Abstract
Background & Aims
Anal carcinoma is thought driven by HPV infection through interrupting function of cell regulatory proteins such as p53 and pRb. JCV expresses a T-antigen (T-Ag) that causes malignant transformation through development of aneuploidy and interaction with some of the same regulatory proteins as HPV. JCV T-Ag is present in brain, gastric and colon malignancies, but has not been evaluated in anal cancers. We examined a cohort of anal cancers for JCV T-Ag and correlated this with clinicopathologic data.
Methods
Archived anal carcinomas were analyzed for JCV T-Ag expression. DNA from tumor and normal tissue was sequenced for JCV with viral copies determined by qPCR and Southern blotting. HPV and MSI status was correlated with JCV T-Ag expression.
Results
Of 21 cases of anal cancer (mean age 49 years, 38% female), 12 (57%) were in HIV-positive individuals. All 21 cancers expressed JCV T-Ag, including 9 HPV-negative specimens. More JCV copies were present in cancer vs. surrounding normal tissue (mean 32.54 copies/μg DNA vs. 2.98 copies/μg DNA, P=0.0267). There was no correlation between disease stage and viral copies, nor between viral copies and HIV-positive or -negative status (28.7 vs. 36.34 copies/μg DNA, respectively, P=0.7804). In subset analysis, we found no association between JCV T-Ag expression and HPV or MSI status.
Conclusions
Anal carcinomas uniformly express JCV T-Ag and contain more viral copies compared to surrounding normal tissue. JCV and its T-Ag oncogenic protein, presumably through interruption of cell regulatory proteins, may play a role in anal cancer pathogenesis.
Keywords: JC virus, anal carcinoma, T-antigen, human papilloma virus, HIV
INTRODUCTION
JC Virus (JCV), a member of the polyomaviridae family, is a 5.13kb closed, supercoiled, double-stranded DNA virus. JCV is thought to ubiquitously infect humans with as much as 60% to 80% of adults in the United States and Europe having JCV-specific antibodies [1,2]. Initial infection is thought to occur in the tonsils [3], or more likely the GI tract [4]. The virus then remains latent in the GI tract [5] and tubular epithelial cells of the kidneys [6]. Classically, JCV association with human pathology has been limited to immunosuppressed patients such as in AIDS and organ transplantation. In the immunosuppressed setting, reactivation of JCV occurs and can induce the fatal demyelinating disease, progressive multifocal leukoencephalopathy (PML) as well as polyomavirus-associated nephropathy [7,8]. More recently, there has been mounting evidence for a potential role of JCV in human cancers in the absence of immunosuppression or PML. JCV DNA sequences and its oncogenic T-antigen (T-Ag) expression have been demonstrated in a variety of human cancers including brain [9], gastric [10,11], esophageal [12] and colon cancer [13-16].
Most anal cancers are squamous in cell origin [17]. Anal cancer is more prevalent in the HIV positive community presumably because of the inherent immunocompromised state that allows reactivation of latent HPV. Anal cancer develops through stepwise transformation of normal squamous cells to dysplastic and then eventually malignant cells [18]. The vast majority of studies have focused on the well-established connection of human papilloma virus (HPV) with invasive anal squamous carcinoma through its potential association with cell cycle regulatory proteins such as p53 and pRb [19-21]. High JC viral load has also been implicated as a potential risk factor for other squamous-based carcinomas including tongue [22] and lung [23]. The precise mechanisms behind JCV mediated cellular oncogenesis are not completely understood but it is believed that T antigen plays a vital role in malignant transformation via interaction with a variety of regulatory and growth signaling pathway proteins including p53 [24], pRb [25] and insulin-like growth factor-1 receptor (IGF-1R) [26]. To our knowledge, there has been no study to date demonstrating an association of JCV and T-Ag expression with anal squamous cancer. It would be of interest to know if there might be a role for JCV and in particular its oncogenic T antigen in the development of anal squamous carcinoma.
Microsatellite instability (MSI) is a hallmark of DNA mismatch repair (MMR) dysfunction and is detected by instability at mono or dinucleotide microsatellite DNA sequences. In sporadic colorectal cancer, MSI is seen in approximately 15% of cases. The prevalence of MSI in sporadic anal carcinoma is less clear with most studies describing loss of heterozygosity (LOH) at chromosomes 5p, 11q and 18q in relation to integration of HPV DNA and subsequent expression of E6 and E7 genes [27,28]. To our knowledge, there are no reports describing the prevalence of MSI in anal cancer.
