Abstract
In the past decade, the zebrafish (Danio rerio) has become a popular model system for the study of vertebrate development, since the embryos and larvae of this species are small, transparent and undergo rapid development ex utero, allowing in vivo analysis of embryogenesis and organogenesis. These characteristics can also be exploited by researchers interested in signaling pathways and disease processes and, accordingly, there is a growing literature on the use of zebrafish to model human disease. This model holds great potential for exploring how autophagy, an evolutionarily conserved mechanism for protein degradation, influences the pathogeneses of a range of different human diseases and for the evaluation of this pathway as a potential therapeutic strategy. Here we summarize what is known about the regulation of autophagy in eukaryotic cells and its role in neurodegenerative disease and highlight how research using zebrafish has helped further our understanding of these processes.
Keywords: Zebrafish, Autophagy, Neurodegeneration
Research highlights
► Zebrafish has become a popular model system for the study of vertebrate development. ► There is a growing literature on the use of zebrafish to model human disease. ► This model has potential for exploring how autophagy affects disease pathogenesis. ► This review considers how zebrafish may help further understanding of these processes.
1. Introduction
1.1. Protein degradation pathways
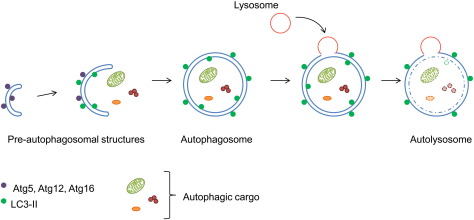
Efficient degradation of proteins is essential to maintain normal cell homeostasis. In eukaryotes, there are two main degradative pathways; the ubiquitin-proteasome pathway and the autophagy-lysosome pathway. The proteasome is a barrel-shaped multi-subunit protein complex, the core of which contains the components necessary for proteolysis. Proteins are generally targeted to the proteasome after they are tagged by a chain of four or more covalently bonded ubiquitin molecules. In addition to the specificity imposed by the requirement of an ubiquitination signal, the narrow core of the proteasome barrel limits the size of proteins that can be degraded via this pathway. Typically, short-lived and long-lived cytosolic and nuclear proteins are degraded by the proteasome. In contrast, macroautophagy (from hereon referred to as autophagy) can mediate non-specific, bulk degradation of long-lived cytosolic proteins and organelles. Autophagic degradation requires the formation of a double-membraned vesicle, the autophagosome, around a portion of the cytoplasm. Ultimately, autophagosomes fuse with lysosomes to form autolysosomes, acidic compartments in which lyosomal hydrolases degrade any proteins contained within the vesicle (see Fig. 1). Autophagy occurs at a basal level in mammalian cells, but is upregulated in response to various physiological stress conditions e.g. starvation. While it is primarily a mechanism to ensure cell survival, there is increasing evidence for the importance of autophagy as a mechanism for cell death, particularly in insect metamorphosis [1].
Fig. 1.
Schematic model of autophagy. Pre-autophagosomal structures form within the cytoplasm. Atg5, Atg12 and Atg16l proteins are recruited to the structure and facilitate elongation. The elongated membranes enwrap a region of the cytoplasm and its contents in a double-membraned autophagosome. Lysosomes ultimately fuse with autophagosomes releasing lysosomal hydrolases into the vesicle resulting in the degradation of its contents.
1.2. Zebrafish models of neurodegeneration
The optical clarity, speed of development, and fecundity of zebrafish have made them a popular vertebrate model for the study of developmental biology, and, more recently, as an animal model to study disease processes [2,3]. The creation of transgenic zebrafish is relatively straightforward [4–7], and has been used to successfully generate models of a range of human neurodegenerative disorders. Diseases caused by dominant mutations can be modeled by expressing the mutated human gene under the control of a zebrafish promoter. Such an approach has been used to model polyglutamine expansion diseases, like Huntington's disease [8], tauopathy [9–11] and amyotrophic lateral sclerosis (ALS) [12]. Furthermore, since zebrafish larvae are transparent, fluorescent transgene constructs can be used in a variety of ways to examine disease pathogenesis, in vivo. For example, reporter lines where particular neurons are fluorescently labeled have been used to investigate the sensitivity of monoaminergic neurons to the neurotoxin MPTP [13]. Bi-directional transgenic constructs have been used to create lines in which a fluorescent protein signal is expressed with the same spatial and temporal control as the disease-causing protein [11]. Similarly, direct fusion of a fluorescent protein to the disease-causing transgene has been employed as a read-out of transgene expression, but can also be used as a marker for protein aggregation. Such an approach has been used to examine huntingtin aggregate clearance in vivo, as a method for validating novel therapeutic strategies [8]. In addition to the creation of stable transgenic lines to model dominant genetic mutations, transient over-expression techniques have been used to this end. In such studies, injection of DNA or mRNA into fertilized eggs results in transient expression of the disease-causing protein during embryogenesis and in early larval stages [14–18]. Although there is an inherent level of variability in gene expression, this method has proved powerful for the study of disease modifiers in models of polyglutamine disease [14] and motor neuron disease [16–19] and in the evaluation of therapeutic strategies [15].
Loss-of-function models of neurodegeneration have also been widely explored in zebrafish (see [20] for review). The most widely used technique for the study of loss-of-function is that of transient knockdown using antisense technologies. Morpholino oligonucleotides are the most commonly used, validated and accepted antisense technique in zebrafish [21], although other antisense technologies, such as peptide nucleic acid mimics (gripNAs) [22], are now gaining popularity. Recently, spatial and temporal control of morpholino knockdown has been described, by combining a neutralizing strand with the morpholino oligonucleotide that is photocleavable by irradiation with UV light [23]. Transient knockdown techniques have been used to develop zebrafish models of Parkinson's disease [24–29], ALS [30] and spinal muscular atrophy (SMA) [31–33]. In addition to their use for developing disease models for loss-of function disorders, antisense knockdown technologies have been used to investigate normal gene function and elucidate novel signaling pathways in a range of neurodegenerative disorders including Huntington's disease [34–37], Alzheimer's disease [38–41] and SMA [32,42–44] and to investigate the role of progranulin and TDP-43 in the pathogenesis of ALS and frontotemporal lobe dementia [17–19,45].
