Abstract
Carbon dioxide (CO2) is a physiological gas found at low levels in the atmosphere and produced in cells during the process of aerobic respiration. Consequently, the levels of CO2 within tissues are usually significantly higher than those found externally. Shifts in tissue levels of CO2 (leading to either hypercapnia or hypocapnia) are associated with a number of pathophysiological conditions in humans and can occur naturally in niche habitats such as those of burrowing animals. Clinical studies have indicated that such altered CO2 levels can impact upon disease progression. Recent advances in our understanding of the biology of CO2 has shown that like other physiological gases such as molecular oxygen (O2) and nitric oxide (NO), CO2 levels can be sensed by cells resulting in the initiation of physiological and pathophysiological responses. Acute CO2 sensing in neurons and peripheral and central chemoreceptors is important in rapidly activated responses including olfactory signalling, taste sensation and cardiorespiratory control. Furthermore, a role for CO2 in the regulation of gene transcription has recently been identified with exposure of cells and model organisms to high CO2 leading to suppression of genes involved in the regulation of innate immunity and inflammation. This latter, transcriptional regulatory role for CO2, has been largely attributed to altered activity of the NF-κB family of transcription factors. Here, we review our evolving understanding of how CO2 impacts upon gene transcription.

Cormac Taylor and Eoin Cummins are leaders of the Hypoxia Research Group at the Conway Institute, University College Dublin, Ireland. Current research is directed towards expanding our understanding of the physiological and pathophysiological mechanisms by which changes in oxygen and carbon dioxide regulate transcriptional and metabolic events in eukaryotic cells.
The natural history of CO2
During the history of metazoan evolution in the Phanerozoic aeon, atmospheric levels of CO2 in dry air ranged from over 6000 ppmv (0.6%) around 600–400 million years ago to 284 ppmv (0.0284%) in the mid 1800s (Berner & Kothavala, 2001; Berner, 2003; Beerling & Berner, 2005; Royer et al. 2007; Vandenbroucke et al. 2010). Current atmospheric 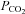 levels are approximately 387 ppmv (0.0387%), representing an increase of approximately 36% since the advent of human industrial activity. While relatively low, this level of CO2 is key in regulation of the Earth's temperature and climate (Lacis et al. 2010). The low background level of atmospheric CO2 can increase dramatically in the presence of respiring organisms or decomposing organic matter (Luo et al. 2009). In fact, a number of species use the presence of environmental CO2 gradients as a behavioural cue including prey-seeking behaviour in mosquitoes and avoidance behaviour in fruitflies and nematodes (Bretscher et al. 2008; Turner & Ray, 2009).
levels are approximately 387 ppmv (0.0387%), representing an increase of approximately 36% since the advent of human industrial activity. While relatively low, this level of CO2 is key in regulation of the Earth's temperature and climate (Lacis et al. 2010). The low background level of atmospheric CO2 can increase dramatically in the presence of respiring organisms or decomposing organic matter (Luo et al. 2009). In fact, a number of species use the presence of environmental CO2 gradients as a behavioural cue including prey-seeking behaviour in mosquitoes and avoidance behaviour in fruitflies and nematodes (Bretscher et al. 2008; Turner & Ray, 2009).
In respiring metazoans, the main source of CO2 is the electron transport chain of mitochondria where the chemical reduction of molecular oxygen is responsible for the generation of CO2 as a by-product. Thus, in contrast to molecular oxygen, the levels of CO2 found in tissues of the body are significantly higher than those found in the external atmosphere. A number of enzymes utilise CO2 during their activity including carbonic anhydrases, a family of ubiquitously expresses metallo-enzymes which are responsible for catalysing the reversible hydration of CO2 and H2O to HCO3− and H+ (De Simone & Supuran, 2010). Remaining CO2 is primarily removed by the blood and is exhaled or diffuses through the skin. Recent advances have demonstrated that organisms contain distinct mechanisms capable of sensing changes in CO2 and eliciting distinct acute responses or changes in gene expression through transcriptional regulation.