The objective of our study was to determine the prevalence of JCV and T-Ag expression within anal cancer specimens compared to matched surrounding normal tissue and to determine any relevant clinicopathological correlation. We also sought to evaluate the association of JCV T-Ag expression with HPV infection and microsatellite instability. We observed that anal squamous cancer tissue had a higher JCV load compared to corresponding surrounding normal squamous tissue. In addition, in our patient cohort, JCV T antigen protein expression was present in all anal squamous cancers including HPV-negative cancers and absent in normal squamous tissue. We did not find any association with JCV T-Ag protein expression, HPV infection, or microsatellite instability in anal cancer tissue.
METHODS
Anal Cancer specimens
Our patient cohort consisted of 21 patients with anal squamous cancer diagnosed at the University of California, San Diego (UCSD) from 2000-2007. Parraffin-embedded tissue slides were obtained for each of the patients under UCSD Institutional Review Board. Our patient cohort consisted of 13 males and 8 females. In addition, 12 of the patients were HIV positive. Pathological staging and histology were all performed by board certified pathologists at UCSD and Baylor University Medical Center, Dallas. Clinicopathological data was obtained via retrospective analysis of our patient cohort.
DNA extraction
The pathologic blocks were cut and marked by one pathologist (KM). Under high power microscopy the area of tumor tissue was marked by the pathologist as separate from normal and dysplastic tissue. This area was then sharply microdissected using a scalpel under high power microscopy. Microdissection under microscopy of paraffin-embedded 5-micron slides was performed in order to isolate anal cancer tissue as well as corresponding normal tissue from the same patient. Genomic DNA was extracted from both anal cancer tissue and normal tissue isolated from the same paraffin-embedded slide using the QIAamp DNA minikit (Qiagen, Valencia, CA) in accordance to the manufacturer’s specifications.
Microsatellite instability analysis
We used 5 NCI recommended microsatellite markers (BAT25, BAT26, D5S346, D2S123, D17S250) [29]. P32 labeled PCR products were separated on 8% polyacrylamide gel containing 7.5M urea and then exposed to x-ray film. Product DNA bands from anal cancer tissue compared against matched normal control tissue from the same patient to determine MSI status. Classification of microsatellite instability was performed in accordance with previously established protocols: tumors were classified as MSI-H if 2 or more loci showed instability compared to normal controls, MSI-L if only 1 locus demonstrated instability [29]. MSS tumors were classified when no instability occurred at any locus.
Determination of HPV status
HPV DNA was amplified via PCR using modified primers from the standard GP5+/GP6+ protocol [30]. The modified primers contained a 17mer 5′ extension sequence (GTTTCCCAGTCACGATC) to the original GP5+/GP6+ primers. HPV DNA was amplified with use of these modified primers via 2 rounds of PCR. Prior to second round of PCR amplification, the initial PCR product was labeled with amino-allyl dUTP. The labeled product was then coupled with Cy-3 NHS ester for array hybridization. The described method allowed for an unbiased amplification of the HPV DNA and increased sensitivity [31,32].
We used a tissue microarray (TMA) that our lab had previously constructed to type 37 different HPV variants (manuscript in preparation) for the determination of HPV status in our patient cohort. The HPV variants included 14 presumed high-risk variants: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 & 68. Also included were 23 presumed low risk variants: 6, 11, 26, 34, 40, 42, 43, 44, 53, 54, 55, 57, 61, 70, 71, 72, 73, 81, 82/MM4, 82/IS39, 83, 84 & CP6108. High risk variants are those HPV serotypes that are known to cause malignant transformation in normal tissue. Low risk variants are serotypes that have low or unknown malignant potential, but are seen in patients who are HPV-positive (21).
Detection of JCV copies
DNA was extracted from anal cancer and normal tissue specimens by careful microdissection of the paraffin embedded tissues. Thereafter, we first performed PCR amplification followed by DNA sequencing to identify and validate the presence of JCV sequences in the anal cancer tissues as described previously [15,33]. Sequencing of the PCR products was performed using an ABI PRISM 3100 Avant Genetic Analyzer (Applied Biosystems, Foster City, CA). The data were aligned to GeneBank reference sequences for JCV.
