Abstract
Craniosynostosis, defined as the premature fusion of the cranial sutures, presents many challenges in classification and treatment. At least 20% of cases are caused by specific single gene mutations or chromosome abnormalities. This article maps out approaches to clinical assessment of a child presenting with an unusual head shape, and illustrates how genetic analysis can contribute to diagnosis and management.
Keywords: craniosynostosis, FGFR, TWIST
In brief
Craniosynostosis is best managed in a multispecialty tertiary referral unit.
Single suture synostosis affects the sagittal suture most commonly, followed by the coronal, metopic and lambdoid sutures.
Both environmental factors (especially intrauterine fetal head constraint) and genes (single gene mutations, chromosome abnormalities and polygenic background) predispose to craniosynostosis.
Most genetically determined craniosynostosis is characterised by autosomal dominant inheritance, but around half of cases are accounted for by new mutations.
Apart from the genetic implications, it is important to recognise cases with a genetic cause because they are more likely to be associated with multiple suture synostosis and extracranial complications.
Genes most commonly mutated in craniosynostosis are FGFR2, FGFR3, TWIST1 and EFNB1.
As well as being associated with syndromes, some clinically non-syndromic synostosis (usually affecting the coronal suture) can be caused by single gene mutations, particularly the Pro250Arg mutation in FGFR3.
In severe cases, initial care should be directed towards maintenance of the airway, support of feeding, eye protection and treatment of raised intracranial pressure.
Introduction
During infancy and childhood, the skull vault (calvaria) expands to accommodate the growing brain. This growth occurs predominantly at the narrow seams of undifferentiated mesenchyme, termed cranial sutures, which lie between different bones. The paired frontal and parietal bones are separated in the midline by the metopic and sagittal sutures, respectively; the frontal and parietal bones are separated by coronal sutures; and the parietal bones are separated from the single occipital bone by lambdoid sutures (Figure 1a and b). Craniosynostosis describes the premature fusion of one or more of the cranial sutures: secondary distortion of skull shape occurs because of a combination of lack of growth perpendicular to the fused suture, and compensatory overgrowth at the non-fused sutures. The overall prevalence of craniosynostosis has been estimated at between 1 in 2100 and 1 in 2500 births.1, 2 Craniosynostosis is important to recognise and treat because it can be associated with many complications affecting sensory, respiratory and neurological function.
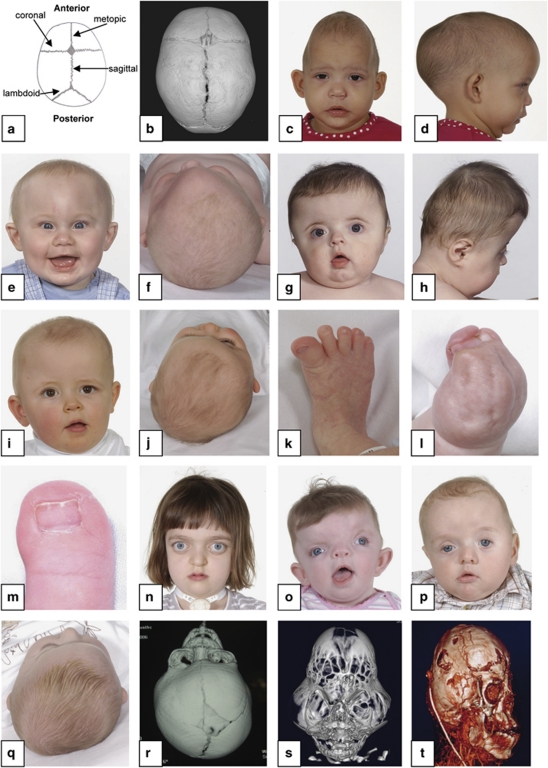
Figure 1.
Diagnostic features of craniosynostosis. (a) Schematic diagram showing positions of the major cranial sutures. (b) CT scan (vertex view of skull) showing major sutures; anterior is at top. (c,d) Sagittal synostosis: note long, narrow head. (e,f) Metopic synostosis: note hypotelorism and triangular profile of forehead. (g,h) Bicoronal synostosis: broad, flattened head. (i,j) Right unicoronal synostosis: note flattened brow and anterior position of ear on affected side, deviation of nasal tip and prominent brow on unaffected side. (k–m), Congenital anomalies of feet or hands characteristic of Pfeiffer syndrome (k), Apert syndrome (l) and craniofrontonasal syndrome (m). (n) Crouzonoid facial appearance. (o) Severe hypertelorism, grooved nasal tip and left unicoronal synostosis in craniofrontonasal syndrome. (p) Ptosis and left unicoronal synostosis in Saethre-Chotzen syndrome. (q) Positional plagiocephaly: prominence on right anteriorly and left posteriorly, with right ear anterior and parallelogram shape to skull. (r) CT reconstruction showing left unicoronal synostosis. (s) CT reconstruction showing cloverleaf skull. (t) CT venogram showing abnormal venous drainage in multisuture syndromic craniosynostosis. See text for further details.
Clinical overview
The aims of clinical assessment are to determine: (1) whether craniosynostosis is present; (2) whether there are additional features suggesting an associated syndrome and (3) to assess whether urgent or elective management is required. Craniosynostosis is very heterogeneous in its causes and presentation, and correspondingly in its management. Most isolated non-syndromic cases present electively, but a minority of syndromic cases present acutely and require immediate intervention. Classifications of craniosynostosis based on the combination of sutures closed, associated features suggesting a syndrome, and identifiable aetiological factors (for example, intrauterine constraint,3, 4 teratogenic exposure and genetic abnormalities) all have validity, and should be considered in combination.