The limitation of such antisense techniques is that knockdown is transient, usually only lasting to 5–7 d.p.f. Therefore efforts have focused on the development of methods for targeted gene knockdown in zebrafish [46–48], with the recent reports of zinc finger nuclease (ZFN) technology holding promise for the widescale and specific generation of heritable loss-of-function alleles [49–51]. In addition, random mutagenesis screens [52,53] have yielded numerous mutant zebrafish lines in which the mutated gene is implicated in a human disease, e.g. muscular dystrophy [54–57], and it is hoped that the continuing screening of mutagenized libraries (e.g. TILLING—Targeting Induced Local Lesions in Genomes) will yield mutations in specific genes of relevance to neurodegenerative disorders.
In addition to the ease of genetic manipulation, zebrafish are also highly amenable to pharmacological manipulation [58]. This allows up and down-regulation of cell signaling pathways by chemical agonists and antagonists, in addition to the use of zebrafish disease models to test therapeutic strategies [8,11] and perform compound screens [15]. The zebrafish offers advantages over equivalent rodent models, since the manifestation of disease phenotypes is typically more rapid than in their rodent equivalent, larvae can be arrayed in multi-well plates, and compound requirement is small. Since zebrafish are highly amenable to both genetic modification [59,60] and direct compound screening in a tractable fashion [61], they are potentially a powerful tool for the investigation of the autophagy pathway and its role in neurodegeneration. The remainder of this article is dedicated to the application of such zebrafish models to the study of autophagy.
1.3. The molecular control of autophagy in eukaryotes
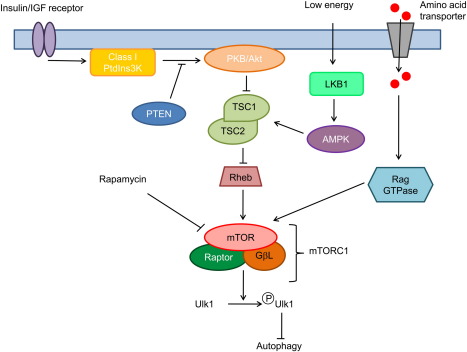
Under normal conditions, autophagy occurs at basal levels, but can be induced rapidly in response to stress conditions and extracellular signals. Target of rapamycin (TOR), a serine/threonine protein kinase, is a central component controlling autophagy, integrating signals from multiple upstream pathways and inhibiting autophagy (see Fig. 2). The regulatory pathways controlling autophagy are well described elsewhere [62], hence this review focuses on aspects of the pathway where the zebrafish model has or could be employed to further our understanding of this process.
Fig. 2.
Simplified schematic of the regulatory pathways controlling autophagy. Additional regulatory pathways exist in mammalian cells (reviewed in [62,73]) but have yet to be investigated in zebrafish.
mTOR forms two distinct complexes (mTORC1 and mTORC2), which vary both in their subunit components and their function. The mTORC1 complex consists of 3 subunits: mTOR, G protein β-subunit-like (mSLT8) and the regulator-associated protein mTOR (Raptor). Under normal conditions, the mTORC1 complex blocks autophagy by phosphorylating Ulk1 [63–65], but this inhibitory activity is repressed by rapamycin treatment (a specific TOR inhibitor) or starvation conditions, leading to an upregulation of autophagy. mTORC1 is itself inhibited by the action of the tuberous sclerosis complex 1 and 2 proteins (TSC1 and TSC2), which together form a complex (TSC1/2) (Fig. 2). The mTORC2 complex consists of 4 subunits: mTOR, mSLT8, Rictor (rapamycin-insensitive companion of mTOR) and mSin1 (mitogen-activated-protein-kinase-associated protein 1). The mTORC2 complex regulates actin cytoskeleton dynamics and is not involved directly in the regulation of autophagy [66].
The conservation of TOR signaling pathway has been explored in zebrafish using both pharmacological manipulation and morpholino gene knockdown [67]. Zebrafish have a single homologue of mTOR which, although expressed ubiquitously during early embryogenesis, becomes localized to the head and developing gut between 35 and 57 hours post-fertilization (h.p.f.). Treatment of zebrafish with rapamycin during early embryogenesis resulted in developmental delay, but did not cause any overt defects. However, longer treatment resulted in specific defects in the growth and morphogenesis of the zebrafish gut. Morpholino knockdown of zebrafish mTOR, raptor and S6 kinase (an mTOR effector that regulates translation but not autophagy) resulted in defects in the development of the digestive tract that phenocopy those observed with rapamycin treatment, whereas knockdown of rictor had only minimal effects in gut growth and morphogenesis [67]. This study demonstrates a critical role for TOR via the TORC1 complex in vertebrate intestinal development. Importantly, since TOR regulates many processes besides autophagy, many of these consequences of mTOR inhibition may be autophagy-independent. The equivalent studies have not been performed in mouse embryos, since knockdown of mTOR results in embryonic lethality [68]. This highlights the advantages that the zebrafish offers over the mouse in the study of gene knockdown effects, as embryonic events can be more readily visualized. However, a recent study using morpholino knockdown of the autophagy-related gene gabarap reported microcephaly and jaw defects in zebrafish morphants [69], whereas gabarap knockout mice are phenotypically normal [70], suggesting that further studies are needed to determine whether the roles of autophagy in mammalian development are conserved in non-mammalian vertebrates. Many more components of the autophagy pathway and aspects of TOR-independent autophagy regulation remain unexplored in zebrafish. A summary of the zebrafish homologs of selected mammalian components of the autophagy regulatory pathway are listed in Table 1. The ability to perform gene knockdown and to temporally control signaling pathways using pharmacological inhibitors, as described above, highlight the potential of this model for the investigation of autophagy regulation.