Following confirmation of JCV sequences in anal cancer tissues, we subsequently performed quantitative analysis for JCV copy number determinations using JCV-specific primers and PCR reactions which consisted of 12.5 μl of Power SYBR green mix (Applied Biosystems, Foster City, CA), 250 nmole of forward and reverse primers, and 2 microliters of each sample DNA. Amplification of β-actin DNA was used as an endogenous control. Standard curves were generated for both JCV and β-actin. Each sample was run in duplicate to ensure quantitative accuracy. The data were expressed in JCV DNA copies per cells assuming two copies of actin per cell. At least two independent experiments were performed for each sample.
Immunohistochemistry for JCV T-Ag
Five-micron paraffin-embedded slide sections consisting of anal squamous cancer and normal squamous epithelium were placed in an oven and heated to 60°C for 40 minutes to melt the paraffin. The tissue sections were then deparaffinized in xylene for 30 minutes. This step was repeated 3 times. The sections were then rehydrated through a graded series of alcohols as previously described [15]. Antigen retrieval was performed via immersion of tissue slide sections in 10mM citrate buffer (pH 6.0) and autoclaved at 100 °C for 15 minutes. Sections were then allowed to cool for 20 minutes at room temperature. To prevent non-specific antibody binding, we blocked the tissue sections with 10% goat serum at room temperature for 1 hour. Slides were incubated overnight with primary mouse monoclonal antibody against SV40 T-Ag, which cross-reacts with JCV T-Ag (clone PAb416, 1:40 dilution, Calbiochem, CA) followed by incubation in Dako EnVision™ labeled polymer (Dako Cytomation Inc., Carpinteria, CA). Staining was developed by incubation of the tissue sections with diaminobenzadine (DAB) chromagen for 5-10 minutes and then further counterstaining with hematoxylin. Presence of brown nuclear staining was indicative of T-Ag expression. Both anal squamous tissue as well as corresponding adjacent normal squamous tissue was analyzed for T-Ag expression. All determinations of T-Ag expression were performed by blinded independent pathologists. All immunohistochemistry against JCV T-Ag were performed in one lab (AG and CRB).
Statistical analysis
Analysis of differences between anal cancer and normal tissue groups were done using students t test or one-way ANOVA when comparing means and fisher exact test for categorical variables. P<0.05 was considered significant.
RESULTS
Patient demographics
The 21 anal cancer patients in our cohort consisted of 13 males (62%) and 8 females (38%). 12 (57%) of the patients were HIV-positive (11 male, 1 female). Their mean age at time of diagnosis of anal cancer was 49 ± 11 years (range 39-77). HPV status was available for all patients. Of these, 9 (43%) patients were negative for HPV. Table 1 illustrates the patient cohort demographic data.
Table 1.
Anal Cancer patient demographics
| HIV + (n=12) | HIV Neg (n=9) | Total (n=21) | |
|---|---|---|---|
| Mean Age ± S.D. | 43 ±(5 | 56 ±12 | 49 ±11 |
| Gender (M/F) | 11/1 (92%/8%) | 2/7 (18%/82%) | 13/8 (62%/38%) |
| HPV status (+/−) | 8/4 (67%/33%) | 4/5 (44%/56%) | 12/9 (57%/43%) |
JCV viral load and T-Ag expression is correlated with anal cancer
All 21 anal cancer specimens demonstrated JCV DNA, and all 21 specimens stained positive for JCV T-Ag protein expression via immunohistochemistry (Figure 1) with expression exclusively nuclear. We obtained matching data of JCV viral copies in both normal and anal cancer tissue for 11 patients. The mean JCV viral load in anal cancer tissue was 32.54 copies/μg DNA compared to 2.98 copies/μg DNA in corresponding normal anal tissue (p=0.0262, CI 3.94 to 56.06). ). Interestingly, we found no correlation between HIV status and mean JCV copies on comparison of mean JCV viral copies in HIV-positive and HIV-negative patients respectively (28.74 vs. 36.34, p=0.7804, CI: −67.8059 - 52.5259). The full breakdown of T-Ag expression and mean JCV viral load data is illustrated in Table 2.
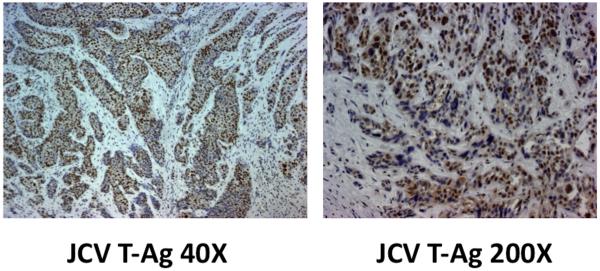
Figure 1.