The most common presentation is with an unusual head shape in the first year of life; the head may be long and narrow (scaphocephaly, dolichocephaly; Figure 1c and d), triangular at the front (trigonocephaly; Figure 1e and f), broad and flattened (brachycephaly; Figure 1g and h) or skewed (plagiocephaly Figure 1i and j). To seek aetiological clues, the history should focus on any family history of unusual head shapes, prenatal exposures (for example, valproate ingestion for maternal epilepsy), evidence of fetal intrauterine constraint (primiparity, multiple pregnancy, abnormal lie and oligohydramnios) and birth history. Obstructed labour and assisted delivery or caesarean sections are frequent, but more likely to be the consequence, rather than the cause of craniosynostosis. In assessing the functional consequences of the condition, the most important clinical information pertains to the airway, feeding, eye protection and raised intracranial pressure (ICP). It is essential to elicit any evidence of breathing difficulty, choking or vomiting on feeds, failure of eyelids to cover eyes during sleep, or irritability, as these may be indications for acute intervention.
The clinical examination should follow a set pattern to avoid overlooking clues. Our own routine is to start with the hands and feet looking for congenital anomalies, for example, a broad radially deviated thumb or big toe in Pfeiffer syndrome (Figure 1k), more extensive syndactyly in Apert syndrome (Figure 1l) and longitudinally split nails in craniofrontonasal syndrome (Figure 1m). Examine the face for dysmorphic features, including hyper- or hypotelorism, exorbitism, midface hypoplasia, asymmetry and ear size, position and shape. The combination of exorbitism, flattened malar region and beaked nose signals a ‘crouzonoid' appearance (Figure 1n), likely to be associated with FGFR2 mutation. If there is hypertelorism, view the nose from above looking for a shallow groove, which suggests craniofrontonasal syndrome (Figure 1o). Ptosis, low frontal hairline and small ears with prominent horizontal crura are features of Saethre-Chotzen syndrome (Figure 1p). Look at the skull shape from front, back, sides and top, feel the sutures for ridging (associated with suture fusion), assess the size, shape and tension in the fontanelles, and measure the head circumference. The cephalic index (ratio of maximum breadth to maximum length of skull, normally between 0.76 and 0.83 (in boys) or 0.84 (in girls))5 provides an objective measure of its overall proportions, but requires calipers for accurate measurement. Finally look in the mouth for a cleft palate and complete the general physical examination. The parents should be assessed similarly for signs suggestive of a carrier state.
It is important, but not always easy, to distinguish synostotic plagiocephaly (caused by unicoronal or, less commonly unilambdoid synostosis) from non-synostotic plagiocephaly. The latter is a very common condition related to deformation and shear stress on the skull during late pregnancy and delivery, and frequently increases in severity before the acquisition of head control, because infants tend to be nursed in a supine position. Clues may be obtained by viewing the head, and particularly the position of the ears, from above (compare Figure 1i, j with q). In synostotic plagiocephaly caused by unicoronal synostosis, the ear on the affected side is shifted forward, and the distance to the flattened brow contour is reduced. The skull when viewed from above is a rhomboid shape. In non-synostotic anterior plagiocephaly, the ear on the flattened side is more posterior (parallelogram shape when viewed from above). If doubt remains, referral to a specialist unit is recommended.
Diagnostic approaches
Computed tomography (CT) scanning and three dimensional reconstruction using both bone and soft tissue windows is the investigation of choice.6 This should clearly reveal the patency, or closure, of each individual suture (Figure 1b, r and s). CT of the brain should also be performed seeking associated anatomical abnormalities (for example, ventriculomegaly and agenesis of corpus callosum) and to check the fluid spaces for evidence of craniocerebral disproportion. CT venography is required in complex cases where abnormal venous drainage is suspected (Figure 1t). Skull radiographs are of limited use as their sensitivity for detecting sutural patency is significantly less than CT. They are most useful when screening cases of plagiocephaly when the clinical findings are not conclusive. Magnetic resonance imaging (MRI), although ideal for the brain, is less good at visualising the cranial sutures.
Craniosynostosis should ideally be managed in a multidisciplinary setting. Full workup should also include baseline psychological, speech/language, hearing and orthoptic assessments. Neurosurgical review with ICP monitoring may be required, although this is more commonly used later in childhood to assess symptoms suggestive of raised ICP.
Molecular and genetic basis of disease
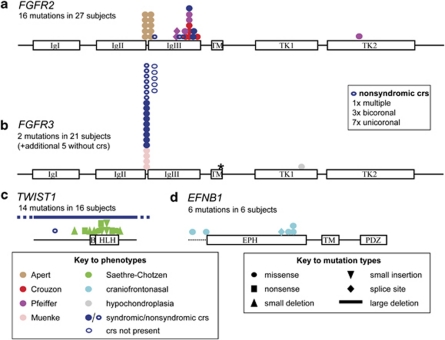
In a recent analysis of a 10-year prospective cohort of craniosynostosis presenting to our unit, a genetic diagnosis was achieved in 21% of cases, comprising 86% single gene disorders and 15% chromosome abnormalities (one patient had both).7 The genes most frequently mutated were FGFR2 (32% of all genetic cases), FGFR3 (25%), TWIST1 (19%) and EFNB1 (7%). Figure 2 illustrates the domain structures of proteins encoded by these four genes, together with the clinical presentation and molecular distribution of mutations in the cohort survey, illustrating the relative prevalence of the major mutations causing craniosynostosis. Much rarer, but well established associations of gene mutations and craniosynostosis are for FGFR1 (mild Pfeiffer syndrome), POR (Antley-Bixler syndrome) and RAB23 (Carpenter syndrome); further information about these genes is provided below. Single-gene mutation associations that are based on only a handful of cases are not further discussed; these include mutations in EFNA4 (non-syndromic coronal synostosis),8 ESCO2 (Roberts syndrome), GLI3 (Greig syndrome), JAG1 (Alagille syndrome), KRAS (Noonan syndrome), RECQL4 (Baller Gerold syndrome) and TGFBR1 or TGFBR2 (Loeys-Dietz syndrome). Mutation in MSX2, the first genetic cause of craniosynostosis to be molecularly determined,9 is exceptionally rare, having been reported to date only in the original family, but several duplications including MSX2 have been associated with craniosynostosis.10
Figure 2.