Table 1.
Zebrafish homologs of key components of the autophagy pathway.
| Mammalian gene | Zebrafish homolog(s) | Accession number(s) | RefSeq status | Notes |
|---|---|---|---|---|
| Akt/Protein kinase B (Akt1) | No sequence homologs for Akt1 identified in Genbank | |||
| AMBRA1 | No sequence homologs identified in Genbank | |||
| ATG10 | atg10 | NM_001037124 | Provisional | |
| ATG12 | atg12 | XM_694510 | Model | Predicted homolog. |
| ATG16L1 | atg16l1 | NM_001017854 | Provisional | |
| ATG3 | apg3l, autophagy 3-like | NM_200022 | Provisional | |
| ATG4A | atg4a | NM_001024434 | Provisional | |
| ATG4B | atg4b | NM_001089352 | Provisional | |
| ATG4C | atg4c | NM_001002103 | Provisional | |
| ATG4D | LOC795933 autophagy-related 4D-like | XM_001333057 | Model | Predicted homolog. |
| ATG5 | atg5 | Isoform 1: NM_001009914 | Provisional | |
| Isoform 2: NM_205618 | Provisional | |||
| ATG7 | atg7 | XM_002663680 | Model | Predicted homolog; partial mRNA sequence. |
| ATG9A | atg9a | NM_001083031 | Provisional | |
| ATG9B | atg9b | NM_001080705 | Provisional | |
| Bcl2 | bcl2 | NM_001030253 | Provisional | |
| Beclin1 | beclin1 | NM_200872 | Provisional | |
| Gabarap | gabarap | NM_001013260 | Validated | |
| GβL/MLST8 | mlst8 | NM_199877 | Provisional | |
| MAP1LC3A | map1lc3a | NM_214739 | Provisional | |
| MAP1LC3B | map1lc3b map1lc3b-like | NM_199604XM_002664472 | Provisional Model | Possible gene duplication in zebrafish. |
| MAP1LC3C | zgc:56565 | NM_200298 | Provisional | |
| PTEN | ptena | NM_200708 | Provisional | ptena and ptenb encode functional enzymes with spatially distinct expression patterns [86,87]. |
| ptenb | NM_001001822 | Provisional | ||
| Raptor | Rptor (raptor-like) | XM_002662358 | Model | Predicted homolog. |
| Rheb | Rheb | NM_001076748 | Validated | |
| Rictor | Rictor | XM_001921872 | Model | Predicted homolog. Homolog previously reported in [67] (XM_685234) has now been removed from Genbank. |
| SQSTM1 | sqstm1 | XM_002662358 | Provisional | |
| TOR | mTOR | NM_001077211 | Provisional | |
| TSC1 | tsc1a | NM_200052 | Provisional | TSC1b reported in [88] now annotated as non-coding RNA. |
| (tsc1b) | (NR_023332) | Provisional | ||
| TSC2 | tsc2 | XM_690820 | Model | Predicted homolog. |
| Ulk1 | Ulk1b | XM_002665925 | Model | Chromosome 21 (predicted homolog). Two zebrafish homologs (Ulk1a and Ulk1b) reported in [72], based on Blast searches but no accession numbers published. |
| Ulk2 | No sequence homologs identified in Genbank | |||
| UVRAG | uvrag | NM_201069 | Provisional | |
| VPS45 | vps45 | XM_002665582 | Model | |
| WIPI1 | wipi1 | NM_200391 | Provisional |
Zebrafish homologs of mammalian genes were identified using NCBI Entrez Nucleotide and NCBI Entrez Gene search engines [89,90]. Due to the incomplete nature of these databases, zebrafish homologs for some mammalian genes do not have entries (e.g. AMBRA, Ulk2). However, search tools such as BLAST can be used to identify zebrafish homolog(s). RefSeq status is a useful indicator of the confidence that the homolog has been correctly assigned [91]. Searches correct as of 30th October 2010.
1.4. Tools for assessing autophagy in zebrafish
In addition to understanding the molecular control of autophagy and its conservation between zebrafish and mammals, it is important to examine the onset of expression of the pathway components. The formation of autophagosomes is assessed by the conversion of LC3-I to LC3-II, since LC3-II is specifically associated with autophagosomes. In contrast to the mouse embryo, where LC3-II is observed in oocytes [71], He et al. demonstrated that zebrafish LC3-II was only detectable from 32 h.p.f. onwards. Although RT-PCR analysis demonstrated the presence of transcripts of the autophagy genes beclin and lc3 at 0 h.p.f., ulk1a and ulk1b (identified as putative zebrafish homologs of the single mammalian ulk1 autophagy gene) and atg9a and atg9b were not expressed until at least 23 h.p.f. [72]. The later expression of these autophagy genes may explain why autophagosome formation (as measured by LC3-II) is delayed in the zebrafish embryo relative to mammalian embryos, and also raises questions about the importance of autophagy in early embryogenesis in this organism.
Gene knockdown studies could be of great value in dissecting the roles of individual pathway components (listed in Table 1) in the regulation of autophagy. This approach has been widely adopted in vitro, using siRNA and shRNA (comprehensively reviewed in [73]). However, little work has been performed to confirm these findings in vivo. Here, gene knockdown in zebrafish may offer advantages over knockout mouse studies, which are, by comparison, lengthy and costly. Such an approach was recently employed by Dowling et al. [74] investigating the work of myotubularins in the regulation of autophagy and in the pathogenesis of centronuclear myopathy. Myotubularins (MTM) and myotubularin-related proteins (MTMR) are family of phosphatases that dephosphorylate phosphinositides. Using siRNA, MTMR14 (also called Jumpy) was previously demonstrated to act as a suppressor of autophagy in vitro, since knockdown resulted in an increase in autophagosome formation [75]. In zebrafish, knockdown of MTMR14 was shown to cause a similar increase in autophagy (as measured by LC3-II levels) and double knockdown studies with MTM1 resulted in a phenotype reminiscent of human centronuclear myopathy [74].