JCV T-Ag expression in anal cancer. Immunohistochemistry was performed as described in the methods. Left panel, 40X; Right panel 200X, and showing nuclear expression. All specimens assessed expressed JCV T-Ag.
Table 2.
Correlation of JCV copies and T-Ag expression in anal cancers
| Anal cancer tissue |
Normal tissue | p value (CI) | ||
|---|---|---|---|---|
| Mean JCV copy (μg/DNA) |
All matched pts (n=11) |
32.54 | 2.98 | 0.0267 (3.94 - 56.06) |
| Matched HIV pts (n=5) |
28.75 | 1.08 | 0.0786 (−4.04 - 60.04) |
|
| Matched HIV neg pts (n=6) |
36.34 | 4.89 | 0.1784 (−16.73 - 78.73) |
|
| T-Ag expression (n=21,%) | 100% | 0% | N/A | |
Relationship of JCV T-Ag expression to anal cancer stage
In our cohort of 21 patients with anal squamous cancer, 8 patients were stage 1 and had mean JCV viral load of 59.91 copies. Ten patients were stage 2 and their mean viral load was slightly increased at 60.92. None of our patients were stage 3 and interestingly the 3 patients with stage 4 disease had the lowest mean viral load (7.37 copies). Utilizing one-way ANOVA for analysis of anal cancer stage data, we found no significant correlation between stage and mean JCV viral load (Table 3).
Table 3.
Anal Cancer Stage and JCV copies/ug DNA
| Stage | N (%) | Mean JCV copy (μg/DNA) | p value |
|---|---|---|---|
| 1 | 8 (38%) | 59.91* | 0.651 |
| 2 | 10 (48%) | 60.92** | |
| 3 | 0 (0%) | N/A | |
| 4 | 3 (14%) | 7.37*** |
JCV copy data available for 7 pts with stage 1 anal cancer
JCV copy data available for 9 pts with stage 2 anal cancer
JCV copy data available for 2 pts with stage 4 anal cancer
Relationship of JCV T-Ag expression to MSI status
MSI data was available for 16 patients (11 HIV-positive, 5 HIV-negative). Only 1 patient (6%) demonstrated MSI-H. One other patient (6%) was MSI-L and the remaining 14 patients (88%) were MSS (Table 4). The one patient that demonstrated MSI-H was HIV-positive. We found no association between HIV status and MSI-H (1/8, 11% MSI-H HIV positive vs. 0/7, 0% MSI-H HIV negative, p=1). No significant association existed between T-Ag expression and MSI status. Thus, in our patient cohort of anal cancer, MSI-H is rare and does not appear to be associated with HIV status or JCV T-Ag expression.
Table 4.
MSI status and JCV T-Ag expression
| MSI-H | MSI-L/MSS | p value | |
|---|---|---|---|
| Total (n=16) | 1 (6%) | 15 (94%) | N/A |
| HIV + (n=9) | 1 (11%) | 8 (89%) | 1.0 |
| HIV neg (n=7) | 0 (0%) | 7 (100%) | |
| T-Ag (n=21) | 1 (5%) | 20 (95%) | N/A |
Relationship of JCV T-Ag expression to HPV status
HPV status was available for all 21 patients in our cohort. Interestingly, only 12 (57%) of our anal cancer patients were HPV-positive (11 high risk HPV, 1 low risk HPV). Of these, 8 (67%) were HIV-positive. In our cohort, there was no significant difference between HIV and HPV status (8/12, 67% vs. 4/9, 44%, p= 0.3964). We also found no association between HPV status and JCV T-Ag expression (Table 5).
Table 5.
HPV status and JCV T-Ag expression
| HPV + | HPV neg | p value | |
|---|---|---|---|
| Total (n=21) | 12 (57%) | 9 (43%) | N/A |
| HIV + (n=12) | 8 (67%) | 4 (33%) | 0.3964 |
| HIV neg (n=9) | 4 (44%) | 5 (56%) | |
| T-Ag (n=21) | 12 (57%) | 9 (43%) | N/A |
DISCUSSION
The oncogenic potential of JCV was initially discovered when Walker et al injected the virus into Syrian hamster brains and induced aneuploid tumors [34]. Since then, numerous studies have linked JCV and in particular, its T-Ag protein expression to a variety of cancers. We were interested in extending this correlation to anal cancer. In this study, we have demonstrated that in our cohort of patients, JCV viral copies are elevated in anal cancer tissue compared to surrounding normal tissue, which suggests a biological and mechanistic role for this virus in the pathogenesis of this disease. Furthermore, JCV T-Ag expression is highly predominant in anal cancer tissue.
