Abstract
Neonatal exposure to endocrine disrupting chemicals (EDCs) such as polychlorinated biphenyls (PCBs) can interfere with hormone-sensitive developmental processes, including brain sexual differentiation. We hypothesized that disruption of these processes by gestational PCB exposure would be detectable as early as the day after birth (postnatal day (P) 1) through alterations in hypothalamic gene and protein expression. Pregnant Sprague-Dawley rats were injected twice, once each on gestational days 16 and 18, with one of the following: DMSO vehicle; the industrial PCB mixture Aroclor 1221 (A1221); a reconstituted mixture of the three most prevalent congeners found in humans: PCB138, PCB153 and PCB180; or estradiol benzoate (EB). On P1, litter composition, anogenital distance (AGD) and body weight were assessed. Pups were euthanized for immunohistochemistry of estrogen receptor α (ERα) or TUNEL labeling of apoptotic cells, or quantitative PCR of 48 selected genes in the preoptic area (POA). We found that treatment with EB or A1221 had a sex-specific effect on developmental apoptosis in the neonatal anteroventral periventricular nucleus (AVPV), a sexually dimorphic hypothalamic region involved in the regulation of reproductive neuroendocrine function. In this region, exposed females had increased numbers of apoptotic nuclei, whereas there was no effect of treatment in males. For ERα, EB treatment increased immunoreactive cell numbers and density in the AVPV of both males and females, while A1221 and the PCB mixture had no effect. PCR analysis of gene expression in the POA identified nine genes that were significantly altered by prenatal EDC exposure, in a manner that varied by sex and treatment. These genes included brain-derived neurotrophic factor, GABAB receptors-1 and -2, IGF-1, kisspeptin receptor, NMDA receptor subunits NR2b and NR2c, prodynorphin, and TGFα. Collectively, these results suggest that the disrupted sexual differentiation of the POA by prenatal EDC exposures is already evident as early as the day after birth, effects that may change the trajectory of postnatal development and compromise adult reproductive function.
Keywords: Polychlorinated biphenyls (PCBs), endocrine disruption, hypothalamus, sexual differentiation, neuroendocrine development, estrogen receptor, developmental apoptosis, anteroventral periventricular nucleus, medial preoptic nucleus
Introduction
Exposure to environmental endocrine disrupting chemicals (EDCs) during critical developmental periods, particularly late gestation and infancy, are consistently linked to impairments in homeostatic, endocrine, and neurobiological processes in adulthood (Dickerson and Gore, 2007). Amid concerns that chronic low-dose exposures to EDCs may be contributing to a decline in fertility in humans (Diamanti-Kandarakis et al., 2009), recent interest has turned to elucidating how reproductive neuroendocrine systems may be perturbed by EDC exposures during early critical life stages. As the hypothalamic control of reproduction develops in a sexually dimorphic manner due to sex differences in gonadal steroid hormone actions in the brain, it is plausible to hypothesize that some of the links between perinatal EDCs and the diminution in reproductive competency may be due at least in part to reprogramming of the neonatal hypothalamus by these compounds.
Sexual differentiation of the hypothalamus of rodents occurs primarily during the third trimester of gestation through the early postnatal period. During these life stages, the number and phenotype of neurons that arise in the sexually dimorphic nuclei of the hypothalamus are sculpted by a number of neurodevelopmental processes, including programmed cell death, called apoptosis (Davis et al., 1996a; Yoshida et al., 2000). Whereas the exposure of the female rodent brain to circulating gonadal hormones is relatively low, the fetal male testis produces much higher levels of testosterone, which are aromatized to estradiol locally in the male brain (Bakker and Brock, 2010). Sex differences in these neural exposures have profound effects on the numbers of cells that ultimately survive in a region-specific manner.
Importantly, there are links between steroid hormone exposures and apoptosis. Depending upon the brain region, ligand-bound estrogen receptors (primarily ERα) may bind to nuclear response elements that promote transcription of factors that either stimulate or inhibit developmental apoptosis (McCarthy, 2008). This point is exemplified by developmental differences in apoptosis in the neonatal anteroventral periventricular nucleus (AVPV), a region that differs in size and cellular phenotype between males and females and is postulated to mediate estradiol positive feedback onto the GnRH/LH surge in females (Clarkson and Herbison, 2006). In the developing AVPV, estradiol stimulates apoptosis during the early postnatal period (Murakami and Arai, 1989; Sumida et al., 1993; Waters and Simerly, 2009), contributing to the development of a smaller AVPV in adult males (Davis et al., 1996b), and presumably, subsequent differences in reproductive physiology and behavior. Another brain region under investigation in this study is the medial preoptic area (MPN), a region important for adult reproductive behaviors (Wu et al., 2009; Wu and Gore, 2010), sexually dimorphic in size (Madeira et al., 1999), and abundant in ERs (Chakraborty et al., 2003). Evidence that EDCs can act upon ERα and other steroid hormone receptors in the nervous system suggests that they may perturb developmental apoptosis in the AVPV and MPN, a hypothesis tested in this current study.
Our laboratory has been using a class of compounds known as polychlorinated biphenyls (PCBs), a family of persistent chemicals once used widely for industrial applications, as a model for neuroendocrine disruption (Salama et al., 2003; Steinberg et al., 2007; Steinberg et al., 2008; Dickerson et al., 2009). Although banned for decades, PCBs are still detectable in most humans (Gladen et al., 2003). EDCs rarely exert their effects by a single mechanism, and PCBs have been shown to be weakly estrogenic (Mortensen and Arukwe, 2008; Nomiyama et al., 2010), as well as anti-estrogenic and anti-androgenic, among other mechanisms (Bonefeld-Jorgensen et al., 2001). We utilized two PCB mixtures that differ in their half-life, degree of chlorination, and properties. Aroclor 1221 (A1221; an estrogenic PCB) is a technical mixture once used commercially, and is comprised of lightly chlorinated congeners with a half-life on the order of days (Matthews and Anderson, 1975). We also used a reconstituted mixture of the three most prevalent congeners detected in human and wildlife samples: PCB138, PCB153, and PCB180, which are more heavily chlorinated and have a half-life on the order of years (Milbrath et al., 2009; Seegal et al., 2010). In vitro studies have shown that these compounds interact with steroid hormone receptors including ERα at low doses (Bonefeld-Jorgensen et al., 2001). We tested the hypotheses that prenatal EDCs would affect developmental apoptosis and expression of ERα in a sexually dimorphic manner, an effect that could be detectable as early as postnatal day (P) 1. We also used a 48-gene real-time PCR array platform to identify novel gene expression targets of developmental PCB exposure on the P1 hypothalamus.
Methods
Animals and perinatal treatment
All protocols on rats were carried out following guidelines from the National Institute of Health Guide for Care and Use of Laboratory Animals, and performed following protocols approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Dams for this study (Harlan Sprague–Dawley Inc.; Houston, Texas; Stock/Strain: Hsd:Sprague–Dawley®™ SD®™) were those described in a sister study on effects of prenatal PCBs on the adult hypothalamus (Dickerson et al., 2011). Rats were housed individually under standard husbandry [12:12 partially reversed light cycled (lights on 2300 h, lights off 1100 h)] and fed low-phytoestrogen Harlan-Teklad 2019 Global Diet ad libitum for at least 3 weeks prior to mating. Rats were handled daily to minimize stress.