Distribution and types of mutation that commonly cause craniosynostosis. The major domains of the proteins encoded by the FGFR2 (a), FGFR3 (b), TWIST1 (c) and EFNB1 (d) genes are shown to scale, together with the position and types of mutation identified, and their associated phenotypes. Dashed line before EPH domain encoded by EFNB1 indicates 5′ untranslated region. The data, which were obtained from the Oxford cohort study,7 convey the relative prevalence of the most common mutations, but many rare mutations were absent in this survey. Asterisk indicates position of Ala391Glu mutation of FGFR3. crs, craniosynostosis. See text for information on protein domains.
FGFR2 (fibroblast growth factor receptor type 2)
The FGFR2 gene encodes a transmembrane receptor tyrosine kinase (Figure 2a) comprising an extracellular ligand-binding region (immunoglobulin-like domains IgI, IgII and IgIII), a single pass transmembrane region (TM) and split tyrosine kinase domain (TK1 and TK2). Heterozygous mutations of FGFR2 cause three classical craniosynostosis syndromes, those of Apert, Crouzon and Pfeiffer. All exhibit a characteristic crouzonoid facial appearance (Figure 1n). Less commonly, mutations may present with non-syndromic synostosis,7 or Beare-Stevenson syndrome (multisuture synostosis associated with cutis gyrata). Mutations in FGFR2 and FGFR3 tend to encode highly localised, recurrent missense substitutions encoding proteins with gain-of-function properties. The cellular consequences of mutation are complex, including enhancement of proliferation, differentiation and apoptosis of osteoblasts bordering the cranial suture mesenchyme; premature differentiation is probably the most important factor leading to craniosynostosis.11, 12
Apert syndrome is characterised by bicoronal synostosis and bilateral symmetrical complex syndactyly of the hands and feet (Figure 1l). Other frequent complications include cleft palate (44%) and learning disability, requiring special needs education (44%).13 Over 98% of cases are caused by specific missense mutations of FGFR2, either Ser252Trp (66%) or Pro253Arg (32%), in the linker between the IgII and IgIII domains (Figure 2a); the former substitution is associated with a higher frequency of cleft palate, but milder syndactyly.13 These substitutions specifically increase the affinity and broaden the specificity of FGF-ligand binding, explaining the exquisite genotype–phenotype correlation.14 Nearly all Apert syndrome mutations arise de novo, and have been shown to originate exclusively from the father. These mutations provide a paradigm for paternal age effect mutations that are enriched in sperm owing to a paradoxical selective advantage to mutant spermatogonial cells in the testis.15, 16
Pfeiffer syndrome is usually characterised by broad, radially deviated thumbs and/or big toes (Figure 1k), sometimes with cutaneous syndactyly, and includes individuals previously classified with a ‘Jackson-Weiss' phenotype. The craniofacial severity is variable, an important subgroup presenting with severe multisuture synostosis (‘Kleeblatschädel') (Figure 1s), which is very challenging to manage and associated with significant mortality. FGFR2 mutations in Pfeiffer syndrome overlap those in Crouzon syndrome (Figure 2a), but the majority of severe cases are caused by a small subset of substitutions encoding Trp290Cys, Tyr340Cys, Cys342Arg or Ser351Cys.17
Crouzon syndrome is usually the mildest of the FGFR2-associated disorders and the clinical diagnosis is suggested by the combination of crouzonoid facies (Figure 1n) and absence of major abnormalities of the hands and feet. Although bicoronal synostosis is most common, Crouzon syndrome can present with late-onset pansynostosis.18, 19 It is important to be aware of this possibility in a child with a crouzonoid appearance, because apparently mild distortion of the skull shape may mask the presence of raised ICP. The association of crouzonoid facies with acanthosis nigricans is caused by a specific FGFR3 mutation (see below).
The distribution of mutations causing Pfeiffer and Crouzon syndromes in FGFR2 overlaps considerably. Most mutations (94%) occur in the third extracellular immunoglobulin-like domain encoded by exons IIIa or IIIc, where they cause constitutive activation by covalent cross-linking of receptor monomers. The remainder of the mutations are scattered in seven other exons of the gene, including several mutations in the tyrosine kinase domain.20 Abnormal splicing of the IIIc exon tends to be associated with more severe limb abnormalities, so these mutations generally present with Pfeiffer or occasionally even Apert syndrome (Alu element insertions).21
FGFR3 (fibroblast growth factor receptor type 3)
FGFR3 encodes a protein that has a domain structure closely resembling its paralogue FGFR2 (Figure 2b). Although FGFR3 mutations are commonly associated with bone dysplasia (hypochondroplasia-achondroplasia-thanatophoric dysplasia series), two heterozygous mutations cause specific craniosynostosis syndromes, Muenke syndrome and Crouzon syndrome with acanthosis nigricans (Figure 2b).