JCV T-Ag is a multifunctional oncogenic protein that has the ability to transform mammalian cells. It does this by binding and inactivating p53 and pRb, two key tumor suppressor proteins that regulate cell cycle progression [35,36]. Through its inactivation of p53 and pRb, JCV sets itself up in an optimal cellular environment for its replication and assembly during its lytic phase of infection and concurrently facilitates transformation of normal cells. Furthermore, through its interaction with insulin receptor substrate 1 (IRS-1), JCV T-Ag has also been shown to inhibit homologous recombination DNA repair, part of the system that maintains genomic stability [37]. Previous work has also demonstrated that the activation of IGF-1R, along with the interaction between JCV T-Ag and IRS-1 could potentially induce carcinogenesis via triggering cell proliferation, anti-apoptotic signaling and inhibition of homologous DNA recombination repair [38].
Our data demonstrating higher JCV viral copies in anal cancer tissue compared to surrounding normal epithelium is consistent with numerous previous studies demonstrating increased JCV sequences in gastric, esophageal and colon cancers [10-16]. To our knowledge, the present study is the first demonstration correlating JCV viral copies and T-Ag protein expression in anal cancer tissue against surrounding normal epithelium. Of note, we did not find any significant correlation between level of JCV viral copies and stage progression in anal cancer. This suggests that JCV, while potentially having a role in carcinogenesis, may not play a big role in prognosis. However, our small sample size could be the other reason that we found no stage correlation with level of JCV copies. To show T-Ag protein expression via IHC in anal cancer is important as the finding of JCV viral copies alone is not sufficient to demonstrate biological activity of this oncogenic protein as JCV DNA sequences have been shown to be frequently present in cells of normal individuals [39]. The finding of JCV T-Ag expression exclusively in the nuclei of anal squamous cells suggests there might be a role for JCV in the pathogenesis of anal cancer. A prior report has illustrated that JCV T-Ag expression in adenomas was lower than in colorectal carcinoma (16% vs. 40-50% respectively) [15]. Given this, it would also be of interest to know if the level of JCV copies as well as predominance of T-Ag expression is attenuated in anal dysplastic compared to anal cancer cells, and with the addition of a control group of benign anorectal disease, to rule out the possibility that JCV T-Ag is an innocent bystander or associated with inflammation or chronic infection. JCV is believed to facilitate carcinogenesis at its early stages, the so-called “hit and run” hypothesis, where late stage tumors do not exhibit high viral numbers after genetic damage has been done [40]. Our observation appears to be similar in our anal cancer cohort.
Our finding that anal cancer tissue in our cohort demonstrated extremely low levels of MSI is to our knowledge a new finding. We found no association between JCV T-Ag protein expression and MSI status in anal cancer tissue, which is also consistent with prior data demonstrating no relation between MSI and JCV T-Ag expression in colon adenomas [15]. A relationship between JCV T-Ag protein expression and promoter methylation of 4 tumor suppressor genes, including hMLH1, has been observed [33]. Hypermethylation of hMLH1 is the main cause of MSI in sporadic colorectal cancers, and in MSI cancers that expressed JCV T-Ag, a higher methylation index, a measure of the degree of methylation, was present [33]. Taken together, the data suggests that the association of hypermethylation, MSI, and JCV T-Ag expression may occur later in the adenoma-to-carcinoma sequence for colorectal cancers. Our results suggest that the underlying genomic instability pattern behind anal squamous cell cancer carcinogenesis is less likely dependent on defects in the DNA mismatch repair system. Of interest, there were 9 (43%) anal cancer patients in our cohort who were HPV negative but had expression of JCV T-Ag thus suggesting a potential for JCV T-Ag to drive carcinogenesis through an HPV-independent process. JCV T-Ag is well known to induce chromosomal instability (aneuploidy) as it’s presumed prime mode of genomic instability, and could account for this mechanism as the dominant one observed in anal cancer [41]. These mechanisms of action may act synergistically or cumulatively to potentiate the transformation of tissue by HPV, but would need be studied in an experimental model to show any conclusive interaction. In bronchial cancers, HPV was detected in 10 of 78 lesions, while JCV sequences was amplified in only 1 of 78 tumors, suggesting a lack of synergistic effect within lung cancer [42]. In colorectal cancers, HPV was detected in 22 of 66 samples while no JCV viral sequences were detected, again suggesting no synergistic effect for this tumor [43].