Another valuable tool in the study of autophagy is the measurement of LC3-II levels. Western blotting to detect LC3-II can be used to determine the number of autophagosomes and to measure changes in autophagic flux. Using such an approach, He et al. [72] demonstrated that rapamycin increases autophagosome synthesis in larval zebrafish, compatible with its effects in other organisms. In addition, the number of autophagosomes (LC3-II levels) in zebrafish can be enhanced by treatment with lysosomal inhibitors such as pepstatinA, E64d [72], or ammonium chloride [76]. These agents reduce the acidity of the lysosome and thereby decrease autophagosome/LC3 degradation. Measuring LC3-II levels in the presence or absence of lysosomal inhibitors provides a useful tool for measuring autophagic flux in cells [77] and has recently been applied to in vivo investigations in zebrafish to assess the effects of antioxidants on autophagy [76]. To further study the process of autophagy in zebrafish, He et al. [72] generated transgenic reporter lines expressing GFP-tagged lc3 and Gabarap, and demonstrated that distribution of the fluorescently tagged proteins changed appropriately following treatment with a variety of known autophagy inducing and inhibiting agents. These lines will be of value in future studies for the validating the mechanism of action of compounds and could be used in combination with the disease models described elsewhere in this issue to evaluate the role of autophagy in the pathogenesis of neurodegeneration.
1.5. Autophagy as a therapeutic strategy for neurodegenerative diseases
A common feature of many late-onset neurodegenerative disorders, including Parkinson's disease, Alzheimer's disease, Huntington's disease, tauopathies, and various spinocerebellar ataxias is the accumulation of misfolded or aggregating proteins within the cell. Under normal conditions, the basal rate of autophagy is not sufficient to prevent the accumulation of cytoplasmic aggregate-prone proteins aggregates over many years. However, induction of autophagy by treatment with rapamycin has proven effective in enhancing the clearance of aggregate-prone proteins in vitro [78–80] and in vivo, in Drosophila models of Huntington's disease and tauopathy [79,80] and mouse models of Huntington's disease and spinocerebellar ataxia type 3 [79,81]. These studies provide proof-of-principle that upregulation of autophagy may be an effective therapeutic strategy for the clearance of aggregate-prone proteins. While rapamycin has been demonstrated to be effective and is prescribed for chronic use in people, it has side effects that make it desirable to find safer and possibly more specific autophagy inducers that can be used to treat patients for many decades. In some cases, patients may be asymptomatic gene carriers of mutations causing conditions like Huntington's disease, where the objective of the treatment would be to delay onset of disease. Several groups have now identified novel compounds that induce autophagy in vitro [82–85], although the challenge remains as to how best to validate these findings in vivo. Traditionally, in vivo validation has been performed on rodent models. However, large scale screens can produce tens or possibly hundreds of “hits” that require further validation, including in vivo testing, in order to select the best therapeutic candidate for further development. Validating all “hit” compounds in rodent models is often not feasible due to the length and cost of trials, in addition to large amounts of compound required for long-term treatment regimes. Here zebrafish models offer a distinct advantage, since many of the neurodegenerative disease models described to date develop disease phenotypes at larval stages [8,9,11]. Williams et al. used such a screening cascade to identify novel inducers of autophagy from a library of FDA-approved drugs. Primary screens were performed using increased clearance of mutant α-synuclein (that causes familial Parkinson's disease) and mutant huntingtin (that causes Huntington's disease) as indicators of increased autophagy. Compounds demonstrated to be effective in cell-based assays were then tested in Drosophila and zebrafish models of Huntington's disease [8]. As screening assays become more sophisticated, with a shift towards high-content read-outs, zebrafish models offer great potential for the development of novel screening assays, using, for example, fluorescent reporters or high-throughput behavioral analysis to identify agents that ameliorate the disease phenotype.
1.6. Future directions
While this review highlights the potential for zebrafish as a model for the study of autophagy, a number of uncertainties or technical limitations remain and should be considered as priorities for future investigation:
-
•
Gene duplications—the conservation of function between mammalian and zebrafish proteins is unclear for genes where zebrafish possess several homologs (e.g. PTEN) and further work is needed to assess the overlapping and/or non-redundant roles of these. In addition, caution is needed in the interpretation of potentially duplicated sequences identified in genomic databases and it is expected that the ongoing annotation of the zebrafish genome will clarify whether previously reported duplications (e.g. tsc1a and tsc1b [88], ulk1a and ulk1b [72]) are genuine or whether these have arisen from incomplete annotation.
-
•
Targeted gene knockouts—although morpholinos provide a powerful tool for transient gene knockdown, an effective technology for permanent gene knockdown would be desirable for some studies.
-
•
Compound uptake and distribution—while zebrafish offer huge potential for in vivo validation of novel therapies, little is known about compound absorption, distribution or metabolism. Of particular relevance to the study of neurological disorders, it is important to consider the timing of zebrafish blood–brain barrier formation and the similarities or differences between this barrier in zebrafish and mammals.
Acknowledgements
We are grateful for a Wellcome Trust Senior Fellowship in Clinical Science (D.C.R.), an MRC Skills Gap Award (A.F.), an M.R.C. Programme Grant, and funding from the NIHR Biomedical Research Centre at Addenbrooke's Hospital.
Footnotes
This article was originally intended for the Zebrafish Models of Neurology Special Issue, Volume 1812, Issue 3, March 2011, guest edited by T. Allison. Due to publishing constraints, the article was not included. The Guest Editor and Publisher regret any inconvenience caused.