Muenke syndrome, defined by identification of the Pro250Arg substitution, is individually the most common genetic abnormality found in craniosynostosis, comprising ∼5% of all cases.7 The associated phenotype is not distinctive and was not properly delineated until the mutation was described in 1996 (ref. 22). Patients usually present with either unicoronal or bicoronal synostosis, but at least 20% of mutation carriers do not have clinically significant craniosynostosis. The facial appearance ranges from normal to a dysmorphic appearance easily mistakable for Saethre-Chotzen syndrome. Minor digital abnormalities (especially brachydactyly) are not characteristic and there should be a low threshold for requesting the genetic test to establish the diagnosis. An important complication is low frequency hearing loss, requiring the fitting of hearing aids in ∼20% of patients.23 The Pro250Arg substitution is the exact equivalent to the Apert Pro253Arg substitution in FGFR2, and also causes ligand-dependent gain-of-function.24 However the reasons for the specific association of this mutation with craniosynostosis are not fully understood.
Crouzon syndrome with acanthosis nigricans is characterised by the Ala391Glu substitution. The acanthosis nigricans, which develops during childhood, is usually not apparent at presentation, so specific testing should be requested in the diagnostic workup of Crouzon syndrome. A positive result should prompt a careful neurosurgical assessment as hydrocephalus is a frequent association.25
TWIST1 (twist homologue 1)
Saethre-Chotzen syndrome is characterised by heterozygous mutations of TWIST1, which encodes a transcription factor belonging to the basic helix-loop-helix family (BHLH motif, Figure 2c). The facial appearance is fairly characteristic, but experience is required in its recognition (Figure 1p). Affected individuals usually present with unicoronal or bicoronal synostosis, but non-penetrance for craniosynostosis occurs. Abnormal extremities (broad, laterally deviated first digits, 2/3 cutaneous syndactyly and brachydactyly) are variable and only occasionally diagnostic. Saethre-Chotzen syndrome results from haploinsufficiency of TWIST1, which may be the consequence of many different mutations, including whole gene deletions, intragenic nonsense and frameshifting mutations, and missense substitutions; note that the last category is largely confined to the highly conserved BHLH motif required for DNA binding and dimerisation (Figure 2c) (ref. 26). No genotype–phenotype correlation is described, except that large deletions are associated with learning disability (not generally a feature of Saethre-Chotzen syndrome),26, 27 and occasional missense mutations in the C-terminal Twist box apparently confer increased risk of a more non-specific synostosis phenotype (with unknown penetrance).28 Rearrangements of a polyglycine-rich tract (amino acids 82–92), which turn up occasionally during mutation screening, seem to be harmless in the heterozygous state.29 Twist1 has a key role in maintaining the boundary between neural crest and cephalic mesoderm at the site of the developing coronal suture.8
EFNB1 (ephrin-B1)
Craniofrontonasal syndrome is an X-linked disorder that presents paradoxically with heterozygous females more severely affected than hemizygous males. Females have severe hypertelorism, unicoronal or bicoronal synostosis, bifid nasal tip, characteristic longitudinal nail splits (Figure 1m and o) and, at lower frequency, sloping shoulders, asymmetric nipples, bifid digits and agenesis of the corpus callosum.30
The identification of causative mutations in EFNB1, which confer loss of function and are correspondingly diverse (whole gene deletions, intragenic nonsense and frameshifting and splice site mutations, and missense substitutions; Figure 2d) (refs. 31,32), has provided an explanation for the paradoxical inheritance pattern. The encoded protein ephrin-B1 (which comprises an EPH domain that interacts with Eph receptor tyrosine kinases, single pass transmembrane (TM) sequence and intracellular PDZ (protein interaction) domain) is involved in cell recognition, so that cells expressing the molecule tend to group together (homophilic sorting). In the female, random X-inactivation generates groups of cells that either express, or do not express ephrin-B1, and the homophilic sorting process increases the patch size of these cells, causing abnormal tissue boundaries (for example, in the coronal sutures and limbs).33 This process has been termed cellular interference.31 In males this cannot occur, and it is presumed that functional redundancy between different ephrin family members mitigates the consequences of complete lack of ephrin-B1.
Other genes
The heterozygous FGFR1 mutation encoding Pro252Arg occurs at the equivalent position in FGFR1 to the Apert (FGFR2) and Muenke (FGFR3) mutations. However it is an unusual cause of Pfeiffer syndrome accounting for fewer than 10% of cases. Syndactyly of the feet, characteristically affecting digits 2–5 with a wide sandal gap with digit 1, seems to be the most characteristic feature.34
Whereas most genetically determined craniosynostosis is characterised by dominant inheritance, Antley-Bixler syndrome (features include disordered sex development and radio-humeral synostosis; mutation in POR encoding cytochrome P450 oxidoreductase)35 and Carpenter syndrome (with polysyndactyly; mutation in RAB23 encoding RAB23, member of RAS oncogene family)36 are recessive disorders. These mutations demonstrate pathogenic roles for steroid metabolism and hedgehog (or possibly Wnt) signalling in cranial suture development, both of which are at present poorly understood.
Chromosome abnormalities
A wide variety of chromosome abnormalities have been associated with craniosynostosis, many in single cases only. The clearest causal link is with deletions of 7p21.1, which includes the TWIST1 gene.26, 27 Other non-random associations that do not show complete penetrance are with deletions of 9p22 and 11q23.3-qter (metopic synostosis).37 Craniosynostosis is recognised as a low frequency association of the 22q11.21 microdeletion. Published studies of the prevalence of submicroscopic chromosomal abnormalities in syndromic craniosynostosis have ranged widely, from 6.7%7 to 28% (ref. 38). The Oxford study7 showed a predominance (85%) of metopic or sagittal synostosis in patients with chromosome abnormalities. Compared with single gene disorders, which often required reoperation (58% of cases), patients with chromosome abnormalities followed a less aggressive course (reoperation rate 17%) suggesting a different, secondary origin of the associated craniosynostosis.