Dams were mated with sexually experienced male rats (males randomly rotated with females), and the day of sperm positive vaginal smears was termed embryonic day (E) 1. The pregnant rats (n=10-12 per treatment group) were randomly assigned to one of four treatment groups and injected i.p. on E16 and E18 with one of the following: 0.1 ml of vehicle (DMSO 99.5%, Sigma, #D4540, Lot# 037K07663); 50 μg/kg estradiol benzoate (Sigma, #E8515, Lot# 125K1029, serving as an estrogenic positive control); 1 mg/kg Aroclor 1221 (AccuStandard, #C-221N, Lot# 083-166, dose based on our published work showing effects on reproductive function in female rats (Steinberg et al., 2007; Steinberg et al., 2008)); or 1 mg/kg reconstituted PCB mixture (referred to as PCB Mix). Rationale for the PCB Mix is that these are the three most prevalent PCB congeners found in mammalian tissue samples (Gladen et al., 2003; Bentzen et al., 2008): PCB138 (2,2′,3,4,4′,5′-Hexachlorobiphenyl; AccuStandard, #C138N, Lot# 082704MS-AC), PCB153 (2,2′,4,4′,5,5′-Hexachlorobiphenyl; AccuStandard, #C153N, Lot# 111804AG-AC), and PCB180 (2,2′,3,4,4′,5,5′- Heptachlorobiphenyl; AccuStandard, #C180N, Lot# 013004MT-AC) at equimolar concentration. It should be noted that they are non-coplanar and do not bind the aryl hydrocarbon receptor (Van den Berg et al., 1998). The dose, age and route of exposure were based on the literature on detectable levels of PCBs in humans (Lanting et al., 1998; Lackmann, 2002), the timing of brain sexual differentiation in rats (Murakami and Arai, 1989; Rhees et al., 1990), and for consistency with other neuroendocrine studies including our own (Chung and Clemens, 1999; Chung et al., 2001; Gore et al., 2002; Salama et al., 2003; Woodhouse and Cooke, 2004; Steinberg et al., 2008). Although we have not measured PCB content in the offspring, we have previously predicted that pups were exposed to approximately 2 μg/kg total PCBs for both the A1221 treatment group and the PCB Mix treatment group (Takagi et al., 1986; Steinberg et al., 2007; Steinberg et al., 2008). On the day after birth, P1, the numbers of live and dead offspring were counted, and sex ratio was determined. Anogenital distance (AGD) was measured using a digital microcaliper (Marois, 1968; Steinberg et al., 2008), and the ratio of AGD to the cube root of body weight (AGD index) was calculated to evaluate AGD index (Marois, 1968; Gallavan et al., 1999).
Tissue collection
The offspring were divided into two groups and either sacrificed at P1 between 0900 – 1000 h to evaluate experimental endpoints reported herein, or allowed to mature for a study reported elsewhere (Dickerson et al., 2011). All work in this current study focuses on the P1 offspring to evaluate early life events following prenatal endocrine disruptor exposures. One male and female F1 rat per litter was euthanized for protein and apoptosis studies (N=8 per sex, each from different litters), and 1 male and female per litter were utilized for gene expression studies (N=6 per sex, each from different litters). For immunohistochemistry studies, pups were deeply anesthetized with 0.05 ml ketamine (100 mg/ml) and 0.05 ml of xylazine (20 mg/ml), and trans-cardially perfused with 0.9% saline (5 ml) at a rate of 5 ml/min, followed by 4% paraformaldehyde (50 ml). The brains were removed and postfixed overnight in 4% paraformaldehyde, and then transferred into PBS with 0.2% sodium azide. A vibrating microtome (Leica VT 1000S, Leica Microsystems, Nussloch, Germany) was used to cut 50 μm-thick sections that were stored in PBS with 0.2% sodium azide at 4 °C until use.
For gene expression analyses, animals were rapidly euthanized by decapitation. Brains were quickly removed and the preoptic area-anterior hypothalamus (POA), which contains the AVPV and medial preoptic nucleus (MPN), was dissected on ice, snap frozen (Dickerson et al., 2008) and stored at -80 C until RNA extraction. Terminal trunk blood samples were collected and centrifuged at 5000 × g for five minutes to separate serum, and serum was stored at -80°C until steroid hormone analysis. Because EDC treatment can affect AGD of treated animals, the sex of each animal was confirmed via visualization of the uteri or testes at the time of euthanasia.
Immunohistochemistry for ER alpha
Hypothalamic tissues were recoded for tissue processing and analyses so that the experimenter was blind to treatment group and sex. For each neonatal F1 animal, two AVPV tissues and four MPN tissues per rat were immunolabeled for ERα. Although the number of sections was too great to process in a single run, animals from each sex and treatment group were equally represented in every run. Sections were rinsed in PBS (Phosphate-buffered saline, pH = 7.4) at room temperature on a shaker, followed by treatment with 3:1 methanol: 3% H2O2 for 20 minutes at room temperature to eliminate endogenous peroxide activity. Sections were then washed, and incubated in the rabbit polyclonal anti-ERα antibody C1355 (1:10,000; Upstate Biotechnology, Waltham, MA) with 10% normal goat serum (NGS) and 0.1% Triton-X for 72 hours at 4°C on a shaker. This antibody was generated against the last 15 amino acids of ERα, which has no homology to the corresponding region of ERβ. The sections were then washed and incubated in 5% normal goat serum and secondary antibody (biotinylated goat anti-rabbit immunoglobulin (Ig)G, 1:600; BA-1000; Vector Laboratories) for one hour, then subjected to peroxidase reaction with nickel-enhanced diaminobenzidine (DAB). Sections were mounted on gelatin-subbed slides, dried, dehydrated in a graded alcohol series, counterstained with methyl green, and coverslipped with DPX (44581; Fluka, Steinheim, Germany). Controls were also run with the primary antibody omitted, and no specific binding was observed.
Quantification of ERα in the MPN was performed using unbiased stereological analysis according to methods described in detail previously (Chakraborty et al., 2003; Chakraborty et al., 2005). A wet-mount of fresh tissue showed that average tissue thickness was 50.6 μm. The MPN region was identified in Nissl-stained sections by comparing anatomical landmarks to an atlas of the developing rat brain (Ashwell, 2008). Contours were drawn around the MPN at low magnification (10× objective) using an Olympus BX-61 microscope. A buffer zone at the top and bottom of sections was set at 3 μm for all experimental stereology. For each rat, the regional volume was extrapolated based on the contours and tissue thickness (Volume = regional area × thickness). The Stereo Investigator® software (MicroBrightField, Williston, VT) randomly placed 75 μm × 120 μm grids (“dissector frames”) within the MPN contour. Within these dissector frames, the DAB-stained ERα-labeled nuclei were counted within a 45 μm × 45 μm counting frame for the MPN (“optical dissectors”). Based on these parameters, the number and density (# immunoreactive cells/volume of each nucleus) of ERα-immunoreactive (ERα-ir) nuclei falling within the region was quantified. The coefficients of error (Cruz-Orive/Geiser) and variation of the estimates were calculated as described previously (Schmitz and Hof, 2000). Photomicrographs were taken to produce the figures, and images subjected to only minor adjustments of contrast using Adobe Photoshop CS4 (Adobe, San Jose, CA), with any adjustments were applied equally to tissues from rats of different treatment groups. Because ERα labeling was sparse and highly variable in the AVPV and did not enable adequate statistical power, stereological analysis for this region was not possible.
Detection of apoptosis
To stain the nucleosomal DNA fragments in apoptotic cells, we used a modification of the TUNEL method described by Bessert and Skoff (Bessert and Skoff, 1999). All reagents were supplied by the Fluorescein-FragEL DNA Fragmentation Detection Kit (EMD Chemicals Inc., Gibbstown, NJ) and applied according to the kit protocol at room temperature (RT) unless otherwise noted. In brief, tissues were pretreated with sodium citrate buffer (10mM Sodium Citrate, 0.05% Tween 20, pH 6.0) at 60°C for 15 min, then washed in 0.1 M PBS, pH 7.4, incubated in 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS for 15 min, and washed in 0.1 M PBS. The sections were then treated with 20 μg/ml proteinase K in 10 mM Tris pH 8 for 15 min and washed in PBS. The sections were incubated in equilibration buffer for 30 min, followed by incubation in labeling reaction mixture in a humidified chamber at 37°C for 1.5 hr. Sections were washed in PBS and mounted onto gelatin subbed slides, and coverslipped with Fluorescein-FragEL™Mounting Media. Positive controls for the TUNEL assay were generated by pretreatment of sections with DNAse I (Applied Biosystems Inc., Foster City, CA). Negative controls for the TUNEL procedure were treated in the same manner as the test samples except that the TdT enzyme was omitted from the reaction mixture and was replaced with dH2O. No labeling was found in the negative controls. Mounted sections were stored in the dark at 4°C until analysis.
A 1:2 series from the AVPV (2 sections total) and 1:2 series from the MPN (4 sections total) were selected from each animal. For apoptosis studies, numbers of labeled cells were counted unilaterally throughout the rostrocaudal extent of the region. Using StereoInvestigator software (MicroBrightField Inc.), contours were drawn for each region at low magnification (10× objective) using anatomical landmarks and a developing rat brain atlas (Ashwell, 2008). Ten counting grids measuring 25 μm × 25 μm per were randomly placed within the left hemisphere for the AVPV and ten counting grids measuring 45 μm × 45 μm were placed on the left side of the third ventricle for the MPN. Total numbers of apoptotic cells were counted for each region at high magnification (100× objective).