Building on the previously described interactions between JCV T-Ag and various proto-oncogenic and tumor suppressor proteins in various cancers, it seems plausible that T-Ag might likewise interact with similar proteins that could then lead to carcinogenesis in anal cancer. However, this has yet to be determined. In conclusion, our results demonstrate that JCV viral copies are increased in anal squamous cancer compared to surrounding normal anal epithelium. In addition, JCV T-Ag protein expression is also highly predominant in anal cancer tissue thus further suggesting a potential role for JCV T-Ag in the development of anal cancer.
CONDENSED ABSTRACT.
The pathogenesis of anal carcinoma is linked to HPV, but this is less clear in HPV-negative anal cancers. JCV is present in 100% of anal cancers and contains a higher viral load in cancer compared to normal tissue. JCV may play a role in the development of anal cancer.
Acknowledgements
Supported by a UCSD Institutional grant from the American Cancer Society (#CCT05SR), the U.S. Public Health Service (DK067287), the UCSD Digestive Diseases Research Development Center (DK080506), the SDSU/UCSD Comprehensive Cancer Center Partnership (CA132379 and CA132384), and a KL2 grant from the UCSD Clinical and Translational Science Award.
Abbreviations
- MSI
microsatellite instability
- MSI-H
microsatellite instability-high
- MSI-L
microsatellite instability-Low
- MSS
microsatellite stable
- HPV
human papilloma virus
- JCV
JC virus
- T-Ag
T-antigen
- IRS-1
insulin receptor substrate 1
- PML
progressive multifocal leukoencephalopathy
- IGF-1R
insulin-like growth factor-1 receptor
- qPCR
quantitative polymerase chain reaction
Footnotes
Disclosure: There are no conflicts of interest for any authors on this manuscript.
REFERENCES
- 1.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–46. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 2.Taguchi F, Kajioka J, Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26:1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 3.Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72:9918–23. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bofill-Mas S, Formiga-Cruz M, Clemente-Casares P, Calafell F, Girones R. Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J Virol. 2001;75:10290–9. doi: 10.1128/JVI.75.21.10290-10299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricciardiello L, Laghi L, Ramamirtham P, Chang CL, Chang DK, Randolph AE, Boland CR. JC virus DNA sequences are frequently present in the human upper and lower gastrointestinal tract. Gastroenterology. 2000;119:1228–35. doi: 10.1053/gast.2000.19269. [DOI] [PubMed] [Google Scholar]
- 6.Boldorini R, Veggiani C, Barco D, Monga G. Kidney and urinary tract polyomavirus infection and distribution: molecular biology investigation of 10 consecutive autopsies. Arch Pathol Lab Med. 2005;129:69–73. doi: 10.5858/2005-129-69-KAUTPI. [DOI] [PubMed] [Google Scholar]
- 7.Focosi D, Marco T, Kast RE, Maggi F, Ceccherini-Nelli L, Petrini M. Progressive multifocal leukoencephalopathy: what’s new? Neuroscientist. 2010;16:308–23. doi: 10.1177/1073858409356594. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch HH, Drachenberg CB, Steiger J, Ramos E. Polyomavirus-associated nephropathy in renal transplantation: critical issues of screening and management. Adv Exp Med Biol. 2006;577:160–73. doi: 10.1007/0-387-32957-9_11. [DOI] [PubMed] [Google Scholar]
- 9.Del Valle L, White MK, Khalili K. Potential mechanisms of the human polyomavirus JC in neural oncogenesis. J Neuropathol Exp Neurol. 2008;67:729–40. doi: 10.1097/NEN.0b013e318180e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin SK, Li MS, Fuerst F, Hotchkiss E, Meyer R, Kim IT, Goel A, Boland CR. Oncogenic T-antigen of JC virus is present frequently in human gastric cancers. Cancer. 2006;107:481–8. doi: 10.1002/cncr.22028. [DOI] [PubMed] [Google Scholar]