References
- 1.Tettamanti G., Salo E., Gonzalez-Estevez C., Felix D.A., Grimaldi A., de Eguileor M. Autophagy in invertebrates: insights into development, regeneration and body remodeling. Curr. Pharm. Des. 2008;14:116–125. doi: 10.2174/138161208783378716. [DOI] [PubMed] [Google Scholar]
- 2.Dooley K., Zon L.I. Zebrafish: a model system for the study of human disease. Curr. Opin. Genet. Dev. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 3.Ingham P.W. The power of the zebrafish for disease analysis. Hum. Mol. Genet. 2009;18:R107–R112. doi: 10.1093/hmg/ddp091. [DOI] [PubMed] [Google Scholar]
- 4.Linney E., Hardison N.L., Lonze B.E., Lyons S., DiNapoli L. Transgene expression in zebrafish: a comparison of retroviral-vector and DNA-injection approaches. Dev. Biol. 1999;213:207–216. doi: 10.1006/dbio.1999.9376. [DOI] [PubMed] [Google Scholar]
- 5.Asakawa K., Kawakami K. Targeted gene expression by the Gal4-UAS system in zebrafish. Dev. Growth Differ. 2008;50:391–399. doi: 10.1111/j.1440-169X.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 6.Soroldoni D., Hogan B.M., Oates A.C. Simple and efficient transgenesis with meganuclease constructs in zebrafish. Methods Mol. Biol. 2009;546:117–130. doi: 10.1007/978-1-60327-977-2_8. [DOI] [PubMed] [Google Scholar]
- 7.Suster M.L., Kikuta H., Urasaki A., Asakawa K., Kawakami K. Transgenesis in zebrafish with the tol2 transposon system. Methods Mol. Biol. 2009;561:41–63. doi: 10.1007/978-1-60327-019-9_3. [DOI] [PubMed] [Google Scholar]
- 8.Williams A., Sarkar S., Cuddon P., Ttofi E.K., Saiki S., Siddiqi F.H., Jahreiss L., Fleming A., Pask D., Goldsmith P., O'Kane C.J., Floto R.A., Rubinsztein D.C. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasiewicz H.G., Flaherty D.B., Soria J.P., Wood J.G. Transgenic zebrafish model of neurodegeneration. J. Neurosci. Res. 2002;70:734–745. doi: 10.1002/jnr.10451. [DOI] [PubMed] [Google Scholar]
- 10.Bai Q., Garver J.A., Hukriede N.A., Burton E.A. Generation of a transgenic zebrafish model of Tauopathy using a novel promoter element derived from the zebrafish eno2 gene. Nucleic Acids Res. 2007;35:6501–6516. doi: 10.1093/nar/gkm608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paquet D., Bhat R., Sydow A., Mandelkow E.M., Berg S., Hellberg S., Falting J., Distel M., Koster R.W., Schmid B., Haass C. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J. Clin. Invest. 2009;119:1382–1395. doi: 10.1172/JCI37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramesh T., Lyon A.N., Pineda R.H., Wang C., Janssen P.M., Canan B.D., Burghes A.H., Beattie C.E. A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis. Model Mech. 2010;3:652–662. doi: 10.1242/dmm.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen L., Wei W., Gu W., Huang P., Ren X., Zhang Z., Zhu Z., Lin S., Zhang B. Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev. Biol. 2008;314:84–92. doi: 10.1016/j.ydbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Miller V.M., Nelson R.F., Gouvion C.M., Williams A., Rodriguez-Lebron E., Harper S.Q., Davidson B.L., Rebagliati M.R., Paulson H.L. CHIP suppresses polyglutamine aggregation and toxicity in vitro and in vivo. J. Neurosci. 2005;25:9152–9161. doi: 10.1523/JNEUROSCI.3001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffer N.W., Broadley S.A., Hirschberger T., Tavan P., Kretzschmar H.A., Giese A., Haass C., Hartl F.U., Schmid B. Identification of anti-prion compounds as efficient inhibitors of polyglutamine protein aggregation in a zebrafish model. J. Biol. Chem. 2007;282:9195–9203. doi: 10.1074/jbc.M607865200. [DOI] [PubMed] [Google Scholar]
- 16.Lemmens R., Van Hoecke A., Hersmus N., Geelen V., D'Hollander I., Thijs V., Van Den Bosch L., Carmeliet P., Robberecht W. Overexpression of mutant superoxide dismutase 1 causes a motor axonopathy in the zebrafish. Hum. Mol. Genet. 2007;16:2359–2365. doi: 10.1093/hmg/ddm193. [DOI] [PubMed] [Google Scholar]
- 17.Kabashi E., Lin L., Tradewell M.L., Dion P.A., Bercier V., Bourgouin P., Rochefort D., Bel Hadj S., Durham H.D., Vande Velde C., Rouleau G.A., Drapeau P. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum. Mol. Genet. 2010;19:671–683. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- 18.Laird A.S., Van Hoecke A., De Muynck L., Timmers M., Van den Bosch L., Van Damme P., Robberecht W. Progranulin is neurotrophic in vivo and protects against a mutant TDP-43 induced axonopathy. PLoS ONE. 2010;5:e13368. doi: 10.1371/journal.pone.0013368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitramuthu B.P., Baranowski D.C., Kay D.G., Bateman A., Bennett H.P. Progranulin modulates zebrafish motoneuron development in vivo and rescues truncation defects associated with knockdown of Survival motor neuron 1. Mol. Neurodegener. 2010;5:41. doi: 10.1186/1750-1326-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S. Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin. Drug Discov. 2009;4:715–726. doi: 10.1517/17460440902988464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisen J.S., Smith J.C. Controlling morpholino experiments: don't stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- 22.Wickstrom E., Urtishak K.A., Choob M., Tian X., Sternheim N., Cross L.M., Rubinstein A., Farber S.A. Downregulation of gene expression with negatively charged peptide nucleic acids (PNAs) in zebrafish embryos. Methods Cell Biol. 2004;77:137–158. doi: 10.1016/s0091-679x(04)77008-9. [DOI] [PubMed] [Google Scholar]