Management
Genetic testing strategy
Although targeted genetic testing is appropriate for patients in whom a specific diagnosis is suspected, the general issue arises whether those with non-syndromic synostosis should be offered genetic testing. In the Oxford study, causative mutations were present in 11% multisuture, 37.5% bicoronal and 17.5% unicoronal synostosis, but were absent in all sagittal, metopic and lambdoid synostosis cases tested. Although the numbers of patients tested in some groups were relatively small, the low success rate of molecular diagnosis in sagittal synostosis has been independently confirmed.39 The numbers of mutations found in the non-syndromic patients were seven in FGFR3, three in the IIIa or IIIc exons of FGFR2 and one in TWIST1. On the basis of these data, we recommend that as a minimum, genetic testing of FGFR3 (for the Pro250Arg mutation) and FGFR2 (mutation hotspots in exons IIIa and IIIc), should be offered for all patients presenting with coronal or multisuture synostosis. The higher genetic load associated with coronal synostosis is likely to reflect the distinct embryological origin of the coronal suture, at the boundary between the developing frontal bone (neural crest origin) and parietal bone (cephalic mesoderm).8, 40
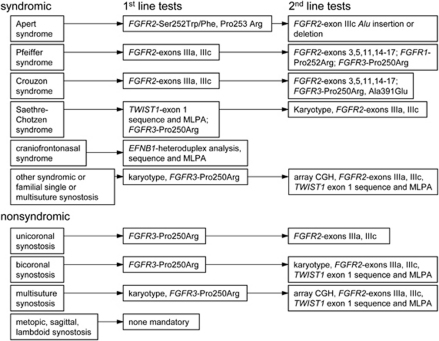
A flow diagram to aid prioritisation of genetic testing is shown in Figure 3.
Figure 3.
Flow diagram for molecular genetic diagnosis of craniosynostosis, showing the minimum tests recommended for each clinical presentation. In practice, the Oxford laboratory bundles sequencing of the FGFR1, FGFR2 (exons IIIa and IIIc), FGFR3 and TWIST1 genes together into a single ‘level 1' screen to simplify the workflow.41 If the suggested tests are negative, the diagnosis should be reviewed.
Genetic counselling/risk assessment
Where no molecular or cytogenetic diagnosis is made and the family history is negative, we suggest a sibling recurrence risk of 2% for sagittal and metopic synostosis, 5% for unicoronal synostosis and 10% for bicoronal and multisuture synostosis. These figures are informed by older epidemiological studies,1, 42, 43, 44 but do involve some guesswork, because these studies included patients in whom mutations could now be identified. Offspring risks are not well documented; they are probably low (∼5%) in the case of non-syndromic sagittal, metopic and unicoronal synostosis, but likely to be substantially higher (30–50%) in bicoronal and possibly multisuture synostosis.
For individuals with identified single gene disorders, the main issue surrounds the recurrence risk that should be given for apparently de novo mutations. In the case of FGF receptor mutations, provided that the blood of both parents is negative for the mutation, the recurrence risk is very low (well under 1%). This is because the vast majority of cases arise from mutations occurring in adult spermatogonia, which although subject to strong positive selection never populate large proportions of the sperm.15, 16 However, a single case of true mosaicism for FGFR2 mutation was recently described.45 At the opposite end of the spectrum, mosaicism was present in 19% of first affected individuals in pedigrees with EFNB1 mutations, so the mutation must be looked for very carefully in the parents (for example, by heteroduplex analysis in addition to DNA sequencing).32 A recurrence risk of 10% for the siblings of sporadic females with CFNS is appropriate. Mosaicism for TWIST1 mutations, although theoretically possible, has not been reported; however, the sample size of de novo TWIST1 mutations is relatively small, so a cautiously low (2%) sibling recurrence risk is appropriate.
Prenatal diagnosis
Where a molecular abnormality has been identified in the family, the issues regarding prenatal or preimplantation diagnosis do not differ substantially from those in other genetic disorders. More problematic is the question whether it is feasible to diagnose craniosynostosis prenatally by ultrasound or other imaging techniques. Cranial sutures form very late (∼16 weeks gestation)46 compared with most other embryonic structures, therefore at the time when most routine ultrasound diagnosis is being undertaken (∼20 weeks), insufficient time has elapsed for growth distortion of the skull to have occurred, except in the most severe cases. Although there have been a few reports of ultrasound diagnosis of craniosynostosis around this time,47 the majority of even syndromic craniosynostosis is not detected during the pregnancy. When the issue is to screen for recurrence of craniosynostosis present in an older sibling, evidence of Apert syndrome can be sought by careful examination of the limbs for syndactyly. In most other situations, a late ultrasound is recommended to check for cephalopelvic disproportion, but caution should be exercised in providing any confident opinion in the prenatal period that craniosynostosis, except for the most severe forms, has been excluded.
Treatment and care
Acute management
Care of neonates and infants with severe multisuture synostosis is directed towards maintenance of the airway, support of feeding, eye protection, and treatment of raised ICP. Respiratory difficulty may require urgent sleep study assessment by specialist paediatric respiratory physicians and ear, nose and throat (ENT) surgeons, necessitating either continuous positive airway pressure support, nasal stenting, tonsillectomy/adenoidectomy, choanal dilatation, early midface advancement or tracheostomy, depending on the anatomical cause. Exposure keratitis occurs if the eyelids are unable to close and an urgent ophthalmology opinion should be sought, with consideration given to tarsorraphies or early midface advancement to provide eye protection.
Raised ICP associated with craniosynostosis has several different causes, which require different treatments. Hydrocephalus needs a shunt; obstructive sleep apnoea is treated by improving the airway (see above). Craniocerebral disproportion (the consequence of craniostenosis) requires calvarial expansion. Foramen magnum decompression might be required if there is tonsillar herniation. In multisuture synostosis, abnormal venous drainage poses a significant hazard when operating on the back of the skull (Figure 1t).