- 11.Murai Y, Zheng HC, Abdel Aziz HO, Mei H, Kutsuna T, Nakanishi Y, Tsuneyama K, Takano Y. High JC virus load in gastric cancer and adjacent non-cancerous mucosa. Cancer Sci. 2007;98:25–31. doi: 10.1111/j.1349-7006.2006.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Valle L, White MK, Enam S, Piña Oviedo S, Bromer MQ, Thomas RM, Parkman HP, Khalili K. Detection of JC virus DNA sequences and expression of viral T antigen and agnoprotein in esophageal carcinoma. Cancer. 2005;103:516–27. doi: 10.1002/cncr.20806. [DOI] [PubMed] [Google Scholar]
- 13.Enam S, Del Valle L, Lara C, Gan DD, Ortiz-Hidalgo C, Palazzo JP, Khalili K. Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002;62:7093–101. [PubMed] [Google Scholar]
- 14.Niv Y, Goel A, Boland CR. JC virus and colorectal cancer: a possible trigger in the chromosomal instability pathways. Curr Opin Gastroenterol. 2005;21:85–9. [PubMed] [Google Scholar]
- 15.Jung WT, Li MS, Goel A, Boland CR. JC virus T-antigen expression in sporadic adenomatous polyps of the colon. Cancer. 2008;112:1028–36. doi: 10.1002/cncr.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosho K, Shima K, Kure S, Irahara N, Baba Y, Chen L, Kirkner GJ, Fuchs CS, Ogino S. JC virus T-antigen in colorectal cancer is associated with p53 expression and chromosomal instability, independent of CpG island methylator phenotype. Neoplasia. 2009;11:87–95. doi: 10.1593/neo.81188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wietfeldt ED, Thiele J. Malignancies of the anal margin and perianal skin. Clin Colon Rectal Surg. 2009;22:127–35. doi: 10.1055/s-0029-1223845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palefsky JM, Rubin M. The epidemiology of anal human papillomavirus and related neoplasia. Obstet Gynecol Clin North Am. 2009;36:187–200. doi: 10.1016/j.ogc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Oh KJ, Kalinina A, Bagchi S. Destabilization of Rb by human papillomavirus E7 is cell cycle dependent: E2-25K is involved in the proteolysis. Virology. 2010;396:118–24. doi: 10.1016/j.virol.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner MS, Laimins LA. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J Virol. 1994;68:4262–73. doi: 10.1128/jvi.68.7.4262-4273.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramamoorthy S, Luo L, Luo E, Carethers JM. Tobacco smoking and risk of recurrence for squamous cell cancer of the anus. Cancer Detect Prev. 2008;32:116–20. doi: 10.1016/j.cdp.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutsuna T, Zheng H, Abdel-Aziz HO, Murai Y, Tsuneyama K, Furuta I, Takano Y. High JC virus load in tongue carcinomas may be a risk factor for tongue tumorigenesis. Virchows Arch. 2008;452:405–10. doi: 10.1007/s00428-007-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel-Aziz HO, Murai Y, Hong M, Kutsuna T, Takahashi H, Nomoto K, Murata S, Tsuneyama K, Takano Y. Detection of the JC virus genome in lung cancers: possible role of the T-antigen in lung oncogenesis. Appl Immunohistochem Mol Morphol. 2007;15:394–400. doi: 10.1097/01.pai.0000213126.96590.64. [DOI] [PubMed] [Google Scholar]
- 24.Poulin DL, Kung AL, DeCaprio JA. p53 targets simian virus 40 large T antigen for acetylation by CBP. J Virol. 2004;78:8245–53. doi: 10.1128/JVI.78.15.8245-8253.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caracciolo V, Reiss K, Khalili K, De Falco G, Giordano A. Role of the interaction between large T antigen and Rb family members in the oncogenicity of JC virus. Oncogene. 2006;25:5294–301. doi: 10.1038/sj.onc.1209681. [DOI] [PubMed] [Google Scholar]
- 26.Novosyadlyy R, Vijayakumar A, Lann D, Fierz Y, Kurshan N, LeRoith D. Physical and functional interaction between polyoma virus middle T antigen and insulin and IGF-I receptors is required for oncogene activation and tumor initiation. Oncogene. 2009;28:3477–86. doi: 10.1038/onc.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muleris M, Salmon RJ, Girodet J, Zafrani B, Dutrillaux B. Recurrent deletions of chromosomes 11q and 3p in anal canal carcinoma. Int J Cancer. 1987;39:595–8. doi: 10.1002/ijc.2910390509. [DOI] [PubMed] [Google Scholar]