Acute cellular sensing of CO2
Many species of animal including nematodes, fruit-flies, rodents and humans possess the ability to sense CO2 in an acute manner leading to a rapid, neuronally mediated response (Chandrashekar et al. 2009; Frommer, 2010). For example CO2 levels can be sensed in D. melanogaster by neurons equipped with Gr63a and Gr21a olfactory receptors leading to avoidance behaviour (Jones et al. 2007). In C. elegans, CO2 avoidance is also mediated by specialized neurons (Bretscher et al. 2008). Furthermore, in humans, acute carbon dioxide sensing has been identified in the synapses of taste receptor cells in a manner dependent upon carbonic anhydrase IV (Chandrashekar et al. 2009). Furthermore, a diverse range of CO2 sensitive potassium channels have been shown to be important in CO2 sensing in maintaining homeostasis in higher species (Tang et al. 2004). The ability of metazoan cells to sense CO2 acutely and initiate rapid neuronal responses is analogous in nature to the acute oxygen-sensing pathways which exist in specialized tissues such as the carotid body (Weir et al. 2005; Lopez-Barneo et al. 2009) leading to neuronal signalling to control rate and depth of breathing. It is likely that in vivo such changes in neuronal activity will lead indirectly to CO2-induced changes in gene transcription as a consequence of altered neuronal activity. Associations between altered neuronal activity and gene expression have been expertly described previously (Kandel, 2001). Furthermore, previous studies have reported that stress responses to hypercapnia in animals result in increased expression of stress hormones (Schaefer et al. 1968), which can also regulate gene expression (De Kloet, 2004). Thus altered neuronal activity and/or increased stress hormone production in vivo in response to high CO2 may be an indirect link between CO2 sensing and gene expression. In this review, however, we will focus on direct mechanisms by which CO2 can regulate gene expression.
Metazoan cells have evolved the capacity to respond to changes in microenvironmental oxygen through the induction of specific adaptive gene cohorts in a manner governed by the hypoxia-inducible factor (Semenza, 2007; Kaelin & Ratcliffe, 2008). Recent advances have indicated that an analogous transcriptional response to CO2 may also exist in metazoan cells. The evidence supporting this will be discussed below.
CO2 and gene expression
In studies investigating the mechanisms underpinning the protective effects of ‘permissive hypercapnia’ in pulmonary disease, gene array analysis experiments were carried out on neonatal mice exposed to atmospheric hypercapnia (Li et al. 2006). This study identified altered levels of pulmonary genes related to cell adhesion, growth, signal transduction and innate immunity (Li et al. 2006). Of note in these studies, genes related to innate immunity were broadly suppressed in response to elevated CO2. In separate studies, exposure of the model organisms C. elegans and D. melanogaster to high CO2 also resulted in altered gene expression (Helenius et al. 2009; Sharabi et al. 2009). C. elegans exposure to hypercapnia impacted upon motility, fertility and lifespan, which was associated with altered expression of over 6% of the transcriptome in whole animals including genes encoding proteins involved in the regulation of innate immunity (Sharabi et al. 2009). In Drosophila, exposure to hypercapnia impaired embryonic morphogenesis, egg laying, egg hatching and innate immune responses resulting in increased susceptibility to bacterial infection which was associated with suppression of genes regulated by Relish (an NF-κB homolog) in adult flies (Helenius et al. 2009). This response was independent of the known neuronal Gr63a CO2 sensor, changes in extracellular pH or nitric oxide. Interestingly, in both D. melanogaster and C. elegans, the CO2 sensing pathway was not directly dependent upon the hydroxylase enzymes known to be important in oxygen sensing mechanisms in the hypoxia-inducible factor (HIF) pathway (reviewed by Kaelin & Ratcliffe, 2008). A further study in mouse macrophages exposed to hypercapnia also demonstrated an immunosuppressive effect of hypercapnia as determined by decreased levels of cytokine release from cells exposed to lippopolysaccharide (Wang et al. 2010). In this study, the authors also demonstrated a suppression of NF-κB signalling which was independent of acidosis and resulted in decreased phagocytic activity of the cells (Wang et al. 2010). A separate study also demonstrated that hypercapnia results in the suppression of NF-κB signalling in mouse embryonic fibroblasts and a number of other mammalian cell types (Cummins et al. 2010).
