3 days of treatment. Results are the percentage of cells positive for bromodeoxyuridine. Panel 3. Apoptosis Levels of cleaved caspase 3/7 were assessed immunohistochemically by use of an antibody against cleaved caspase 3/7 after day 3 of treatment. Apoptosis was measured at least 8 tumors from each treatment group. Results are the percentage of cells positive for cleaved caspase 3/7.
In all panels, error bars represent the standard error.
Levels of p-MAPK were assessed in MCF7/HER2-18 (figure 4A, 4B, 4D) and BT474 tumors (figure 4C). Levels of p-MAPK were significantly reduced in tumors from mice receiving L, or T in addition to endocrine therapy in both models (figure 4A; p=0.008, 0.05, figure 4C; p=0.02, 0.002 respectively, when compared to endocrine therapy alone, Wilcoxon rank sum test). L+T also significantly reduced p-MAPK levels in both tumor models regardless of endocrine therapy (figure 4A; p=0.02 compared to Tam, figure 4B; p=0.05 compared to E2, figure 4C; p=0.002 compared to ED, Wilcoxon rank sum test). Interestingly, figure 4A shows that L reduced p-MAPK more than L+T. However, p-MAPK assessment by IHC (figure 4D) shows that in tumors from mice treated with Tam+L+T, p-MAPK levels were significantly reduced than those observed in Tam, Tam+L, and Tam+T (p=0.005, 0.019, 0.039 respectively, Wilcoxon rank sum test). Similar significant reductions in p-MAPK levels were observed in tumors from mice treated with Tam+L+T in the presence of estrogen (data not shown). In BT474 tumors p-MAPK levels were suppressed in tumors treated with both T and L as single agents, and even more so with the combination (figure 4C). These results indicate that L+T is the most effective regimen in reducing levels of p-MAPK.
Levels of p-Akt were assessed by Western blot and were notably reduced in tumors receiving L, T, or their combination in both tumor models; MCF7/HER2-18 and BT474 (figure 4A, 4B, and 4C).
The relative effect of L+T on proliferation and apoptosis was investigated. Proliferation was assessed using BrDU uptake after 3 days of treatment in MCF/HER2-18 tumor xenografts (figure 4D). Neither L nor T significantly reduced the proliferative fraction when added to Tam (p=0.54, 0.10, respectively, Wilcoxon rank sum test). However, the L+T combination caused a significant reduction in BrDU uptake in comparison to Tam alone, Tam+L, or Tam+T (p=0.007, 0.007, 0.016 respectively, Wilcoxon rank sum test). Similar results were noted BT474 xenografts as well (data not shown).
Apoptosis was assessed by IHC at day 3 by an antibody that detects cleaved caspase 3 and 7. Figure 4D shows that in MCF7/HER2-18 xenografts, neither L nor T resulted in any significant change in apoptosis (p=0.57, 0.61 respectively, Wilcoxon rank sum test). However, when L+T were used in combination, a significant increase in apoptosis was observed over Tam alone or Tam+L (p=0.032, 0.0097 respectively, Wilcoxon rank sum test). In BT474 xenografts, a trend of increased apoptosis in the T and L+T treatment groups over Tam or Tam+L was noted, but was not statistically significant.
DISCUSSION
Trastuzumab is effective in several clinical settings (11, 12), and recent data suggest that the population of patients that benefit from trastuzumab therapy may be expanding (32). However, de novo and acquired resistance to trastuzumab remain a challenge in clinical management. One strategy to overcome resistance is a more complete blockade of HER2 signaling using a combination of HER2 inhibitors. At the same time, identifying optimal duration of this blockade could lead to reduced treatment cost and enhanced quality of life if prolonged therapy is not necessary.
Lapatinib, a dual tyrosine kinase inhibitor of HER1 and 2, should block the HER receptors more completely than trastuzumab which is most effective in inhibiting HER2 homodimers. Furthermore, lapatinib inhibits p95, the constitutively active short form of HER2 against which trastuzumab is ineffective. The data presented here using two different in vivo model systems show that neither lapatinib nor trastuzumab alone are as effective as their combination in antagonizing HER2 related signaling pathways or inducing tumor regression. The combination of the two drugs provides more potent inhibition of downstream signals, more effective inhibition of cell proliferation and possibly greater induction of apoptosis than each as a single agent. Our findings support our prior report of the remarkable efficacy of a three-drug cocktail of HER inhibitors in tumor xenografts in mice (21) and they provide additional rationale for a combination regimen of the two approved drugs now in clinical testing.