- 23.Tomasini A.J., Schuler A.D., Zebala J.A., Mayer A.N. PhotoMorphs: a novel light-activated reagent for controlling gene expression in zebrafish. Genesis. 2009;47:736–743. doi: 10.1002/dvg.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bretaud S., Allen C., Ingham P.W., Bandmann O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease. J. Neurochem. 2007;100:1626–1635. doi: 10.1111/j.1471-4159.2006.04291.x. [DOI] [PubMed] [Google Scholar]
- 25.Anichtchik O., Diekmann H., Fleming A., Roach A., Goldsmith P., Rubinsztein D.C. Loss of PINK1 function affects development and results in neurodegeneration in zebrafish. J. Neurosci. 2008;28:8199–8207. doi: 10.1523/JNEUROSCI.0979-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flinn L., Mortiboys H., Volkmann K., Koster R.W., Ingham P.W., Bandmann O. Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio) Brain. 2009;132:1613–1623. doi: 10.1093/brain/awp108. [DOI] [PubMed] [Google Scholar]
- 27.Xi Y., Ryan J., Noble S., Yu M., Yilbas A.E., Ekker M. Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function. Eur. J. Neurosci. 2010;31:623–633. doi: 10.1111/j.1460-9568.2010.07091.x. [DOI] [PubMed] [Google Scholar]
- 28.Sheng D., Qu D., Kwok K.H., Ng S.S., Lim A.Y., Aw S.S., Lee C.W., Sung W.K., Tan E.K., Lufkin T., Jesuthasan S., Sinnakaruppan M., Liu J. Deletion of the WD40 domain of LRRK2 in zebrafish causes Parkinsonism-like loss of neurons and locomotive defect. PLoS Genet. 2010;6:e1000914. doi: 10.1371/journal.pgen.1000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallinen V., Kolehmainen J., Priyadarshini M., Toleikyte G., Chen Y.C., Panula P. Dopaminergic cell damage and vulnerability to MPTP in Pink1 knockdown zebrafish. Neurobiol. Dis. 2010;40:93–101. doi: 10.1016/j.nbd.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Gros-Louis F., Kriz J., Kabashi E., McDearmid J., Millecamps S., Urushitani M., Lin L., Dion P., Zhu Q., Drapeau P., Julien J.P., Rouleau G.A. Als2 mRNA splicing variants detected in KO mice rescue severe motor dysfunction phenotype in Als2 knock-down zebrafish. Hum. Mol. Genet. 2008;17:2691–2702. doi: 10.1093/hmg/ddn171. [DOI] [PubMed] [Google Scholar]
- 31.McWhorter M.L., Monani U.R., Burghes A.H., Beattie C.E. Knockdown of the survival motor neuron (Smn) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. J. Cell Biol. 2003;162:919–931. doi: 10.1083/jcb.200303168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler C., Eggert C., Gradl D., Meister G., Giegerich M., Wedlich D., Laggerbauer B., Fischer U. Reduced U snRNP assembly causes motor axon degeneration in an animal model for spinal muscular atrophy. Genes Dev. 2005;19:2320–2330. doi: 10.1101/gad.342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boon K.L., Xiao S., McWhorter M.L., Donn T., Wolf-Saxon E., Bohnsack M.T., Moens C.B., Beattie C.E. Zebrafish survival motor neuron mutants exhibit presynaptic neuromuscular junction defects. Hum. Mol. Genet. 2009;18:3615–3625. doi: 10.1093/hmg/ddp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumsden A.L., Henshall T.L., Dayan S., Lardelli M.T., Richards R.I. Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum. Mol. Genet. 2007;16:1905–1920. doi: 10.1093/hmg/ddm138. [DOI] [PubMed] [Google Scholar]
- 35.Diekmann H., Anichtchik O., Fleming A., Futter M., Goldsmith P., Roach A., Rubinsztein D.C. Decreased BDNF levels are a major contributor to the embryonic phenotype of huntingtin knockdown zebrafish. J. Neurosci. 2009;29:1343–1349. doi: 10.1523/JNEUROSCI.6039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Futter M., Diekmann H., Schoenmakers E., Sadiq O., Chatterjee K., Rubinsztein D.C. Wild-type but not mutant huntingtin modulates the transcriptional activity of liver X receptors. J. Med. Genet. 2009;46:438–446. doi: 10.1136/jmg.2009.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henshall T.L., Tucker B., Lumsden A.L., Nornes S., Lardelli M.T., Richards R.I. Selective neuronal requirement for huntingtin in the developing zebrafish. Hum. Mol. Genet. 2009;18:4830–4842. doi: 10.1093/hmg/ddp455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krawitz P., Haffner C., Fluhrer R., Steiner H., Schmid B., Haass C. Differential localization and identification of a critical aspartate suggest non-redundant proteolytic functions of the presenilin homologues SPPL2b and SPPL3. J. Biol. Chem. 2005;280:39515–39523. doi: 10.1074/jbc.M501645200. [DOI] [PubMed] [Google Scholar]
- 39.Campbell W.A., Yang H., Zetterberg H., Baulac S., Sears J.A., Liu T., Wong S.T., Zhong T.P., Xia W. Zebrafish lacking Alzheimer presenilin enhancer 2 (Pen-2) demonstrate excessive p53-dependent apoptosis and neuronal loss. J. Neurochem. 2006;96:1423–1440. doi: 10.1111/j.1471-4159.2006.03648.x. [DOI] [PubMed] [Google Scholar]
- 40.Zetterberg H., Campbell W.A., Yang H.W., Xia W. The cytosolic loop of the gamma-secretase component presenilin enhancer 2 protects zebrafish embryos from apoptosis. J. Biol. Chem. 2006;281:11933–11939. doi: 10.1074/jbc.M512521200. [DOI] [PubMed] [Google Scholar]
- 41.Nornes S., Newman M., Verdile G., Wells S., Stoick-Cooper C.L., Tucker B., Frederich-Sleptsova I., Martins R., Lardelli M. Interference with splicing of Presenilin transcripts has potent dominant negative effects on Presenilin activity. Hum. Mol. Genet. 2008;17:402–412. doi: 10.1093/hmg/ddm317. [DOI] [PubMed] [Google Scholar]