Thus, a common feature of the transcriptional response to elevated CO2 in mice, fruit-flies and nematodes is the suppression of the genes associated with innate immunity. This suppression of immunity has been linked to decreased activity of the NF-κB signalling pathway. A summary of the studies linking hypercapnia to altered NF-κB signalling is provided in Table 1. NF-κB is a master regulator of the genes involved in innate immunity and inflammation. The NF-κB pathway is complex and has been expertly reviewed recently (Gilmore 2006). Briefly, NF-κB is sequestered in the cytosol of a cell by inhibitory molecules known as IκBs. In response to a range of inflammatory stimuli, activation of receptor-specific upstream signalling cascades leads to the phosphorylation of IκB by the IKK complex (composed of three subunits IKKα/IKKβ/NEMO). This leads to the degradation of IκB, thus liberating NF-κB and allowing its translocation to the nucleus where it drives the expression of genes involved in driving innate immune and inflammatory responses.
Table 1.
Summary table of the evidence for NF-κB involvement in response to CO2
| Experimental model | Cellular Effect | Evidence of NF-κB involvement | Reference |
|---|---|---|---|
| Rat hepatic IRI | ↓ TNFα | ↓ NF-κB staining by IHC | Li et al. |
| ↑ IL-10 | |||
| ↓ Apoptosis | |||
| ↓ Liver injury | |||
| In vitro buffered hypercapnia (MEF, A549 lung epithelial cells and others) | ↓ TNFα, ICAM-1 and CCL2 | ↓ NF-κB luciferase promoter reporter | Cummins et al. |
| ↑ IL-10 | ↓ Nuclear p65 accumulation | ||
| ↓ IκBα degradation | |||
| ↑ Nuclear lKKα | |||
| In vitro hypercapnic acidosis (pulmonary endothelial cells) | ↓ ICAM-1, IL-8 | ↓ Nuclear p65 binding (EMSA) | Takeshita et al. |
| ↓ Neutrophil adherence | ↓ IκBα degradation | ||
| In vitro hypercapnia (macrophages) | ↓ IL-6, TNFα | No change in p65 or IκBα | Wang et al. |
| IL-10 unaffected | ↓ IL-6 promoter activity | ||
| ↓ Phagocytosis | |||
| In vitro hypercapnia acidosis (wound healing model in A549 lung epithelial cells) | ↓ Wound healing | ↓ IκBα degradation | O’Toole et al. |
| ↓ Cell migration | ↓ NF-κB luciferase promoter reporter | ||
| Effect of HCA lost when NF-κB inhibited | |||
| Drosophila (flies +/− pathogen at a range of CO2 concentrations) | ↑ Mortality | Proteolytic cleavage of Relish unchanged | Helenius et al. |
| ↓ Antimicrobial peptide genes | Hypercapnia inhibits Rel targets in parallel or downstream of proteolytic activation of Rel |
While the effects of in vivo hypercapnia on gene expression are likely to occur in part through indirect mechanisms such as altered neuronal activity or the release of stress hormones, recent evidence suggests that CO2 may also directly regulate gene expression through the NF-κB pathway (Cummins et al. 2010). Some insight into a possible mechanism underpinning the suppression of NF-κB activity by hypercapnia was recently provided by the demonstration of CO2-induced nuclear localization of the IKKα subunit (Cummins et al. 2010). In a non-canonical manner, nuclear IKKα has previously been shown to repress NF-κB activity (Lawrence et al. 2005) and have a nucleosomal role in the expression of NF-κB target genes including the repressor protein IκBα (Yamamoto et al. 2003). Consistent with the studies outlined above, Cummins and colleagues also demonstrated the immunosuppressive effects of high CO2 were not directly dependent upon the oxygen sensing hydroxylases which regulate HIF, extracellular pH or pathways which mediate acute CO2 sensing in nematodes and flies (Cummins et al. 2010).