Serum Hormone Assays
Because of the small amount of serum obtainable from a P1 rat, serum samples from 2-3 same-sex siblings were pooled for littermates (N=8 pooled samples per sex per assay), and samples run in single testosterone, progesterone, or estradiol RIAs as described previously (Dickerson et al., 2011). Briefly, total serum testosterone was determined using the Active® Testosterone coated well EIA kit (Catalog # DSL-10-4000, Lot # 08035-B, Diagnostic Systems Laboratories, Inc., Webster, TX, USA) on duplicate volumes of 50 μl serum. The assay limit of detection was 0.04 ng/ml, and the intra-assay CV based on duplicate samples for the assay was 2.97%. Progesterone concentrations were determined using the ACTIVE® Progesterone Coated-Tube Radioimmunoassay Kit (Catalog # DSL-3900, Lot # 07076, Diagnostic Systems Laboratories, Inc., Webster, TX, USA), on duplicate volumes of 25 μl. The sensitivity of the assay was 0.12 ng/ml, and the intra-assay CV was 3.26%. Estradiol concentrations were determined using an ultrasensitive double-antibody RIA kit (Catalog # DSL-4800, Lot # 07076, Diagnostic Systems Laboratories, Inc., Webster, TX, USA), on duplicate volumes of 200μL. The assay limit of detection was 2.2 pg/mL, and the intra-assay coefficient of variability based on duplicate samples was 7.97%. For all assays, a few samples for which the CV between duplicates was 10% or greater were excluded from analysis.
RNA extraction
Messenger RNA from frozen POA dissections was extracted using our in-house double detergent lysis buffer system as described previously (Dickerson et al., 2008; Walker et al., 2009). In brief, samples were homogenized and the cytoplasmic RNA was treated with proteinase K, followed by extraction with phenol chloroform and precipitation in isopropanol. Genomic DNA contamination was removed using the TURBO DNA-free kit (Applied Biosystems Inc., Cat. No. AM1907, Foster City, CA) according to the manufacturer's protocol. The concentration of resulting cytoplasmic RNA was determined using a Nanodrop (ND-1000, Nanodrop Technologies, Inc., Wilmington, DE).
Taqman low-density arrays (TLDA)
Samples were run as described by our laboratory (Walker et al., 2009) on a custom rat neuroendocrine TLDA (Applied Biosystems Inc., Foster City, CA), a panel of 48 candidate neuroendocrine genes. Cytoplasmic RNA (2 μg) was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's instructions. The resulting cDNA was diluted 1:5 before PCR reactions were performed using Applied Biosystems' Taqman reagents on an ABI 7900 real-time PCR machine using the following parameters: 50 C for 2 min, 94.5 C for 10 min, 45 cycles of 97 C for 30 sec, and 59.7 C for 1 min. Relative expression for each gene was determined using the comparative Ct method (Pfaffl, 2001). Each sample was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression (Walker et al., 2009), and the data were calibrated to the average change in Ct for the treatment group with the lowest expression.
Statistical Analysis
SPSS statistical software (17.0 for Macintosh, SPSS Inc., Chicago, IL) was used to evaluate the effects of treatment on litter composition, body weight, AGD, serum hormones, AVPV and MPN volume, stereological results of ERα immunoreactive cells, and apoptosis endpoints. We had independent a priori hypotheses for each sex; thus, statistics were performed separately for males and females. Datasets were examined for homogeneity of variance and normality. For datasets that met these criteria, comparisons were made by one-way ANOVA (factor: treatment) followed by Fisher's LSD post-hoc analysis when indicated by a significant main effect. When variance between treatment groups was unequal, datasets were compared using the nonparametric Kruskal-Wallis test. In all these cases p-values < 0.05 were considered statistically significant. For the gene expression data, statistics were conducted using the normalized Ct (δ-Ct) for each sample (before transformation to fold change). Because the TLDA measures expression of 48 genes, a Bonferroni correction was used to set the cut off for significance at p < 0.001.
Results
Litter composition, birth weight and anogenital distance
On postnatal day P1, litter composition was determined, and birth weight and anogenital distance (AGD) were recorded for all pups in each litter. There was no effect of treatment upon total pups (DMSO: 13.0 ± 0.43; EB: 12.3 ± 0.85; A1221: 12.4 ± 1.0; PCB: 10.8 ± 1.3) or live pups (DMSO: 10.9 ± 0.81; EB: 10.9 ± 0.97; A1221: 11.8 ± 1.0; PCB: 10.2 ± 1.5) per litter, or upon sex ratio (Percentage female: DMSO: 49 ± 3%; EB: 40 ± 3%; A1221: 48 ± 3%; PCB: 48 ± 7%). Likewise, there was no main effect of treatment upon body weight in either sex, although we did observe a trend for increased body weight in females treated with EB or A1221 (Fig. 1A, p = 0.06). We observed a sex-dependent effect of treatment on AGD, with EB-, A1221-, and PCB-treated females having reduced AGD (p<0.001), and EB- and A1221-treated males having increased AGD (p<0.05) compared to their vehicle counterparts (Fig. 1B).
Figure 1.
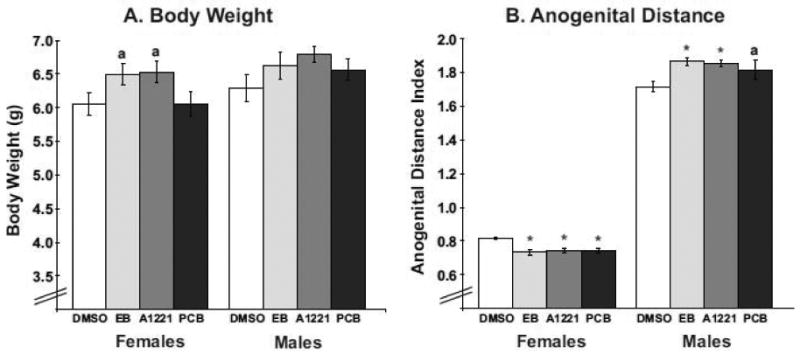
Data for body weight (A) and anogenital distance (B) on P1 are shown for female and male rats (mean ± S.E.M). Treatment with estradiol benzoate (EB) or Aroclor 1221 (A1221) caused a non-significant trend for increased body weight in females (ap=0.06). For anogenital distance (AGD), treatment with EB, A1221, or PCB Mix caused a decrease in female AGD (**p<0.001), while EB- or A1221-treatment increased male AGD (*p<0.05). Treatment with PCB Mix caused a non-significant trend for increased AGD in males (ap=0.06). AGD index is the ratio of AGD (mm) to the cube root of body weight. On the x-axis of this and other figures, the label PCB refers to the PCB Mix group.
Serum hormones
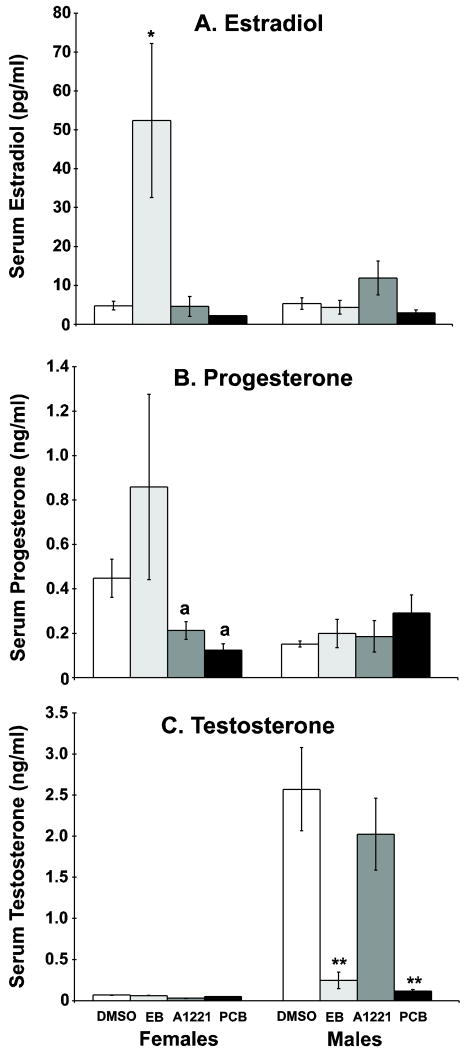
We observed a significant effect of treatment upon serum estradiol in EB-treated females (Fig. 2A, p<0.005), a group with higher estradiol levels than all other groups. A significant main effect of treatment upon serum progesterone was noted in females (Fig. 2B) but not in males, with A1221- and PCB Mix-treated females having reduced progesterone compared to EB- (p<0.05) but not DMSO-treated females. However, no group differed from control in progesterone concentrations. There was a main effect of treatment upon serum testosterone in males (Fig. 2C, p<0.001), but not females, with a reduction observed in EB and PCB-treated males compared to the control and A1221 males.
Figure 2.