- 42.Carrel T.L., McWhorter M.L., Workman E., Zhang H., Wolstencroft E.C., Lorson C., Bassell G.J., Burghes A.H., Beattie C.E. Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. J. Neurosci. 2006;26:11014–11022. doi: 10.1523/JNEUROSCI.1637-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McWhorter M.L., Boon K.L., Horan E.S., Burghes A.H., Beattie C.E. The SMN binding protein Gemin2 is not involved in motor axon outgrowth. Dev. Neurobiol. 2008;68:182–194. doi: 10.1002/dneu.20582. [DOI] [PubMed] [Google Scholar]
- 44.Oprea G.E., Krober S., McWhorter M.L., Rossoll W., Muller S., Krawczak M., Bassell G.J., Beattie C.E., Wirth B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320:524–527. doi: 10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shankaran S.S., Capell A., Hruscha A.T., Fellerer K., Neumann M., Schmid B., Haass C. Missense mutations in the progranulin gene linked to frontotemporal lobar degeneration with ubiquitin-immunoreactive inclusions reduce progranulin production and secretion. J. Biol. Chem. 2008;283:1744–1753. doi: 10.1074/jbc.M705115200. [DOI] [PubMed] [Google Scholar]
- 46.Amacher S.L. Emerging gene knockout technology in zebrafish: zinc-finger nucleases. Brief Funct. Genomic Proteomic. 2008;7:460–464. doi: 10.1093/bfgp/eln043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong M., Fu Y.F., Du T.T., Jing C.B., Fu C.T., Chen Y., Jin Y., Deng M., Liu T.X. Heritable and lineage-specific gene knockdown in zebrafish embryo. PLoS ONE. 2009;4:e6125. doi: 10.1371/journal.pone.0006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang S.L., Yan S., Niu R.L., Lin X.K. VEGF gene silencing by cytomegalovirus promoter driven shRNA expression vector results in vascular development defects in zebrafish. Genetika. 2009;45:1187–1193. [PubMed] [Google Scholar]
- 49.Meng X., Noyes M.B., Zhu L.J., Lawson N.D., Wolfe S.A. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat. Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doyon Y., McCammon J.M., Miller J.C., Faraji F., Ngo C., Katibah G.E., Amora R., Hocking T.D., Zhang L., Rebar E.J., Gregory P.D., Urnov F.D., Amacher S.L. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foley J.E., Yeh J.R., Maeder M.L., Reyon D., Sander J.D., Peterson R.T., Joung J.K. Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN) PLoS ONE. 2009;4:e4348. doi: 10.1371/journal.pone.0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D., Jao L.E., Zheng N., Dolan K., Ivey J., Zonies S., Wu X., Wu K., Yang H., Meng Q., Zhu Z., Zhang B., Lin S., Burgess S.M. Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proc. Natl Acad. Sci. USA. 2007;104:12428–12433. doi: 10.1073/pnas.0705502104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moens C.B., Donn T.M., Wolf-Saxon E.R., Ma T.P. Reverse genetics in zebrafish by TILLING. Brief Funct. Genomic Proteomic. 2008;7:454–459. doi: 10.1093/bfgp/eln046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bassett D., Currie P.D. Identification of a zebrafish model of muscular dystrophy. Clin. Exp. Pharmacol. Physiol. 2004;31:537–540. doi: 10.1111/j.1440-1681.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 55.Hall T.E., Bryson-Richardson R.J., Berger S., Jacoby A.S., Cole N.J., Hollway G.E., Berger J., Currie P.D. The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin alpha2-deficient congenital muscular dystrophy. Proc. Natl Acad. Sci. USA. 2007;104:7092–7097. doi: 10.1073/pnas.0700942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffen L.S., Guyon J.R., Vogel E.D., Howell M.H., Zhou Y., Weber G.J., Zon L.I., Kunkel L.M. The zebrafish runzel muscular dystrophy is linked to the titin gene. Dev. Biol. 2007;309:180–192. doi: 10.1016/j.ydbio.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacoby A.S., Busch-Nentwich E., Bryson-Richardson R.J., Hall T.E., Berger J., Berger S., Sonntag C., Sachs C., Geisler R., Stemple D.L., Currie P.D. The zebrafish dystrophic mutant softy maintains muscle fibre viability despite basement membrane rupture and muscle detachment. Development. 2009;136:3367–3376. doi: 10.1242/dev.034561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- 59.Fishman M.C. Genomics. Zebrafish—the canonical vertebrate. Science. 2001;294:1290–1291. doi: 10.1126/science.1066652. [DOI] [PubMed] [Google Scholar]
- 60.Skromne I., Prince V.E. Current perspectives in zebrafish reverse genetics: moving forward. Dev. Dyn. 2008;237:861–882. doi: 10.1002/dvdy.21484. [DOI] [PubMed] [Google Scholar]
- 61.Murphey R.D., Zon L.I. Small molecule screening in the zebrafish. Methods. 2006;39:255–261. doi: 10.1016/j.ymeth.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 62.Ravikumar B., Futter M., Jahreiss L., Korolchuk V.I., Lichtenberg M., Luo S., Massey D.C., Menzies F.M., Narayanan U., Renna M., Jimenez-Sanchez M., Sarkar S., Underwood B., Winslow A., Rubinsztein D.C. Mammalian macroautophagy at a glance. J. Cell Sci. 2009;122:1707–1711. doi: 10.1242/jcs.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganley I.G., Lam Test H. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., Guan J.L., Oshiro N., Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung C.H., Jun C.B., Ro S.H., Kim Y.M., Otto N.M., Cao J., Kundu M., Kim D.H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacinto E., Loewith R., Schmidt A., Lin S., Ruegg M.A., Hall A., Hall M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 67.Makky K., Tekiela J., Mayer A.N. Target of rapamycin (TOR) signaling controls epithelial morphogenesis in the vertebrate intestine. Dev. Biol. 2007;303:501–513. doi: 10.1016/j.ydbio.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gangloff Y.G., Mueller M., Dann S.G., Svoboda P., Sticker M., Spetz J.F., Um S.H., Brown E.J., Cereghini S., Thomas G., Kozma S.C. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell. Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Komoike Y., Shimojima K., Liang J.S., Fujii H., Maegaki Y., Osawa M., Fujii S., Higashinakagawa T., Yamamoto T. A functional analysis of GABARAP on 17p13.1 by knockdown zebrafish. J. Hum. Genet. 2010;55:155–162. doi: 10.1038/jhg.2010.1. [DOI] [PubMed] [Google Scholar]