In summary, the studies outlined above provide evidence that metazoan cells possess the capability to sense changes in microenvironmental CO2 levels and activate a transcriptional response which results in the suppression of innate immunity and inflammatory signalling. While the specific CO2 sensor mediating this transcriptional response has yet to be identified, the sensing mechanism appears to be independent of extracellular pH, oxygen sensing enzymes associated with the HIF pathway and mechanisms that mediate acute neuronal CO2 sensing (Fig. 1). The experiments excluding a role for altered pH have relied mainly on the buffering of extracellular pH or the manipulation of pH in the absence of changing CO2, neither of which elicits a transcriptional response comparable to hypercapnia (Cummins et al. 2010). However, this does not exclude possible roles for changes in intracellular pH in mediating the response to hypercapnia. Furthermore, alternative oxygen sensing mechanisms such as alterations in intracellular reactive oxygen species (ROS) production could also be implicated. Additionally, altered CO2 levels are likely to impact upon metabolic processes such as glycolysis. Whether such changes in metabolism mediate transcriptional changes also requires experimental investigation. Finally, whether enzymatically mediated post-translational modifications such as carboxylation of target proteins (Bandyopadhyay et al. 2002) plays a role in CO2 sensing remains to be determined. Future studies will be aimed at answering these important questions and identifying the CO2 sensor which regulates the cellular response to hypercapnia.
Figure 1. Transcriptional regulation by oxygen and carbon dioxide.
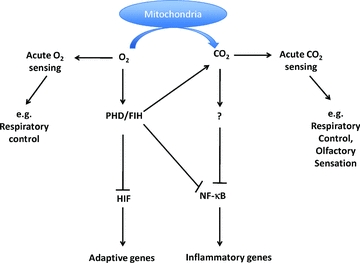
Metazoan cells have evolved to be capable of sensing levels of physiological gasses in the microenvironment through highly conserved pathways in the control of gene expression. Acute O2 and CO2 sensing leads to neuronally mediated changes in processes such as respiratory control and olfactory sensation, respectively. Molecular oxygen (O2) is sensed by prolyl and aspariginyl hydroxylases, which confer oxygen-dependent instability upon the HIF transcription factor. Activation of this pathway in hypoxia leads to the expression of adaptive genes. The sensor for regulation of NF-κB-dependent gene expression in response to changes in CO2 has yet to be defined. However, elevated CO2 leads to repression of the NF-κB pathway and decreased levels of genes which promote innate immunity and inflammation.
Crosstalk between oxygen and carbon dioxide sensing
Because of the close association between oxygen consumption and CO2 production, the capacity of a cell to sense changes in CO2 is likely to be closely linked to its ability to sense changes in oxygen. However, the nature of this crosstalk is poorly understood and is an area in need of further investigation. As outlined above, the oxygen sensing hydroxylases responsible for conferring oxygen/hypoxia sensitivity to the HIF pathway do not appear to play a direct role on conferring CO2 sensitivity to the NF-κB pathway. However, it should be noted that these enzymes produce CO2 during their oxygen sensing enzymatic activity in the regulation of HIF and therefore may contribute to the intracellular levels of CO2 (Fig. 1). Furthermore, studies in hyperoxia have demonstrated a possible role for reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the regulation of NF-κB (reviewed by D’Angio & Finkelstein, 2000). Because both O2 and CO2 play an important role in determining ROS/RNS levels in cells (Dean, 2010), altered intracellular redox potential may be a point of convergence between O2 and CO2 in the regulation of gene expression through NF-κB.
CO2 in health and disease
In normal conditions, 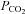 levels in the body are likely to vary between tissues and individual cells. Typical arterial blood
levels in the body are likely to vary between tissues and individual cells. Typical arterial blood 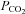 values are in the range of 35–45 mmHg. A thorough review of the contribution of CO2 to physiological and pathophysiological processes has recently been published elsewhere (Curley et al. 2010).
values are in the range of 35–45 mmHg. A thorough review of the contribution of CO2 to physiological and pathophysiological processes has recently been published elsewhere (Curley et al. 2010).