Serum levels of estradiol (A), progesterone (B), and testosterone (C) in neonatal F1 animals. For estradiol, EB-treatment caused an increase in estradiol levels in females (*p<0.005), while males were unaffected by any treatment. No effect of treatment upon progesterone was observed in males or females, although the A1221- and PCB Mix-treated females had lower progesterone levels than the EB females (a, p<0.05). For testosterone, EB- and PCB Mix-treated males had lower serum testosterone compared to DMSO- and A1221-treated males (**p<0.001 vs. both). N=8 pooled samples per sex per assay. The bars are the mean ± S.E.M.
Apoptosis in AVPV and MPN
Cells undergoing apoptotic cell death display distinct morphological changes, characterized by cellular shrinkage, nuclear pyknosis, chromatin condensation and membrane blebbing. To ascertain whether prenatal EDC exposure disrupts developmental apoptosis in the neonatal hypothalamus, we used a TUNEL assay and DAPI labeling to visualize apoptotic nuclei in the AVPV and MPN. A representative photomicrograph of TUNEL labeling with DAPI counterstaining is shown for the MPN (Fig. 3A); similar apoptotic cells were seen in the AVPV (not shown). Quantification of apoptotic cell numbers showed that in the female AVPV, EB- and A1221-treatment increased the number of apoptotic nuclei (Fig. 3B, p<0.001), while no effect of treatment was observed in the male AVPV. In contrast, no effect of treatment upon apoptosis was observed in either females or males in the MPN (Fig. 3C).
Figure 3.
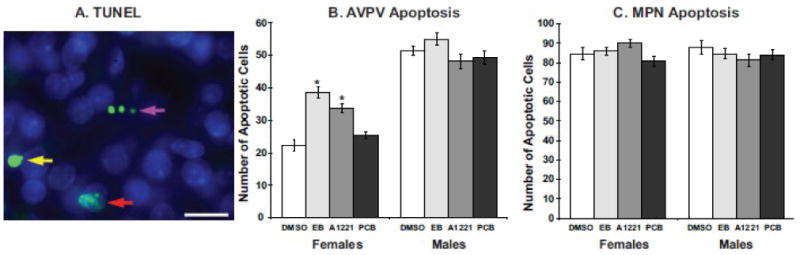
A representative photomicrograph shows TUNEL labeling results for early (red arrow), middle (yellow arrow), and late (purple arrow) phase apoptosis the MPN (A). TUNEL-positive cells appear bright green (arrows) and DAPI counterstained nuclei appear blue. Scale bar = 25 μm. Quantification of apoptotic nuclei was performed in the AVPV (B) and MPN (C). In females, EB- and A1221-treated females have a higher incidence of apoptotic nuclei compared to DMSO control (*p<0.01), while males were unaffected by treatment. No effect of treatment upon MPN apoptosis was observed in either sex.
ER alpha expression in the AVPV and MPN
In the AVPV, ERα labeling was sparse and highly variable; thus, stereological analysis for this region was not possible due to inadequate statistical power. However, qualitative observations suggested that ERα immunoreactivity (ir) in the AVPV is greater in control females compared to control males, and it did not appear that either males or females were affected by treatment with EB, A1221, or PCB Mix in this region on P1.
Representative photomicrographs of ERα-ir in the MPN are shown in P1 male and female rats for the four treatments (Fig. 4). Stereological cell counting showed a significant main effect of treatment on ERα-ir cell numbers, with EB-treated males and females having significantly more of these cells than DMSO-, A1221-, and PCB Mix-treated counterparts (p<0.05; Fig. 5A). No effect of treatment upon MPN regional volume was observed in males or females (Fig. 5B). Density of ERα was increased by EB-treatment in MPN of P1 males and females (p<0.05; Fig. 5C).
Figure 4.
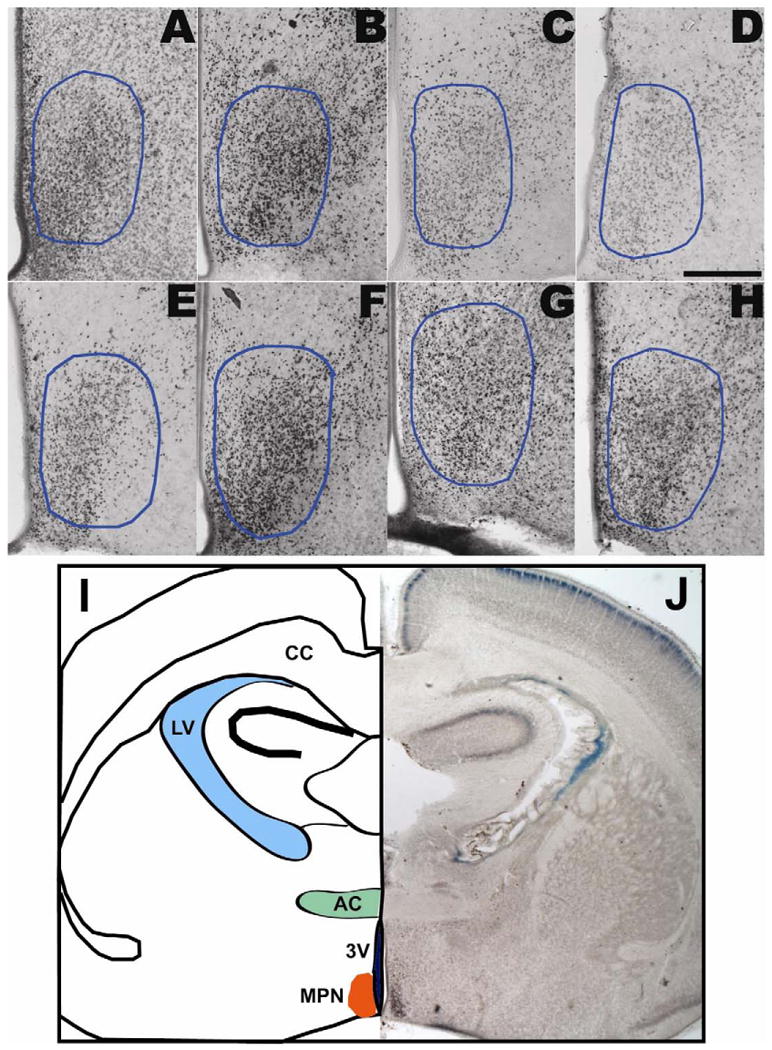
Photomicrographs of estrogen receptor alpha (ERα) immunoreactivity in the MPN (A-H; outlined in blue) of P1 female and male rats prenatally exposed to DMSO vehicle (A,E), EB (B,F), A1221 (C,G), or PCB Mix (D,H). The third ventricle is at the lower left of each micrograph. Although the images presented here were photographed at low magnification (10×), quantification and analysis of ERα-immunoreactivity was performed at high-power magnification (40×). ERα nuclei are labeled with dark brown nickel-enhanced DAB product. Scale bar = 250 μm. I, J: A lower magnification micrograph at the level of the MPN was reflected and drawn to indicate landmarks. Abbreviations: 3V, third ventricle; AC: anterior commisure; CC: corpus callosum; LV: lateral ventricle; MPN: medial preoptic nucleus.
Figure 5.
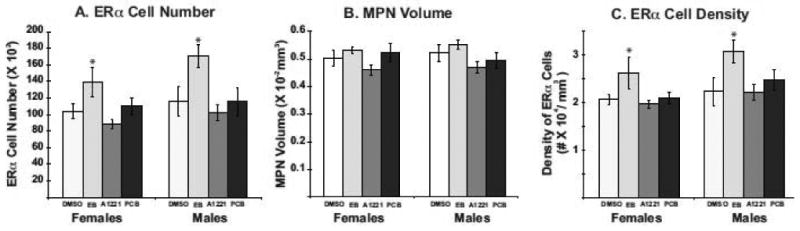
Stereologic analysis results for ERα-immunoreactive cell numbers in the MPN (A), regional volume of the MPN (B) and ERα-immunoreactive cell density (C) are shown for neonatal P1 rats (n = 6-8 rats per treatment group). Data are the mean ± S.E.M. ERα cell number was significantly increased in EB-treated males and females compared to DMSO control. MPN volume was unaffected by EDC treatment in either sex. Cell density was increased by EB treatment in both sexes. *p < 0.05.
Effects of developmental PCB exposure on POA neuroendocrine gene expression
Forty-seven of the candidate genes on the neuroendocrine TLDA were detectable following real-time PCR reactions (Table 1). As described previously (Walker et al., 2009), POA genes were normalized to GAPDH for analyses, as this gene did not vary by treatment. Of the remaining detectable genes, relative gene expression of nine genes was significantly affected by developmental EDC exposure in P1 males or females following the Bonferroni correction (Fig. 6): brain-derived neurotrophic factor (BDNF), GABAB receptors 1 and 2, IGF-1, kisspeptin receptor (GPR54), NMDA receptor subtypes NR2b and NR2c, prodynorphin, and TGFα.