Elective management
Elective surgical management of craniosynostosis has three major objectives, which are to correct the skull deformity, prevent its progression and reduce the future risk of raised ICP. At the Oxford unit, the majority (∼60%) of primary surgical procedures are performed between the ages of 6 months and 2 years. Attention must be given to secondary sensory deficits, for example, resulting from ptosis (Saethre-Chotzen syndrome), strabismus (unicoronal synostosis and craniofrontonasal syndrome), hearing loss (conductive in the case of FGFR2 mutations and sensorineural in the case of Muenke syndrome) and dental malocclusion (especially FGFR2 mutations). Associated malformations, such as syndactyly and cleft palate (Apert syndrome) also require surgery.
Regular follow-up throughout childhood is advisable, particularly to monitor for symptoms of raised ICP, such as headaches, behaviour change, or decline in school performance. Information on the presence of specific mutations is of prognostic value: for example, coronal synostosis has a worse prognosis (higher risk of repeat surgery and persistent deformity) in the presence of the Muenke syndrome mutation;48, 49 subjects with TWIST1 mutations are at higher risk for secondary development of raised ICP;50 large TWIST1 deletions are associated with a higher risk of learning disability.26, 27
Conclusions
Genetic workup should be an integral part of the management of craniosynostosis as it contributes both to risk assessment for the family and prognostic information for the patient. Although the molecular bases of the common craniosynostosis syndromes have been defined, it is likely that a single molecular aetiology remains to be identified in a further 10–15% of individuals. Whole genome assessment of copy number changes and DNA sequencing are likely to identify further predisposing loci, and consideration should be given to other mechanisms of disease such as mosaicism and imprinting defects. Although surgery is expected to remain the mainstay of management for the foreseeable future, the identification of signalling pathways pathologically activated in the cranial suture (such as the RAS-ERK pathway activated by Apert syndrome mutations)51 raises the possibility of the use of adjuvant medical therapies in the future.
Acknowledgments
Research into craniosynostosis in AOMW's laboratory has been supported by the Wellcome Trust, Medical Research Council, EPA Cephalosporin Fund and Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health. We thank all members of the craniofacial unit in Oxford for supporting the work on which this article is based.
The authors declare no conflict of interest.
References
- Lajeunie E, Le Merrer M, Bonaïti-Pellie C, Marchac D, Renier D. Genetic study of nonsyndromic coronal craniosynostosis. Am J Med Genet. 1995;55:500–504. doi: 10.1002/ajmg.1320550422. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Rasmussen SA, Honein MA. A population-based study of craniosynostosis in metropolitan Atlanta, 1989–2003. Am J Med Genet. 2008;146A:984–991. doi: 10.1002/ajmg.a.32208. [DOI] [PubMed] [Google Scholar]
- Lajeunie E, Crimmins DW, Arnaud E, Renier D. Genetic considerations in nonsyndromic midline craniosynostoses: a study of twins and their families. J Neurosurg (Pediatrics 4) 2005;103:353–356. doi: 10.3171/ped.2005.103.4.0353. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lara PA, Carmichael SL, Graham JM, Jr, et al. Fetal constraint as a potential risk factor for craniosynostosis. Am J Med Genet. 2010;152A:394–400. doi: 10.1002/ajmg.a.33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LL. Roentgenological skull measurements and their diagnostic applications. Am J Roentgenol Radium Ther Nucl Med. 1952;67:197–209. [PubMed] [Google Scholar]
- Kirmi O, Lo SJ, Johnson D, Anslow P. Craniosynostosis: a radiological and surgical perspective. Semin Ultrasound CT MR. 2009;30:492–512. doi: 10.1053/j.sult.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Wilkie AOM, Byren JC, Hurst JA, et al. Prevalence and complications of single gene and chromosomal disorders in craniosynostosis. Pediatrics. 2010;126:e391–e400. doi: 10.1542/peds.2009-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AE, Bochukova EG, Brugger SM, et al. Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Hum Mol Genet. 2006;15:1319–1328. doi: 10.1093/hmg/ddl052. [DOI] [PubMed] [Google Scholar]
- Jabs EW, Müller U, Li X, et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993;75:443–450. doi: 10.1016/0092-8674(93)90379-5. [DOI] [PubMed] [Google Scholar]
- Kariminejad A, Kariminejad R, Tzschach A, et al. Craniosynostosis in a patient with 2q37.3 deletion 5q34 duplication: association of extra copy of MSX2 with craniosynostosis. Am J Med Genet A. 2009;149A:1544–1549. doi: 10.1002/ajmg.a.32949. [DOI] [PubMed] [Google Scholar]
- Iseki S, Wilkie AOM, Morriss-Kay GM. Fgfr1 and Fgfr2 have distinct differentiation and proliferation-related roles in the developing mouse skull vault. Development. 1999;126:5611–5620. doi: 10.1242/dev.126.24.5611. [DOI] [PubMed] [Google Scholar]