Our data also provide mechanistic insights into the optimal method to block the HER pathway at the receptor level. Lapatinib was expected to be a more complete inhibitor of the pathway but the antitumor effects of the drug were insufficient as a single agent in both MCF7/HER2-18 and BT474 tumors. Adding gefitinib to lapatinib for added HER1 inhibition, doubling the lapatinib dose, and substituting pertuzumab for trastuzumab to block HER2 heterodimerization were much less effective in causing complete tumor regressions and delaying TTR than was the combination of lapatinib with trastuzumab.
Data from our group evaluating human HER2-overexpressing breast cancer samples of patients obtained from 2 neoadjuvant clinical trials treated with single agent trastuzumab or lapatinib showed that tumors with low PTEN or mutated PI3KCA are resistant to trastuzumab but sensitive to lapatinib (33). These findings along with evidence from other groups and the present data indicate that lapatinib and trastuzumab may exert their effect via distinct but complementary mechanisms of action (34, 35).
After demonstrating the efficacy of the combination of lapatinib and trastuzumab, we investigated the effect of dose reduction, intermittent delivery, or shortened duration of the standard combination. Given the promising results from a clinical study in patients treated with short term trastuzumab (36) and our prior observation that trastuzumab, like chemotherapy, induces apoptosis in tumors from patients undergoing neoadjuvant therapy (37), in the current study we reasoned that a brief course of combined L+T might still be very effective.
Our results demonstrated that even when treatment was given for a short course (14 days), tumor regression continued after stopping therapy, and the majority of mice had a CR with 40% of mice without tumor progression at 315 days. When treatment duration was extended to 42 days, the proportion of mice without tumor progression at 315 days increased to 72%. Thus, while longer therapy may be optimal, these very short exposures were still very effective; suggesting that shorter treatment durations should be explored in patients.
We also demonstrate that a reduction of the dose of both L and T (½ L + ½ T) effectively blocks the HER pathway and is as effective as full doses in eradicating tumors. Furthermore, the schedule of intermittent therapy which lowered the dose intensity by half was just as effective.
While it is difficult to extrapolate these treatment doses and durations directly to patients, the cumulative data suggests these or similar strategies should be tested in patients. There is increasing emphasis on reducing toxicity from unnecessary treatment. A recent clinical trial of L+T with paclitaxel had to be stopped early due to significant toxicity especially diarrhea (38). This, together with economic implications of the rising cost of cancer care, highlights the potential impact of our results. Clinical trials comparing the standard 1 year approach to shorter and intermittent dosing schedules should be explored. If as effective as standard therapy, these alternative schedules would significantly lower the cost and toxicity of treatment.
Our molecular studies show that the combination of lapatinib and trastuzumab reduces phosphorylated HER more effectively than either agent alone.
Additionally, the (L+T) combination inhibits downstream signaling through two important cell proliferation and survival pathways mediated through AKT and MAPK, thus explaining the increased anti-tumor activity of the combination. Our finding of a significant reduction in proliferation and increased apoptosis in the (L+T) treated tumors over those treated with a single agent also supports this conclusion.
In addition to direct potent inhibition of HER signaling, the combination may be working by other mechanisms. There are data showing that lapatinib stabilizes different HER dimers which are then internalized after trastuzumab binding to HER2 (19). Additionally, there is mounting evidence that both agents are active against tumor initiating cells which may explain tumor xenograft elimination in some animals in our study (39, 40)
Our data, derived from experimental models, may not represent the totality of HER2-overexpressing breast cancers in patients. Both our models are ER-positive, although established BT474 xenografts are not estrogen dependent (21). However, our findings have important and compelling clinical implications.
In addition to lending support for clinical trials studying the combination of lapatinib and trastuzumab, our findings strongly argue for timely clinical testing of intermittent dosing of this combination. As personalized cancer therapy becomes the new standard of care in oncology, it is a high priority to combine optimal therapeutic strategies with minimal toxicity and cost.