Table 1. Low-density PCR array gene expression results.
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Gene | Name | EB | A1221 | PCB | EB | A1221 | PCB |
| Ahr | Aryl hydrocarbon receptor | 0.01 ↓ | -- | 0.05 ↓ | -- | -- | 0.009 ↑ |
| AR | Androgen receptor | -- | -- | -- | -- | -- | -- |
| Arnt | Aryl hydrocarbon nuclear translocator | -- | -- | 0.06 ↓ | 0.004 ↑ | 0.022 ↑ | 0.026 ↑ |
| Bdnf | Brain-derived neurotrophic factor | <0.001 ↓ | 0.01 ↓ | 0.01 ↓ | -- | -- | -- |
| Cyp 17a1 | Cytochrome P450, 17a1 (17-alpha hydroxylase) | -- | -- | -- | -- | -- | -- |
| Cyp 19a1 | Cytochrome P450, 19a1 (aromatase) | -- | -- | -- | 0.037 ↑ | -- | 0.026 ↑ |
| Cyp 1b1 | Cytochrome P450, 1b1 | -- -- | -- | 0.04 ↑ | 0.075 ↓ | 0.062 ↓ | 0.078 ↑ |
| Esr1 | Estrogen receptor alpha | -- | -- | -- | 0.067 ↑ | -- | 0.079 ↑ |
| Esr2 | Estrogen receptor beta | -- | -- | -- | -- | -- | -- |
| Gab br1 | GABA-B receptor 1 | 0.07 ↓ | -- | 0.028 ↓ | <0.001 ↑ | <0.001 ↑ | <0.004 ↑ |
| Gab br2 | GABA-B receptor 2 | 0.011 ↓ | -- | <0.001 ↑ | -- | -- | -- |
| Gal | Galanin | -- | -- | -- | -- | -- | -- |
| Gnrh 1 | Gonadotropin-releasing hormone 1 | -- | -- | -- | -- | -- | -- |
| Gnrh r | Gonadotropin-releasing hormone receptor | 0.037 ↓ | -- | -- | -- | -- | -- |
| Gper | G-protein coupled receptor 30 | -- | -- | -- | -- | -- | -- |
| Gria 1 | GluR1 | -- | -- | -- | -- | -- | -- |
| Gria 2 | GluR2 | 0.072 ↓ | -- | 0.042 ↓ | -- | -- | -- |
| Gria 3 | GluR3 | -- | -- | -- | -- | -- | -- |
| Grik 2 | Kainate 2 receptor | 0.01 ↓ | -- | 0.07 ↓ | -- | -- | -- |
| Hsd 17b1 | Hydroxysteroid 17-beta dehydrogenase 1 | -- | -- | -- | -- | -- | -- |
| Hsd 17b2 | Hydroxysteroid 17-beta dehydrogenase 2 | -- | -- | -- | -- | -- | -- |
| Hsd 17b3 | Hydroxysteroid 17-beta dehydrogenase 3 | -- | -- | -- | -- | -- | -- |
| Hsd 17b8 | Hydroxysteroid 17-beta dehydrogenase 8 | -- | -- | -- | -- | -- | -- |
| Igf1 | Insulin-like growth factor 1 | -- | <0.001 ↑ | -- | -- | -- | -- |
| Igf1r | Insulin-like growth factor 1 receptor | 0.03 ↓ | -- | 0.04 ↓ | -- | -- | -- |
| Kiss 1 | Kisspeptin | -- | -- | -- | -- | -- | -- |
| Kiss 1r | Kisspeptin receptor (GPR54) | <0.001 ↓ | 0.01 ↓ | <0.001 ↓ | 0.009 ↑ | 0.047 ↑ | 0.05 ↑ |
| Pgr mc1 | Membrane progesterone receptor | -- | -- | -- | -- | -- | -- |
| Nmd ar1 | N-methyl-D-aspartate receptor (NMDAR) subunit 1 (NR1) | -- | -- | -- | -- | -- | -- |
| Grin 2a | NMDAR subunit 2a | 0.066 ↓ | -- | 0.033 ↓ | -- | -- | -- |
| Grin 2b | NMDAR subunit 2b | -- | -- | -- | < 0.001 ↑ | < 0.001 ↑ | -- |
| Grin 2c | NMDAR subunit 2c | 0.001 ↑ | -- | -- | -- | -- | 0.03 ↑ |
| Grin 2d | NMDAR subunit 2d | -- | -- | -- | -- | -- | -- |
| Pdyn | Prodynorphin | -- | -- | -- | 0.008 ↓ | -- | 0.01 ↓ |
| Pgr | Progesterone receptor | -- | -- | -- | -- | -- | -- |
| Slc1 7a1 | Vesicular glutamate transporter 1 | -- | -- | 0.006 ↓ | -- | -- | -- |
| Slc1 7a6 | Vesicular glutamate transporter 2 | -- | -- | -- | -- | -- | -- |
| Srd5 a1 | Steroid 5-alpha reductase 1 | -- | -- | -- | -- | -- | -- |
| Stat5 b | Signal transducer and activator of transcription 5B | -- | -- | -- | -- | -- | -- |
| Sts | Steroid sulfatase | -- | -- | -- | -- | -- | -- |
| Tac2 | Neurokinin B | -- | -- | -- | -- | -- | -- |
| Tgfa | Transforming growth factor alpha | -- | -- | < 0.001 ↑ | -- | -- | -- |
| Tgfb 1 | Transforming growth factor beta-1 | -- | -- | -- | -- | -- | -- |
| Ucp 2 | Uncoupling protein 2 | -- | -- | -- | -- | -- | -- |
| Vdr | Vitamin D receptor | -- | -- | -- | -- | 0.005 ↑ | 0.016 ↑ |
Low-density PCR arrays were used to measure expression of 48 genes in whole POAs of P1 female and male rats treated prenatally with EDCs. The full list of detected neuroendocrine genes is shown. Statistical results are shown for significant effects and for trends in data. Because of the Bonferroni correction, the cut-off for a significant effect was set at p < 0.001. Not shown are results for internal controls 18S, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In addition, one gene was not detected in the assay: 3ß-hydroxysteroid dehydrogenase (HSD3b).
Figure 6.
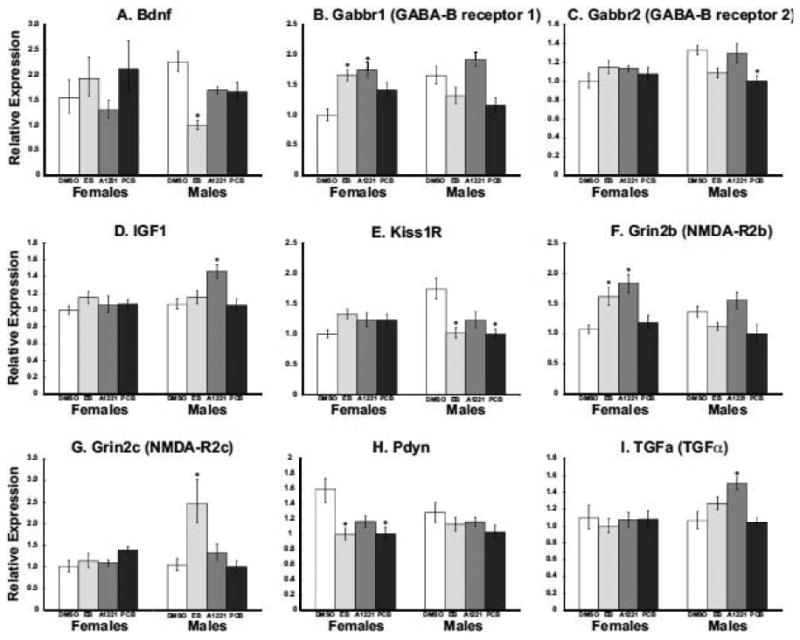
Gene expression data are shown for the nine neuroendocrine genes that were significantly affected in the POA of neonatal P1 male or female rats. Data shown are mean ± S.E.M. Relative expression for each gene was determined using the comparative Ct method, with each sample normalized to GAPDH, and data calibrated to the average change in Ct for the treatment group with the lowest expression. N = 6 rats per group; *p<0.001 vs. DMSO control of the same sex. Gene abbreviations appear in Table 1.