Hypocapnia is the condition which arises when the mean arterial 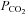 is lower than normal. It usually occurs as a consequence of hyperventilation in conditions such as asthma and acute lung injury. While hypocapnia is associated with adverse outcomes in diabetic ketoacidosis (Glaser et al. 2001), therapeutic hypocapnia is used in the management strategy for brain injury associated with increased intracranial pressure (Neumann et al. 2008).
is lower than normal. It usually occurs as a consequence of hyperventilation in conditions such as asthma and acute lung injury. While hypocapnia is associated with adverse outcomes in diabetic ketoacidosis (Glaser et al. 2001), therapeutic hypocapnia is used in the management strategy for brain injury associated with increased intracranial pressure (Neumann et al. 2008).
Hypercapnia arises when the mean arterial 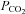 is elevated above normal levels and can occur as a consequence of respiratory failure (e.g. in chronic obstructive pulmonary disease), but clinically it is commonly seen as a consequence of a low tidal volume ventilation strategy for acute respiratory distress syndrome (ARDS). Environmental hypercapnia may also occur in the natural habitats of burrowing animals (Lechner, 1976). Mechanical ventilation strategies to elicit normal physiological blood gas levels have been associated with stretch-induced mechanical lung damage. In the Acute Respiratory Distress Syndrome Network (ARDSnet), multi-centre trial examining lower tidal volumes as compared to traditional tidal volumes, there was a significant decrease in patient mortality in the lower tidal volume group (ARDSnet, 2000). Hypercapnic acidosis (HCA), which can be a consequence of patient hypoventilation, was also identified as being associated with decreased mortality in a subset of the ARDSnet patient cohort (patients receiving 12 ml kg−1 tidal volumes who were defined as having hypercapnic acidosis on day 1 of the study) independent of changes in mechanical ventilation (Kregenow et al. 2006). Taken together these data are suggestive of elevated CO2 levels being protective in the critically ill patient.
is elevated above normal levels and can occur as a consequence of respiratory failure (e.g. in chronic obstructive pulmonary disease), but clinically it is commonly seen as a consequence of a low tidal volume ventilation strategy for acute respiratory distress syndrome (ARDS). Environmental hypercapnia may also occur in the natural habitats of burrowing animals (Lechner, 1976). Mechanical ventilation strategies to elicit normal physiological blood gas levels have been associated with stretch-induced mechanical lung damage. In the Acute Respiratory Distress Syndrome Network (ARDSnet), multi-centre trial examining lower tidal volumes as compared to traditional tidal volumes, there was a significant decrease in patient mortality in the lower tidal volume group (ARDSnet, 2000). Hypercapnic acidosis (HCA), which can be a consequence of patient hypoventilation, was also identified as being associated with decreased mortality in a subset of the ARDSnet patient cohort (patients receiving 12 ml kg−1 tidal volumes who were defined as having hypercapnic acidosis on day 1 of the study) independent of changes in mechanical ventilation (Kregenow et al. 2006). Taken together these data are suggestive of elevated CO2 levels being protective in the critically ill patient.
Therapeutic hypercapnia has been reported to be of benefit in ischaemia–reperfusion injury in the mesentery (Laffey et al. 2003) and recently in the liver (Li et al. 2010). The mechanisms for this protection are not yet fully elucidated in vivo, but the latter study reports attenuated IRI-mediated pro-inflammatory gene expression (TNFα), enhanced anti-inflammatory cytokine production (IL-10), decreased apoptosis and decreased immunohistochemical staining for NF-κB in the hypercapnia treated groups. These studies are consistent with the observations described above for CO2 (independent of extracellular pH) having a suppressive effect on NF-κB signalling (Cummins et al. 2010; Wang et al. 2010) and of hypercapnic acidosis blunting endotoxin-stimulated NF-κB signalling, resulting in decreased ICAM-1 and IL-8 expression in pulmonary endothelial cells (Takeshita et al. 2003).