STATEMENT OF TRANSLATIONAL RELEVANCE
We have previously shown that combination anti-HER therapies are more effective than single agents in HER2-overexpressing breast cancer models by providing more potent blockade of downstream signaling. The present pre-clinical study investigates drugs targeting the pathway by different mechanisms and examines different doses and schedules in HER2-overexpressing xenografts. Lapatinib plus trastuzumab was the most potent combination in blocking HER signaling, and it led to tumor eradication in most mice. Interestingly, intermittent or reduced dosing schedules were just as effective as continuous therapy.
These data provide a rationale for clinical trials of intermittent or reduced doses of lapatinib plus trastuzumab therapy, strategies that, if as effective as continuous full dose treatment, would dramatically reduce the toxicity and cost of treatment.
Acknowledgments
Supported by: This work was supported in part by NCI grants P50 CA58183 (Breast Cancer SPORE) and P01 CA30195, The Breast Cancer Research Foundation (BCRF), Entertainment Industry Foundation/Lee Jeans Breast Cancer Program, and a research grant from GSK.
REFERENCES
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 3.Maurer CA, Friess H, Kretschmann B, et al. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol. 1998;29:771–777. doi: 10.1016/s0046-8177(98)90444-0. [DOI] [PubMed] [Google Scholar]
- 4.Alimandi M, Romano A, Curia MC, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 5.Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. Embo J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 7.Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122–130. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 9.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 10.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 14.Vogel C, Cobleigh MA, Tripathy D, et al. First-line, single-agent Herceptin(trastuzumab) in metastatic breast cancer: a preliminary report. Eur J Cancer. 2001;37 Suppl 1:S25–S29. [PubMed] [Google Scholar]
- 15.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 16.Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 17.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib ( GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 18.Ritter CA, Perez-Torres M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 19.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 20.Rimawi MF, Weiss HL, Arpino G, Morris GS, Soliz RD, Ward RM, Gilmer TM, Osborne CK, Schiff R. Potent inhibition of EGFR(ErbB1)/HER2 (ErbB2) pathway plus estrogen deprivation is a superior therapeutic combination in ER positive HER2-neu Overexpressing breast tumor xenografts. Breast Cancer Research and Treatment. 2006;100:S1. [Google Scholar]
- 21.Arpino G, Gutierrez C, Weiss H, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 22.Chang JC, Wooten EC, Tsimelzon A, et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. J Clin Oncol. 2005;23:1169–1177. doi: 10.1200/JCO.2005.03.156. [DOI] [PubMed] [Google Scholar]
- 23.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 24.Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1993;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa H, Lenferink AE, Simpson JF, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 26.Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SH, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. Journal of the National Cancer Institute. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 27.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 28.Massarweh S, Osborne CK, Jiang S, et al. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 29.Scott GK, Marden C, Xu F, Kirk L, Benz CC. Transcriptional repression of ErbB2 by histone deacetylase inhibitors detected by a genomically integrated ErbB2 promoter-reporting cell screen. Mol Cancer Ther. 2002;1:385–392. [PubMed] [Google Scholar]
- 30.Moasser MM, Wilmes LJ, Wong CH, et al. Improved tumor vascular function following high-dose epidermal growth factor receptor tyrosine kinase inhibitor therapy. J Magn Reson Imaging. 2007;26:1618–1625. doi: 10.1002/jmri.21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chien AJ, Illi JA, Ko AH, et al. A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res. 2009;15:5569–5575. doi: 10.1158/1078-0432.CCR-09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migliaccio I, Gutierrez MC, Wu M-F, Wong H, Pavlick A, Hilsenbeck SG, Horlings HM, Rimawi M, Berns K, Bernards R, Osborne CK, Arteaga CL, Chang JC. San Antonio Breast Cancer Symposium; 2008. San Antonio, TX: Breast Cancer Research and Treatment; 2008. PI3 kinase activation and response to trastuzumab or lapatinib in HER-2 overexpressing locally advanced breast cancer (LABC) [Google Scholar]
- 34.Johnston S, Trudeau M, Kaufman B, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26:1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 35.Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170–1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 36.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 37.Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–2468. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 38.Esteva FJ, Franco S, Hagan MK, Brewster A, Williams W, Florance AM, Koch K, Turner SJ, Ridderheim M, Perez AT. Updated efficacy and safety assessment of first-line therapy with lapatinib, trastuzumab, and paclitaxel in HER2+ metastatic breast cancer. ASCO Annual Meeting; 2010; Chicago, Il. 2010. [Google Scholar]
- 39.Magnifico A, Albano L, Campaner S, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–2021. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]