- 28.Heselmeyer K, du Manoir S, Blegen H, Friberg B, Svensson C, Schröck E, Veldman T, Shah K, Auer G, Ried T. A recurrent pattern of chromosomal aberrations and immunophenotypic appearance defines anal squamous cell carcinomas. Br J Cancer. 1997;76(10):1271–8. doi: 10.1038/bjc.1997.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 30.Van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40:779–87. doi: 10.1128/JCM.40.3.779-787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YT, Carson DA. A novel approach for determining cancer genomic breakpoints in the presence of normal DNA. PLoS One. 2007;2:e380. doi: 10.1371/journal.pone.0000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Q, Nunez E, Lin C, Christensen K, Downs T, Carson DA, Wang-Rodriguez J, Liu YT. A sensitive array-based assay for identifying multiple TMPRSS2:ERG fusion gene variants. Nucleic Acids Res. 2008;36:e130. doi: 10.1093/nar/gkn585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goel A, Li MS, Nagasaka T, Shin SK, Fuerst F, Ricciardiello L, Wasserman L, Boland CR. Association of JC virus T-antigen expression with the methylator phenotype in sporadic colorectal cancers. Gastroenterology. 2006;130:1950–61. doi: 10.1053/j.gastro.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 34.Walker DL, Padgett BL, ZuRhein GM, Albert AE, Marsh RF. Human papovavirus (JC): Induction of brain tumors in hamsters. Science. 1973;181:674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- 35.Bollag B, Chuke WF, Frisque RJ. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: Identification of sequences important for efficient transformation. J Virol. 1989;63:863–872. doi: 10.1128/jvi.63.2.863-872.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyson N, Bernards R, Friend SH, Gooding LR, Hassell JA, Major EO, Pipas JM, Vandyke T, Harlow E. Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990;64:1353–6. doi: 10.1128/jvi.64.3.1353-1356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trojanek J, Croul S, Ho T, Wang JY, Darbinyan A, Nowicki M, Valle LD, Skorski T, Khalili K, Reiss K. T-antigen of the human polyomavirus JC attenuates faithful DNA repair by forcing nuclear interaction between IRS-1 and Rad51. J Cell Physiol. 2006;206:35–46. doi: 10.1002/jcp.20425. [DOI] [PubMed] [Google Scholar]
- 38.Reiss K, Khalili K, Giordano A, Trojanek J. JC virus large T-antigen and IGF-I signaling system merge to affect DNA repair and genomic integrity. J Cell Physiol. 2006;206:295–300. doi: 10.1002/jcp.20455. [DOI] [PubMed] [Google Scholar]
- 39.Laghi L, Randolph AE, Chauhan DP, Marra G, Major EO, Neel JV, Boland CR. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc Natl Acad Sci U S A. 1999;96:7484–9. doi: 10.1073/pnas.96.13.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricciardiello L, Baglioni M, Giovannini C, Pariali M, Cenacchi G, Ripalti A, Landini MP, Sawa H, Nagashima K, Frisque RJ, Goel A, Boland CR, Tognon M, Roda E, Bazzoli F. Induction of chromosomal instability in colonic cells by the human polyomavirus JC virus. Cancer Res. 2003;63:7256–62. [PubMed] [Google Scholar]
- 41.Ricciardiello L, Chang DK, Laghi L, Goel A, Chang CL, Boland CR. Mad-1 is the exclusive JC virus strain present in the human colon, and its transcriptional control region has a deleted 98-base-pair sequence in colon cancer tissues. J Virol. 2001;75:1996–2001. doi: 10.1128/JVI.75.4.1996-2001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giuliani L, Jaxmar T, Casadio C, Gariglio M, Manna A, D’Antonio D, Syrjanen K, Favalli C, Ciotti M. Detection of oncogenic viruses SV40, BKV, JCV, HCMV, HPV and p53 codon 72 polymorphism in lung carcinoma. Lung Cancer. 2007;57:273–81. doi: 10.1016/j.lungcan.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Giuliani L, Ronci C, Bonifacio D, Di Bonito L, Favalli C, Perno CF, Syrjänen K, Ciotti M. Detection of oncogenic DNA viruses in colorectal cancer. Anticancer Res. 2008;28:1405–10. [PubMed] [Google Scholar]