The human brain has evolved compared with that of other vertebrates, yet we pay a substantial energetic bill for its use. This high-energy consumption is evidenced by some oft-cited values: despite being only 2–3% of total body weight, the brain receives 15% of cardiac output, and consumes ∼20% of the body's oxygen, and 15% of total body glucose. But it is precisely this high-energy demand, married especially with a limited capacity for energy storage, that necessitates complex but effective regulation of regional blood delivery. If the supply of glucose to the brain were theoretically abolished, the brain's total energy pool – from free glucose, lactate, and astrocytic glycogen – would only allow brain tissue to continue functioning for ∼12 min (Barros & Deitmer, 2010). The consequent requirement for a steady energy-substrate and oxygen supply relies on effective regional regulation. This elegant coupling was reported as early as 1880 (Mosso, 1880) where – in a man with a skull defect that allowed measurement of cerebral blood volume – it was observed that cerebral blood volume increased in concert with an emotional response to innuendoes made about the man's wife! This cerebrovascular response to increased neuronal activity has since been explicated to result from coordinated nervous input, local paracrine regulation, and metabolism in active regions of the brain.
Relative to other organs in the body, the brain is particular in its requirement for a constant supply of specific energy substrates. It has traditionally been held that the brain obligatorily metabolizes glucose. At rest, this is largely the case, with blood-derived glucose being the primary metabolic substrate for brain tissue; it is a current topic for debate as to how this glucose is specifically metabolized within neurons and astrocytes. For example, glucose may be taken up directly into neurons that derive ATP through glycolysis, releasing lactate that is in turn utilized by astrocytes. Alternatively, astrocytes metabolize glucose, and neurons oxidize the lactate. Regardless, at rest there is a small lactate efflux of ∼50 μmol min−1 from the cerebral vasculature, suggestive of negligible blood-derived lactate use for resting brain metabolism. The situation during exercise is decidedly different. Glucose intake actually decreases and lactate influx may be as high as ∼150 μmol min−1, accounting for 11% of total-body lactate uptake – influx rates second only to skeletal muscle tissue.
Lactate transport in tissues throughout the body is directly affected by pH because cell membrane permeability to lactate is facilitated by a number of putative H+ linked membrane translocators. In an article in a recent edition of The Journal of PhysiologyVolianitis et al. (2011) set out to control arterial pH during high-intensity whole body exercise while measuring brain lactate consumption. In this elegant study, arterial and jugular venous bloods were examined in order to quantify lactate and glucose arterio-venous (a-v) differences across the brain in a group of elite rowers. These metabolites were measured at rest and during high intensity exercise with saline or sodium bicarbonate infusion (in order to limit exercise related acidosis). With rowing, the lactate a-v difference was increased from −0.03 ± 0.01 mm at rest to 3.2 ± 0.9 mm during saline-infusion rowing (indicating a net lactate influx to brain tissue with exercise), whereas glucose a-v difference remained the same. Because lactate does not accumulate in brain tissue or cerebrospinal fluid, the cerebral metabolic ratio decreased by ∼4.1 during exercise, which was due exclusively to an increase in brain lactate metabolism. Although it was shown that bicarbonate infusion significantly increased exercise performance by ∼2%, brain lactate metabolism was unaffected by pH. These data contrast with previous opinions that lactate transport across the blood–brain barrier is likely to be increased with lower pH (Oldendorf et al. 1979). Given that pH certainly does affect lactate metabolism in other organs (such as skeletal muscle) but apparently not in the brain, this unexpected finding underscores the importance of research into the unique mechanisms of cerebral metabolic control during exercise.
Although brain lactate metabolism may not be pH dependant, regulation of brain blood flow is highly sensitive to pH. Indeed, the authors reported a significant decrease in minute ventilation with HCO3− infusion from 156 ± 14 to 145 ± 13 litres and a consequent increase in 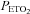 from 35.9 ± 2 to 46.5 ± 2.5 mmHg. The cerebrovasculature is highly sensitive to CO2, with an approximate 3.8% increase in cerebral blood flow (CBF) per mmHg increase in
from 35.9 ± 2 to 46.5 ± 2.5 mmHg. The cerebrovasculature is highly sensitive to CO2, with an approximate 3.8% increase in cerebral blood flow (CBF) per mmHg increase in 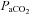 within a
within a 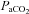 range of 35–55 mmHg. Thus, although Volianitis et al did not explicitly report cerebral blood flow in their study, it is likely that there was a dramatically higher CBF with bicarbonate infusion than with saline infusion. This is supported by the reported a-v oxygen content difference that decreased from 96 ± 9 to 75 ± 7 ml l−1 during exercise with saline or bicarbonate infusion, respectively. Assuming that the total cerebral metabolic rate was similar between the saline and bicarbonate exercise trials, CBF should have increased by approximately ∼22%.