- Holmes G, Rothschild G, Roy UB, Deng C-X, Mansukhani A, Basilico C. Early onset of craniosynostosis in an Apert mouse model reveals critical features of this pathology. Dev Biol. 2009;328:273–284. doi: 10.1016/j.ydbio.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaney SF, Oldridge M, Hurst JA, et al. Differential effects of FGFR2 mutations on syndactyly and cleft palate in Apert syndrome. Am J Hum Genet. 1996;58:923–932. [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi OA, Chiu ES, McCarthy JG, Mohammadi M. Understanding the molecular basis of Apert syndrome. Plast Reconstr Surg. 2005;115:264–270. [PubMed] [Google Scholar]
- Goriely A, McVean GAT, van Pelt AMM, et al. Gain-of-function amino acid substitutions drive positive selection of FGFR2 mutations in human spermatogonia. Proc Natl Acad Sci USA. 2005;102:6051–6056. doi: 10.1073/pnas.0500267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely A, Hansen RMS, Taylor IB, et al. Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat Genet. 2009;41:1247–1252. doi: 10.1038/ng.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunie E, Heuertz S, El Ghouzzi V, et al. Mutation screening in patients with syndromic craniosynostoses indicates that a limited number of recurrent FGFR2 mutations accounts for severe forms of Pfeiffer syndrome. Eur J Hum Genet. 2006;14:289–298. doi: 10.1038/sj.ejhg.5201558. [DOI] [PubMed] [Google Scholar]
- Hoefkens MF, Vermeij-Keers C, Vaandrager JM. Crouzon syndrome: phenotypic signs and symptoms of the postnatally expressed subtype. J Craniofac Surg. 2004;15:233–240. doi: 10.1097/00001665-200403000-00013. [DOI] [PubMed] [Google Scholar]
- Connolly JP, Gruss J, Seto ML, et al. Progressive postnatal craniosynostosis and increased intracranial pressure. Plast Reconstr Surg. 2004;113:1313–1323. doi: 10.1097/01.prs.0000111593.96440.30. [DOI] [PubMed] [Google Scholar]
- Kan S-H, Elanko N, Johnson D, et al. Genomic screening of fibroblast growth-factor receptor 2 reveals a wide spectrum of mutations in patients with syndromic craniosynostosis. Am J Hum Genet. 2002;70:472–486. doi: 10.1086/338758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochukova EG, Roscioli T, Hedges DJ, et al. Rare mutations of FGFR2 causing Apert syndrome: identification of the first partial gene deletion, and an Alu element insertion from a new subfamily. Hum Mutat. 2009;30:204–211. doi: 10.1002/humu.20825. [DOI] [PubMed] [Google Scholar]
- Muenke M, Gripp KW, McDonald-McGinn DM, et al. A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am J Hum Genet. 1997;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- Mansour SL, Twigg SRF, Freeland RM, Wall SA, Li C, Wilkie AOM. Hearing loss in a mouse model of Muenke syndrome. Hum Mol Genet. 2009;18:43–50. doi: 10.1093/hmg/ddn311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi OA, Zhang F, Eliseenkova AV, Linhardt RJ, Mohammadi M. Proline to arginine mutations in FGF receptors 1 and 3 result in Pfeiffer and Muenke craniosynostosis syndromes through enhancement of FGF binding affinity. Hum Mol Genet. 2004;13:69–78. doi: 10.1093/hmg/ddh011. [DOI] [PubMed] [Google Scholar]
- Schweitzer DN, Graham JM, Jr, Lachman RS, et al. Subtle radiographic findings of achondroplasia in patients with Crouzon syndrome with acanthosis nigricans due to an Ala391Glu substitution in FGFR3. Am J Med Genet. 2001;98:75–91. [PubMed] [Google Scholar]
- Kress W, Schropp C, Lieb G, et al. Saethre-Chotzen syndrome caused by TWIST 1 gene mutations: functional differentiation from Muenke coronal synostosis syndrome. Eur J Hum Genet. 2006;14:39–48. doi: 10.1038/sj.ejhg.5201507. [DOI] [PubMed] [Google Scholar]
- Johnson D, Horsley SW, Moloney DM, et al. A comprehensive screen for TWIST mutations in patients with craniosynostosis identifies a new microdeletion syndrome of chromosome band 7p21.1. Am J Hum Genet. 1998;63:1282–1293. doi: 10.1086/302122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto ML, Hing AV, Chang J, et al. Isolated sagittal and coronal craniosynostosis associated with TWIST box mutations. Am J Med Genet. 2007;143A:678–686. doi: 10.1002/ajmg.a.31630. [DOI] [PubMed] [Google Scholar]
- Elanko N, Sibbring JS, Metcalfe KA, et al. A survey of TWIST for mutations in craniosynostosis reveals a variable length polyglycine tract in asymptomatic individuals. Hum Mutat. 2001;18:535–541. doi: 10.1002/humu.1230. [DOI] [PubMed] [Google Scholar]
- Grutzner E, Gorlin RJ. Craniofrontonasal dysplasia: phenotypic expression in females and males and genetic considerations. Oral Surg Oral Med Oral Pathol. 1988;65:436–444. doi: 10.1016/0030-4220(88)90358-1. [DOI] [PubMed] [Google Scholar]
- Wieacker P, Wieland I. Clinical and genetic aspects of craniofrontonasal syndrome: towards resolving a genetic paradox. Mol Genet Metab. 2005;86:110–116. doi: 10.1016/j.ymgme.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Twigg SRF, Matsumoto K, Kidd AMJ, et al. The origin of EFNB1 mutations in craniofrontonasal syndrome: frequent somatic mosaicism and explanation of the paucity of carrier males. Am J Hum Genet. 2006;78:999–1010. doi: 10.1086/504440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Rossi M, Jones RL, Norbury G, Bloch-Zupan A, Winter RM. The appearance of the feet in Pfeiffer syndrome caused by FGFR1 P252R mutation. Clin Dysmorphol. 2003;12:269–274. doi: 10.1097/00019605-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Huang N, Pandey AV, Agrawal V, et al. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76:729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D, Seelow D, Jehee FS, et al. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehee FS, Johnson D, Alonso LG, et al. Molecular screening for microdeletions at 9p22-p24 and 11q23-q24 in a large cohort of patients with trigonocephaly. Clin Genet. 2005;67:503–510. doi: 10.1111/j.1399-0004.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- Jehee FS, Krepischi-Santos ACV, Rocha KM, et al. High frequency of submicroscopic chromosomal imbalances in patients with syndromic craniosynostosis detected by a combined approach of microsatellite segregation analysis, multiplex ligation-dependent probe amplification and array-based comparative genome hybridisation. J Med Genet. 2008;45:447–450. doi: 10.1136/jmg.2007.057042. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA. International craniosynostosis consortium: genetic analysis of non-syndromic craniosynostosis. Orthod Craniofacial Res. 2007;10:129–137. doi: 10.1111/j.1601-6343.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Wilkie AOM. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie AOM, Bochukova EG, Hansen RM, et al. Clinical dividends from the molecular genetic diagnosis of craniosynostosis. Am J Med Genet. 2007;143A:1941–1949. doi: 10.1002/ajmg.a.31905. [DOI] [PubMed] [Google Scholar]
- Carter CO, Till K, Fraser V, Coffey A family study of craniosynostosis, with probable recognition of a distinct syndrome. J Med Genet. 1982;19:280–285. doi: 10.1136/jmg.19.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunie E, Le Merrer M, Bonaïti-Pellie C, Marchac D, Renier D. Genetic study of scaphocephaly. Am J Med Genet. 1996;62:282–285. doi: 10.1002/(SICI)1096-8628(19960329)62:3<282::AID-AJMG15>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lajeunie E, Le Merrer M, Marchac D, Renier D. Syndromal and nonsyndromal primary trigonocephaly: analysis of a series of 237 patients. Am J Med Genet. 1998;75:211–215. doi: 10.1002/(sici)1096-8628(19980113)75:2<211::aid-ajmg19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Goriely A, Lord H, Lim J, et al. Germline and somatic mosaicism for FGFR2 mutation in the mother of a cild with Crouzon syndrome: Implications for genetic testing in ‘paternal age-effect' syndromes. Am J Med Genet. 2010;152A:2067–2073. doi: 10.1002/ajmg.a.33513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathijssen IMJ, van Splunder J, Vermeij-Keers C, et al. Tracing craniosynostosis to its developmental stage through bone center displacement. J Craniofac Genet Dev Biol. 1999;19:57–63. [PubMed] [Google Scholar]
- David AL, Turnbull C, Scott R, et al. Diagnosis of Apert syndrome in the second-trimester using 2D and 3D ultrasound. Prenat Diagn. 2007;27:629–632. doi: 10.1002/pd.1758. [DOI] [PubMed] [Google Scholar]
- Thomas GPL, Wilkie AOM, Richards PG, Wall SA. FGFR3 P250R mutation increases the risk of reoperation in apparent ‘nonsyndromic' coronal craniosynostosis. J Craniofac Surg. 2005;16:347–352. doi: 10.1097/01.scs.0000157024.56055.f2. [DOI] [PubMed] [Google Scholar]
- Arnaud E, Meneses P, Lajeunie E, Thorne JA, Marchac D, Renier D. Postoperative mental and morphological outcome for nonsyndromic brachycephaly. Plast Reconstr Surg. 2002;110:6–12. doi: 10.1097/00006534-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Woods RH, Ul-Haq E, Wilkie AOM, et al. Reoperation for intracranial hypertension in TWIST1-confirmed Saethre-Chotzen syndrome: a 15-year review. Plast Reconstr Surg. 2009;123:1801–1810. doi: 10.1097/PRS.0b013e3181a3f391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V, Coumoul X, Wang R-H, Kim H-S, Deng C-X. RNA interference and inhibition of MEK-ERK signaling prevent abnormal skeletal phenotypes in a mouse model of craniosynostosis. Nat Genet. 2007;39:1145–1150. doi: 10.1038/ng2096. [DOI] [PubMed] [Google Scholar]
Further Reading
- Jabs EW.TWIST1 and the Saethre-Chotzen syndromein Epstein CJ, Erickson RP, Wynshaw-Boris A (eds): Inborn Errors of Development Oxford: Oxford University Press; 2008474–481. [Google Scholar]
- Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- Passos-Bueno MR, Sertié AL, Jehee FS, Fanganiello R. Genetics of craniosynostosis: genes, syndromes, mutations and genotype-phenotype correlations. Front Oral Biol. 2007;12:107–143. doi: 10.1159/000115035. [DOI] [PubMed] [Google Scholar]
- Twigg SRF, Wilkie AOM.EFNB1 and EFNA4 in craniofrontonasal syndrome and craniosynostosisin Epstein CJ, Erickson RP, Wynshaw-Boris A (eds): Inborn Errors of Development Oxford: Oxford University Press; 20081476–1482. [Google Scholar]
- Webster MK, Donoghue DJ. FGFR activation in skeletal disorders: too much of a good thing. Trends Genet. 1997;13:178–182. doi: 10.1016/s0168-9525(97)01131-1. [DOI] [PubMed] [Google Scholar]
- Wilkie AOM. Bad bones, absent smell, selfish testes: the pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev. 2005;16:187–203. doi: 10.1016/j.cytogfr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wilkie AOM.FGF receptor mutations: bone dysplasia, craniosynostosis, and other syndromesin Epstein CJ, Erickson RP, Wynshaw-Boris A (eds): Inborn Errors of Development Oxford: Oxford University Press; 2008461–470. [Google Scholar]
- Yu JE, Jeong SY, Yang JA, Park MS, Kim HJ, Yoon SH. Genotypic and phenotypic analyses of Korean patients with syndromic craniosynostosis. Clin Genet. 2009;76:287–291. doi: 10.1111/j.1399-0004.2009.01201.x. [DOI] [PubMed] [Google Scholar]