- 70.O'Sullivan G.A., Kneussel M., Elazar Z., Betz H. GABARAP is not essential for GABA receptor targeting to the synapse. Eur. J. Neurosci. 2005;22:2644–2648. doi: 10.1111/j.1460-9568.2005.04448.x. [DOI] [PubMed] [Google Scholar]
- 71.Tsukamoto S., Kuma A., Murakami M., Kishi C., Yamamoto A., Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321:117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- 72.He C., Bartholomew C.R., Zhou W., Klionsky D.J. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy. 2009;5:520–526. doi: 10.4161/auto.5.4.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W., Jimenez-Sanchez M., Korolchuk V.I., Lichtenberg M., Luo S., Massey D.C., Menzies F.M., Moreau K., Narayanan U., Renna M., Siddiqi F.H., Underwood B.R., Winslow A.R., Rubinsztein D.C. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 74.Dowling J.J., Low S.E., Busta A.S., Feldman E.L. Zebrafish MTMR14 is required for excitation–contraction coupling, developmental motor function and the regulation of autophagy. Hum. Mol. Genet. 2010;19:2668–2681. doi: 10.1093/hmg/ddq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vergne I., Roberts E., Elmaoued R.A., Tosch V., Delgado M.A., Proikas-Cezanne T., Laporte J., Deretic V. Control of autophagy initiation by phosphoinositide 3-phosphatase Jumpy. EMBO J. 2009;28:2244–2258. doi: 10.1038/emboj.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Underwood B.R., Imarisio S., Fleming A., Rose C., Krishna G., Heard P., Quick M., Korolchuk V.I., Renna M., Sarkar S., Garcia-Arencibia M., O'Kane C.J., Murphy M.P., Rubinsztein D.C. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum. Mol. Genet. 2010;19:3413–3429. doi: 10.1093/hmg/ddq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubinsztein D.C., Cuervo A.M., Ravikumar B., Sarkar S., Korolchuk V., Kaushik S., Klionsky D.J. In search of an “autophagomometer”. Autophagy. 2009;5:585–589. doi: 10.4161/auto.5.5.8823. [DOI] [PubMed] [Google Scholar]
- 78.Ravikumar B., Duden R., Rubinsztein D.C. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 79.Ravikumar B., Vacher C., Berger Z., Davies J.E., Luo S., Oroz L.G., Scaravilli F., Easton D.F., Duden R., O'Kane C.J., Rubinsztein D.C. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 80.Berger Z., Ravikumar B., Menzies F.M., Oroz L.G., Underwood B.R., Pangalos M.N., Schmitt I., Wullner U., Evert B.O., O'Kane C.J., Rubinsztein D.C. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum. Mol. Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 81.Menzies F.M., Huebener J., Renna M., Bonin M., Riess O., Rubinsztein D.C. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain. 2010;133:93–104. doi: 10.1093/brain/awp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balgi A.D., Fonseca B.D., Donohue E., Tsang T.C., Lajoie P., Proud C.G., Nabi I.R., Roberge M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS ONE. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarkar S., Perlstein E.O., Imarisio S., Pineau S., Cordenier A., Maglathlin R.L., Webster J.A., Lewis T.A., O'Kane C.J., Schreiber S.L., Rubinsztein D.C. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat. Chem. Biol. 2007;3:331–338. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L., Yu J., Pan H., Hu P., Hao Y., Cai W., Zhu H., Yu A.D., Xie X., Ma D., Yuan J. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl Acad. Sci. USA. 2007;104:19023–19028. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farkas T., Hoyer-Hansen M., Jaattela M. Identification of novel autophagy regulators by a luciferase-based assay for the kinetics of autophagic flux. Autophagy. 2009;5:1018–1025. doi: 10.4161/auto.5.7.9443. [DOI] [PubMed] [Google Scholar]
- 86.Croushore J.A., Blasiole B., Riddle R.C., Thisse C., Thisse B., Canfield V.A., Robertson G.P., Cheng K.C., Levenson R. Ptena and ptenb genes play distinct roles in zebrafish embryogenesis. Dev. Dyn. 2005;234:911–921. doi: 10.1002/dvdy.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faucherre A., Taylor G.S., Overvoorde J., Dixon J.E., Hertog J. Zebrafish pten genes have overlapping and non-redundant functions in tumorigenesis and embryonic development. Oncogene. 2008;27:1079–1086. doi: 10.1038/sj.onc.1210730. [DOI] [PubMed] [Google Scholar]
- 88.DiBella L.M., Park A., Sun Z. Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum. Mol. Genet. 2009;18:595–606. doi: 10.1093/hmg/ddn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.http://www.ncbi.nlm.nih.gov/sites/entrez?db=nuccore&itool=toolbar.
- 90.http://www.ncbi.nlm.nih.gov/gene.
- 91.http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=handbook&part=ch18&rendertype=table&id=ch18.T18.2.