range of 35–55 mmHg. Thus, although Volianitis et al did not explicitly report cerebral blood flow in their study, it is likely that there was a dramatically higher CBF with bicarbonate infusion than with saline infusion. This is supported by the reported a-v oxygen content difference that decreased from 96 ± 9 to 75 ± 7 ml l−1 during exercise with saline or bicarbonate infusion, respectively. Assuming that the total cerebral metabolic rate was similar between the saline and bicarbonate exercise trials, CBF should have increased by approximately ∼22%.
Their data also show that during exercise brain lactate influx is not affected by arterial lactate concentration. Despite arterial lactate being greater during bicarbonate infusion (versus the saline trial) at each exercise intensity, the lactate a-v difference across the brain was not different. Perhaps it is possible that the difference in CBF between the bicarbonate and saline trials is responsible here. Certainly brain metabolism exerts a potent influence on regional CBF, but the literature is unclear as to the reciprocal relationship – that is, what effect CBF per se might exert on substrate metabolism that is thought to be at least partially arterial concentration dependant.
Net cerebral lactate uptake increases with raised arterial lactate during lactate infusion and during high intensity exercise (Van Hall et al. 2009; Volianitis et al. 2011). Yet, it is interesting to note that Volianitis et al report that during post-exercise recovery, when arterial lactate remains elevated 13-fold above resting levels, the lactate a-v difference returns to near baseline levels. Clearly, at least following high intensity exercise, brain lactate metabolism is not driven principally by arterial lactate concentration, but perhaps follows brain tissue energy requirement; upon cessation of exercise neural activity is decreased and brain tissue reverts to a higher degree of oxidative metabolism. Regardless, that these data from during and following exercise appear to support a lack of coupling between arterial lactate concentration and brain lactate metabolism warrants further consideration, as there is certainly evidence available to the contrary.
A relevant study by Van Hall et al. (2009) showed that lactate infusion during exercise increased brain lactate consumption more than just lactate infusion. However, lack of an exercise control group where arterial [lactate] was maintained at exercising levels while at rest makes it difficult to differentiate the role of arterial [lactate] and exercise-induced increased in brain activity in lactate metabolism. The reason for increased lactate metabolism during exercise – whether this is due to increased arterial [lactate]per se, increased brain metabolism with exercise, or both – remains to be elucidated in humans, as do the regulatory mechanisms responsible. Volianitis et al. have contributed an important piece of this regulatory puzzle – that unlike other tissues, the brain's use of lactate is not dependant on the prevailing arterial pH. But given the obvious importance of the sophisticated mechanisms that facilitate efficacious energy supply to the brain, further research is certainly merited. Specifically, the question of the relative importance of arterial lactate concentration, versus neuronal metabolism, to the brain's choice of substrate needs to be addressed. Such a definitive study should take into account influencing variables such as lactate origin (e.g. exercising skeletal muscle versus infusion); resting, exercise, or post-exercise states; and prevailing cerebral blood flow.
Acknowledgments
Both authors are ever grateful for the continued support, supervision, and critical feedback of Dr Philip N. Ainslie. C.K.W. is funded by an NSERC CGS Masters Scholarship. Important references to work within the field of brain metabolism have unfortunately been excluded due to the truncated Journal Club format.
References
- Barros LF, Deitmer JW. Glucose and lactate supply to the synapse. Brain Res Rev. 2010;63:149–159. doi: 10.1016/j.brainresrev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Mosso A. Sulla circolazione del cervello dell’uomo. Att R Accad Lincei. 1880;5:237–358. [Google Scholar]
- Oldendorf W, Braun L, Cornford E. pH dependence of blood-brain barrier permeability to lactate and nicotine. Stroke. 1979;10:577–581. doi: 10.1161/01.str.10.5.577. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Strømstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen H. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Rasmussen P, Seifert T, Nielsen HB, Secher NH. Plasma pH does not influence the cerebral metabolic ratio during maximal whole body exercise. J Physiol. 2011;589:423–429. doi: 10.1113/jphysiol.2010.195636. [DOI] [PMC free article] [PubMed] [Google Scholar]