Discussion
We recently reported that prenatal PCB exposure impairs gene and protein expression in the adult female hypothalamus (Dickerson et al., 2011), and alters paced mating behavior (Steinberg et al., 2007). As sexual differentiation begins during late gestation, we hypothesized that PCB exposure at E16 and E18 causes effects on developing sexually dimorphic regions of the neonatal brain that would be manifested very shortly after exposure. In support of our hypothesis, we found that low doses of PCBs, relevant for human exposures (Lackmann, 2002; Lackmann et al., 2004) had sex-dependent effects on developmental apoptosis in the P1 hypothalamus, as well as neuroendocrine gene and protein expression. The implications of these findings are discussed below.
Litter composition, AGD and body weight
In the current study, we selected doses of A1221 and PCB Mix to approximate human and environmental exposures (Lackmann, 2002; Lackmann et al., 2004), anticipating that these sub-toxic levels would not cause any gross morphological effects. Rather, our aim was to assess the early postnatal neuroendocrine outcomes of prenatal PCB treatment. This would enable us to establish just how early the brain is altered and the relationship between these early life outcomes to those we have reported for animals later in life (Steinberg et al., 2007; Steinberg et al., 2008; Dickerson et al., 2011). As predicted, EB, A1221, and PCB Mix had no significant effects on total or live births in the exposed F1 generation, nor did they affect sex ratio or body weight.
In most mammalian species the AGD is sexually dimorphic, with males having a longer AGD than females. Neonatal anogenital distance is largely determined by the action of androgens (Marois, 1968), and thus can be an external indicator of masculinization (Gray et al., 1994). In the current study, we found a sex-dependent effect upon neonatal AGD, although not in the direction that we predicted: EB-, A1221-, and PCB Mix-treatment decreased (hyperfeminized) female AGD, while EB- and A1221- exposure increased (hypermasculinized) male AGD. We are not certain how to interpret this result. One possibility for the observed reduction in female AGD may be related to direct or indirect PCB effects on the androgen receptor (AR), although it is important to note that their relative binding affinity for the receptor is several orders of magnitude lower than endogenous androgens (Portigal et al., 2002; Fang et al., 2003). However, this cannot explain the larger AGD in EB-treated males. In the context of reduced serum testosterone observed in EB- and PCB Mix-treated males, the increase in male AGD may seem further counterintuitive, but it is consistent with reports by our lab and others of increased AGD following developmental PCB exposure throughout postnatal development (Kuriyama and Chahoud, 2004; Dickerson et al., 2011).
Developmental apoptosis and ERα protein expression in the neonatal POA
In the current study, we investigated the effects of gestational PCB exposure on sexual differentiation and developmental apoptosis in the neonatal hypothalamus of males and females. In the female AVPV, treatment with EB or A1221, but not the PCB mix, increased the number of apoptotic nuclei, while no effect of treatment was observed in the male AVPV. In addition, no effect of treatment was detected in the MPN of either sex. The similar results for EB and A1221 suggest that these effects are likely exerted through an estrogenic mechanism to masculinize the female AVPV via increased apoptosis. In addition, the differences between A1221 and the PCB mix may be attributable to their difference in relative estrogenicity at the ER. While A1221 has been consistently shown to have estrogenic properties, the di-ortho substituted non-coplar congeners that comprise the reconstituted PCB mixture have demonstrated anti-estrogenic properties in several in vitro studies (Oh et al., 2007). The lack of treatment effect in males may be attributable to the higher baseline exposure of their developing hypothalamus to steroid hormones. The consequences of altering developmental apoptosis in the female AVPV may affect the AVPV's ability to generate the estrogen-induced GnRH/LH surge and ovulation in adulthood (Wiegand et al., 1978; Wiegand and Terasawa, 1982; Gu and Simerly, 1997; Le et al., 2001). In our companion study (Dickerson et al., 2011), we found that EB- and A1221-treatment reduced AVPV volume in female littermates of the animals used herein who had been allowed to mature to early adulthood and were studied at P60. Together, these results suggest that increased AVPV cell loss at P1 may be a contributing factor to the reduction of the regional AVPV volume observed in adult (P60) females.
There are developmental sex differences in the number of cells in the AVPV that are immunopositive for ERα (Davis et al., 1996b; Orikasa and Sakuma, 2003). Thus, we also ascertained the effect of prenatal EDC exposure on this endpoint in the neonatal AVPV and MPN. In both regions, qualitative observations suggested no clear sex difference in ERα-ir at P1, consistent with reports from other labs (Yokosuka et al., 1997). Although we could not quantify ERα-ir in the AVPV, in the MPN, stereological analysis showed that treatment with EB, but not PCBs, increased the number of cells immunopositive for ERα in both male and female P1 pups. These results were unexpected, as estradiol has been shown to down-regulate ERα at the level of gene expression (Lauber et al., 1991; Simerly and Young, 1991) as well as immunoreactivity in the adult (Koch, 1990) and neonatal (P10) (Orikasa et al., 1994; Orikasa et al., 1995; Orikasa et al., 1996) rodent brain. Other studies investigating the effects of PCBs upon hypothalamic estrogen receptor expression are quite limited. In one, Lichtensteiger et al. (2003) found that gestational exposure to A1254 increased ERα gene expression in the ventromedial nucleus of the hypothalamus (VMN), a region important for feminine sexual behavior, in female rat embryos (Lichtensteiger et al., 2003). Because sex differences in ERα in the AVPV and MPN regions do not appear until postnatal day 10, it is likely that future studies evaluating later developmental time points will provide temporal resolution to the endocrine disrupting effects of PCBs upon hypothalamic ERα. Although our experimental design did not enable us to collect tissues from rats at ages other than P1 (this study) and P60 (Dickerson et al., 2011), ongoing work is including a more systematic analysis of developmental postnatal profiling of the POAs of male and female rats prenatally exposed to PCBs.
Neuroendocrine gene expression in the neonatal POA
Using a custom-designed 48-gene real-time PCR array, we identified nine genes whose expression changed significantly with neonatal PCB exposure: brain-derived neurotrophic factor (BDNF), GABAB receptors 1 and 2, IGF-1, kisspeptin receptor, NMDA receptor subunits NR2b and NR2c, prodynorphin, and TGFα. Each of these identified genes is an important contributing factor to hypothalamic development, differentiation and function (Gore, 2001; Daftary and Gore, 2004; Walker et al., 2009). For instance, BDNF stimulates migration of neurons during development, is highly expressed in discrete regions of the hypothalamus during postnatal development, and its release from certain cell types is regulated by gonadal steroid hormones [reviewed in (Tobet et al., 2009)]. In our study, expression of BDNF was reduced 50% by EB treatment in males, while females were not affected by treatment. Similarly, several neurotransmitters, including GABA, are thought to act as neurotrophic factors in hypothalamus (McClellan et al., 2008). The GABAB receptor subunits B1 and B2 guide cell migration and positioning in the ventromedial nucleus (VMN). In females prenatally exposed to either EB or A1221, we observed an increase in the expression GABAB1, while a decrease in the expression of GABAB2 was observed in males treated with the PCB mix. This observation could have implications for proper establishment of sexually dimorphic circuitry and connections within this region.
Other significantly affected genes are involved in brain sexual differentiation by modulating cell survival and developmental apoptosis. For example, the growth hormone IGF-1 plays a crucial role in somatic growth, as well as proliferation and inhibition of apoptosis [reviewed in (D'Ercole and Ye, 2008)]. Similarly, NR2b and NR2c are each subunits of the ionotropic NMDA receptor, whose activation not only has actions on GnRH neurons and the reproductive axis (Gore, 2001; Maffucci et al., 2008), but also play an important role in sexually dimorphic apoptosis (Hsu et al., 2000; Hsu et al., 2001).
Kisspeptin signaling is important for many aspects of reproductive maturation and function, including a recently discovered role in the guidance of GnRH neurites to the median eminence at the base of the hypothalamus (Fiorini and Jasoni, 2010). In males treated with either EB or PCB Mix, we observed a decrease in POA kisspeptin receptor expression. Interestingly, in littermates of these animals used for a related study, we observed a delay in male pubertal onset (Dickerson et al., 2011) that may reflect improper targeting of kisspeptin fibers. The neuropeptide dynorphin is co-expressed in kisspeptin neurons, and also acts to modulate GnRH secretion (Navarro et al., 2009). In females treated with EB or PCB Mix, we observed a decrease in prodynorphin gene expression, and a non-significant trend (p=0.006) in A1221-treated females. This result is consistent with our related study, in which we observed decreased kisspeptin protein expression and impaired GnRH neuron activation in adult female littermates in the EB, A1221, and PCB treatment groups (Dickerson et al., 2011). It is possible that disruption of this neuronal circuitry begins during neonatal development. Finally, TGFα, expression of which was increased in male-A1221 rats, is a cytokine involved in a number of cellular functions such as cell growth, proliferation, differentiation, and apoptosis (Galbiati et al., 2003). Moreover, this growth factor is released by hypothalamic glial cells, and plays a role in regulation of GnRH release. As a whole, these gene expression data identify a group of candidates for further study as early developmental targets of EDCs.
The neural, neuroendocrine, and/or behavioral changes observed in the adult offspring of EDC-exposed females may be the result of indirect effects secondary to changes in maternal care (Cummings et al., 2005; Cummings et al., 2010). Although we did not assess maternal behavior in the current study, our results provide evidence that suggest a direct effect of PCBs on the developing neuroendocrine system of exposed neonatal offspring, independent of the effects of PCBs on maternal care. Ongoing studies in our lab are evaluating the effect of these PCBs on aspects of maternal behavior, and will help clarify the contribution of changes in maternal care to neuroendocrine deficits in the developing offspring.
Comparison of Treatment Effects
PCBs are typically found as complex mixtures in environmental, human, and wildlife samples. We therefore utilized two mixtures of PCBs that differ in degree of chlorination, half-life, and chemical properties to study the effects of PCBs on the developing neuroendocrine system. Although this experimental approach has inherent limitations, including difficulty in discerning individual mechanisms of action, we selected these compounds in an effort to represent ecologically relevant conditions. While A1221 is thought to be mainly estrogenic with regard to its interaction with the ER, congeners that comprise the reconstituted PCB mixture have been shown to be estrogenic, anti-estrogenic as well as anti-androgenic (Bonefeld-Jorgensen et al., 2001). Moreover, while A1221 contains congeners of all three classifications (coplanar, dioxin-like coplanar, and non-coplanar), the reconstituted PCB mixture congeners are all non-coplanar, and do not bind the arylhydrocarbon receptor with significant affinity (Van den Berg et al., 1998).
We noted some similarities and other differences in the actions of the three treatment groups upon the neuroendocrine and somatic endpoints. The profiles of these effects are summarized in Table 2. Prenatal EB treatment was associated with the greatest number of effects in both sexes, followed by A1221 and finally the PCB Mix. There were more similarities between profiles of endpoints affected by A1221 and EB, especially in females, consistent with A1221's greater estrogenic potential compared to the PCB Mix. The relatively few effects of the reconstituted PCB mixture on the endpoints evaluated may reflect a weaker interaction with neuroendocrine components. It is clear that further work is necessary to understand the underlying mechanisms of these EDCs on targets in the hypothalamus.
Table 2. Summary of treatment effects at P1 for each significant endpoint.
| Endpoint | Females | Males |
|---|---|---|
| Body weight | EB, A1221 > DMSO, PCB Mix | No effect |
| Anogenital distance | EB, A1221, PCB Mix < DMSO | EB, A1221(trend for PCB Mix) > DMSO |
| Serum Estradiol | EB > DMSO, A1221, PCB Mix | No effect |
| Serum Progesterone | A1221, PCB Mix < DMSO | No effect |
| Serum Testosterone | No effect | EB, PCB Mix < DMSO, A1221 |
| Apoptotic cells (AVPV) | EB, A1221 > DMSO, PCB Mix | No effect |
| ERα cell number and density (MPN) | EB > DMSO | EB > DMSO |
| BDNF mRNA | No effect | EB < DMSO |
| GABAB-R1 mRNA | EB, A1221 > DMSO | No effect |
| GABAB-R2 mRNA | No effect | PCB Mix < DMSO |
| IGF-1 mRNA | No effect | A1221 > DMSO |
| Kiss1R mRNA | No effect | EB, PCB Mix < DMSO |
| NMDA-R2b mRNA | EB, A1221 > DMSO | No effect |
| NMDA-R2c mRNA | No effect | EB > DMSO |
| Prodynorphin mRNA | EB, PCB < DMSO | No effect |
| TGFα mRNA | No effect | A1221 > DMSO |
Summary and Conclusions
Collectively, these data show that endocrine disruption by gestational PCBs causes changes to the hypothalamic neural circuitry controlling reproduction in the exposed offspring, a process that is detectable as early as the day after birth. We found that PCBs cause changes to sexually dimorphic brain regions underlying sex-specific reproductive physiology and behavior through the perturbation of normal developmental apoptosis, and by altering gene expression of neurotransmitters and receptors known to play important roles in differentiation and migration of hypothalamic neurons. Although there may not be an easy or obvious interpretation of the nine genes affected by prenatal PCB exposure, the gene expression data add to the story of the effects of developmental EDC exposure on reproductive neuroendocrine function. In fact, the sex differences in gene expression profiles and differential regulation by the various EDCs suggest that subtle mechanistic differences underlie the physiological phenotypic differences such as hormonal regulation, reproductive development, and adult reproductive function.
Acknowledgments
This work was supported by NIH ES018139, ES012272, ES07784 (ACG), NIH T32 ES07247 (SMD), and NSF 04-615 (SMD).
Footnotes
Conflict of Interest Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashwell KWS, Paxinos George. Atlas of the Developing Rat Nervous System. 3rd. Academic Press; 2008. [Google Scholar]
- Bakker J, Brock O. Early estrogens in shaping reproductive networks: Evidence for a potential organizational role of estradiol in female brain development. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.02016.x. [DOI] [PubMed] [Google Scholar]
- Bentzen TW, Muir DC, Amstrup SC, O'Hara TM. Organohalogen concentrations in blood and adipose tissue of Southern Beaufort Sea polar bears. Sci Total Environ. 2008;406:352–367. doi: 10.1016/j.scitotenv.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Bessert DA, Skoff RP. High-resolution in situ hybridization and TUNEL staining with free-floating brain sections. J Histochem Cytochem. 1999;47:693–702. doi: 10.1177/002215549904700511. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158:141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Rajendren G, Gore AC. Expression of estrogen receptor {alpha} in the anteroventral periventricular nucleus of hypogonadal mice. Exp Biol Med (Maywood) 2005;230:49–56. doi: 10.1177/153537020523000106. [DOI] [PubMed] [Google Scholar]
- Chung YW, Clemens LG. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol. 1999;62:664–670. doi: 10.1007/s001289900925. [DOI] [PubMed] [Google Scholar]
- Chung YW, Nunez AA, Clemens LG. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol Behav. 2001;74:363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Clemens LG, Nunez AA. Mother counts: how effects of environmental contaminants on maternal care could affect the offspring and future generations. Front Neuroendocrinol. 2010;31:440–451. doi: 10.1016/j.yfrne.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Nunez AA, Clemens LG. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol Behav. 2005;85:83–91. doi: 10.1016/j.physbeh.2005.04.001. [DOI] [PubMed] [Google Scholar]
- D'Ercole JA, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol. 2004;16:160–169. doi: 10.1111/j.0953-8194.2004.01149.x. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996a;734:10–18. [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996b;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011 doi: 10.1210/en.2010-1103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Guevara E, Woller MJ, Gore AC. Cell death mechanisms in GT1-7 GnRH cells exposed to polychlorinated biphenyls PCB74, PCB118, and PCB153. Toxicol Appl Pharmacol. 2009;237:237–245. doi: 10.1016/j.taap.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Walker DM, Reveron ME, Duvauchelle CL, Gore AC. The recreational drug ecstasy disrupts the hypothalamic-pituitary-gonadal reproductive axis in adult male rats. Neuroendocrinol. 2008;88:95–102. doi: 10.1159/000119691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, Xie Q, Perkins R, Owens W, Sheehan DM. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003;16:1338–1358. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- Fiorini Z, Jasoni CL. A novel developmental role for kisspeptin in the growth of gonadotrophin-releasing hormone neurites to the median eminence in the mouse. J Neuroendocrinol. 2010;22:1113–1125. doi: 10.1111/j.1365-2826.2010.02059.x. [DOI] [PubMed] [Google Scholar]
- Galbiati M, Saredi S, Melcangi RC. Steroid hormones and growth factors act in an integrated manner at the levels of hypothalamic astrocytes: a role in the neuroendocrine control of reproduction. Ann N Y Acad Sci. 2003;1007:162–168. doi: 10.1196/annals.1286.016. [DOI] [PubMed] [Google Scholar]
- Gallavan RH, Jr, Holson JF, Stump DG, Knapp JF, Reynolds VL. Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol. 1999;13:383–390. doi: 10.1016/s0890-6238(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Gladen BC, Doucet J, Hansen LG. Assessing human polychlorinated biphenyl contamination for epidemiologic studies: lessons from patterns of congener concentrations in Canadians in 1992. Environ Health Perspect. 2003;111:437–443. doi: 10.1289/ehp.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC. Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Brain Res Rev. 2001;37:235–248. doi: 10.1016/s0165-0173(01)00121-7. [DOI] [PubMed] [Google Scholar]
- Gore AC, Wu TJ, Oung T, Lee JB, Woller MJ. A novel mechanism for endocrine-disrupting effects of polychlorinated biphenyls: direct effects on gonadotropin-releasing hormone neurones. J Neuroendocrinol. 2002;14:814–823. doi: 10.1046/j.1365-2826.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol. 1994;129:46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- Hsu C, Hsieh YL, Rei-Cheng Y, Hseng-Kuang H. Blockage of N-Methyl-D-Aspartae receptors decreases testosterone levels and enhances postnatal neuronal apoptosis in the preoptic area of male rats. Neuroendocrinology. 2000;71:301–307. doi: 10.1159/000054550. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Yang RC, Shih HC, Hsieh YL, Chen UY, Hsu C. Prenatal exposure of testosterone prevents SDN-POA neurons of postnatal male rats from apoptosis through NMDA receptor. J Neurophysiol. 2001;86:2374–2380. doi: 10.1152/jn.2001.86.5.2374. [DOI] [PubMed] [Google Scholar]
- Koch M. Effects of treatment with estradiol and parental experience on the number and distribution of estrogen-binding neurons in the ovariectomized mouse brain. Neuroendocrinology. 1990;51:505–514. doi: 10.1159/000125384. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Chahoud I. In utero exposure to low-dose 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) impairs male fertility and alters neurobehavior in rat offspring. Toxicology. 2004;202:185–197. doi: 10.1016/j.tox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lackmann GM. Polychlorinated biphenyls and hexachlorobenzene in full-term neonates. Reference values updated. Biol Neonate. 2002;81:82–85. doi: 10.1159/000047188. [DOI] [PubMed] [Google Scholar]
- Lackmann GM, Schaller KH, Angerer J. Organochlorine compounds in breast-fed vs. bottle-fed infants: preliminary results at six weeks of age. Sci Total Environ. 2004;329:289–293. doi: 10.1016/j.scitotenv.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Lanting CI, Huisman M, Muskiet FA, van der Paauw CG, Essed CE, Boersma ER. Polychlorinated biphenyls in adipose tissue, liver, and brain from nine stillborns of varying gestational ages. Pediatr Res. 1998;44:222–225. doi: 10.1203/00006450-199808000-00014. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129:3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE. Parallel declines in fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology. 2001;142:4976–4982. doi: 10.1210/endo.142.11.8470. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Ceccatelli R, Faass O, Fleischmann I, Schlumpf M. Effects of polybrominated diphenylether (PBDE) on reproductive organ and brain development and gene expression in rats. Toxicological Sciences. 2003;72:133–133. [Google Scholar]
- Madeira MD, Leal S, Paula-Barbosa MM. Stereological evaluation and Golgi study of the sexual dimorphisms in the volume, cell numbers, and cell size in the medial preoptic nucleus of the rat. J Neurocytol. 1999;28:131–148. doi: 10.1023/a:1007076206828. [DOI] [PubMed] [Google Scholar]
- Maffucci JA, Walker DM, Ikegami A, Woller MJ, Gore AC. The NMDA receptor subunit NR2b: Effects on LH release and GnRH gene expression in young and middle-aged female rats, with modulation by estradiol. Neuroendocrinology. 2008;87:129–141. doi: 10.1159/000111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois G. Action of progesterone, testosterone and estradiol on the anogenital distance and somatic sexual differentiation in rats. Biol Med (Paris) 1968;57:44–90. [PubMed] [Google Scholar]
- Matthews HB, Anderson MW. The distribution and excretion of 2,4,5,2′, 5′-pentachlorobiphenyl in the rat. Drug Metab Dispos. 1975;3:211–219. [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KM, Calver AR, Tobet SA. GABAB receptors role in cell migration and positioning within the ventromedial nucleus of the hypothalamus. Neuroscience. 2008;151:1119–1131. doi: 10.1016/j.neuroscience.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, Jolliet O. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen AS, Arukwe A. Activation of estrogen receptor signaling by the dioxin-like aryl hydrocarbon receptor agonist, 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) in salmon in vitro system. Toxicol Appl Pharmacol. 2008;227:313–324. doi: 10.1016/j.taap.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: effect of neonatal androgen treatment. Neurosci Lett. 1989;102:185–190. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama K, Nomura Y, Takahashi T, Uchiyama Y, Arizono K, Shinohara R. Hydroxylated polychlorinated biphenyls (OH-PCBs) induce vitellogenin through estrogenic activity in primary-cultured hepatocytes of the Xenopus laevis. Chemosphere. 2010;78:800–806. doi: 10.1016/j.chemosphere.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Oh SM, Ryu BT, Lee SK, Chung KH. Antiestrogenic potentials of ortho-PCB congeners by single or complex exposure. Arch Pharm Res. 2007;30:199–209. doi: 10.1007/BF02977695. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Mizuno K, Sakuma Y, Hayashi S. Exogenous estrogen acts differently on production of estrogen receptor in the preoptic area and the mediobasal hypothalamic nuclei in the newborn rat. Neurosci Res. 1996;25:247–254. doi: 10.1016/0168-0102(96)01050-4. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Okamura H, Hayashi S. Estrogen receptor found in the facial nucleus of the newborn rat is suppressed by exogenous estrogen: immuno- and in situ hybridization histochemical studies. Brain Res Dev Brain Res. 1994;82:9–17. doi: 10.1016/0165-3806(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Sakuma Y. Possible involvement of preoptic estrogen receptor beta positive cells in luteinizing hormone surge in the rat. Domest Anim Endocrinol. 2003;25:83–92. doi: 10.1016/s0739-7240(03)00047-x. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Yokosuka M, Hayashi S. Expression of estrogen receptor in the facial nucleus is suppressed by estradiol, but not by testosterone, indicating a lack of requirement for aromatization. Brain Res. 1995;693:112–117. doi: 10.1016/0006-8993(95)00723-4. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC. Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol Appl Pharmacol. 2002;179:185–194. doi: 10.1006/taap.2002.9371. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- Salama J, Chakraborty TR, Ng L, Gore AC. Effects of polychlorinated biphenyls on estrogen receptor-beta expression in the anteroventral periventricular nucleus. Environ Health Perspect. 2003;111:1278–1282. doi: 10.1289/ehp.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons P, Molho ES, Higgins DS, Factor SA, Marek KL, Seibyl JP, Jennings DL, McCaffrey RJ. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. J Expo Sci Environ Epidemiol. 2010 doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–432. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–1101. doi: 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neurosci Lett. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Aburada S, Hashimoto K, Kitaura T. Transfer and distribution of accumulated (14C)polychlorinated biphenyls from maternal to fetal and suckling rats. Arch Environ Contam Toxicol. 1986;15:709–715. doi: 10.1007/BF01054917. [DOI] [PubMed] [Google Scholar]
- Tobet S, Knoll JG, Hartshorn C, Aurand E, Stratton M, Kumar P, Searcy B, McClellan K. Brain sex differences and hormone influences: a moving experience? J Neuroendocrinol. 2009;21:387–392. doi: 10.1111/j.1365-2826.2009.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld AT, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Juenger TE, Gore AC. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology. 2009;150:2308–2316. doi: 10.1210/en.2008-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE. Persistent estrus and blockade of progesterone-induced LH release follows lesons which do not damage the suprachiasmatic nucleus. Endocrinology. 1978;102:1645–1648. doi: 10.1210/endo-102-5-1645. [DOI] [PubMed] [Google Scholar]
- Woodhouse AJ, Cooke GM. Suppression of aromatase activity in vitro by PCBs 28 and 105 and Aroclor 1221. Toxicol Lett. 2004;152:91–100. doi: 10.1016/j.toxlet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wu D, Gore AC. Changes in androgen receptor, estrogen receptor alpha, and sexual behavior with aging and testosterone in male rats. Horm Behav. 2010;58:306–316. doi: 10.1016/j.yhbeh.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor α in male rats. J Comp Neurol. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yuri K, Kizaki Z, Sawada T, Kawata M. The distributions of apoptotic cells in the medial preoptic areas of male and female neonatal rats. Neurosci Res. 2000;36:1–7. doi: 10.1016/s0168-0102(99)00100-5. [DOI] [PubMed] [Google Scholar]