The role of intracellular acidosis in the protective effects of hypercapnia remains to be fully elucidated. In vitro experiments provide evidence that CO2 driven anti-inflammatory gene expression is independent of changes in extracellular pH (Cummins et al. 2010; Wang et al. 2010), while in vivo studies indicate the protective effects of HCA to be dependent on acidosis in experimental models of lung injury (Laffey et al. 2000).
Hypercapnic acidosis is associated with better prognosis in inflammatory conditions such as ARDS (Amato et al. 1998; ARDSnet, 2000), ischaemia–reperfusion injury (Li et al. 2010) and models of acute lung injury (Laffey et al. 2004). Conversely, experiments examining bacterial-induced lung injury in mice (Nichol et al. 2009) and in pathogen-challenged Drosophila (Helenius et al. 2009) demonstrate that exposure to high CO2 results in a worse prognosis. Furthermore, the initial benefit of HCA in the case of attenuated lung damage may be negated through a reduced ability of pulmonary epithelial cells to promote wound healing (via NF-κB) (O’Toole et al. 2009). Taken together with the recent data demonstrating the blunting of NF-κB pathway-dependent genes with elevated CO2, these outcomes are perhaps not as unexpected as they first seem. CO2 through its modulation of NF-κB signalling has the ability to both suppress inflammatory signalling and diminish innate immune responses. Depending on the nature of the challenge, CO2 and/or HCA can both blunt inflammation driven tissue damage as in the case of LPS-induced lung injury and exacerbate lung damage in response to pathogen infection. This has clear implications for the potential therapeutic applications of CO2 in the clinic where CO2 suppresses inflammation but also the ability to fight infection.
Therapeutic applications of CO2
Given the increasing evidence for a potent effect of CO2 on gene expression and in directing the course of human and animal models of disease, it is important to consider the opportunities for the therapeutic manipulation of CO2 levels.
The ARDS net tidal volume study provides clear evidence for the benefit of lower tidal volumes in patients with acute lung injury and ARDS (ARDSnet, 2000). Use of lower tidal volumes in the ICU results in associated permissive HCA and evidence from models of HCA points to the importance of elevated CO2 in contributing to the beneficial patient outcome. However, concerns over the potential deleterious effects of CO2 with respect to immune suppression and wound healing have stunted the progression of trials for therapeutic hypercapnia (where CO2 is actually administered via the ventilator).
With this in mind there is perhaps more potential for therapeutic strategies directed towards controlled and localized delivery of CO2 to an acute inflammatory locus, e.g. to the gut in IBD, or where organ injury can be predicted and controlled, e.g in IRI or transplantation. Recent studies have pointed to the beneficial effects of CO2 pneumoperitonieum post-laparoscopy (Ikechebelu et al. 2005) and in animal models of LPS-induced sepsis (Hanly et al. 2006). This suggests that laparoscopic surgery with CO2 used as the insufflating gas as may be of particular benefit in surgical procedures with a high risk of developing sepsis, e.g. stomach trauma.
Summary and conclusions
Metazoans evolved to consume oxygen and generate carbon dioxide during the process of aerobic metabolism to maximize the efficiency of metabolic processes. As a consequence, it is not surprising that animals have evolved the ability to sense and respond to alterations in the availability of these important physiological gasses via altered gene transcription in order to maintain metabolic homeostasis. The ability of cells to sense oxygen deprivation (hypoxia) via reduced activity of hydroxylase enzymes resulting in the induction of the hypoxia-inducible factor (HIF) have been well characterized (Semenza, 2007; Kaelin & Ratcliffe, 2008). However, the mechanisms by which cells sense changes in CO2 leading to altered gene transcription remain less well understood. Recent advances have identified the repression of the NF-κB transcriptional pathway by CO2 in a manner which may be of therapeutic benefit in chronic inflammatory disease. Interestingly, NF-κB is also a hypoxia-responsive transcription factor (Fig. 1). Future studies will be aimed at identifying the CO2 sensor and determining whether crosstalk between O2 and CO2 sensing pathways exists at the level of transcription factors such as NF-κB.
References
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- ARDSnet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay PK, Garrett JE, Shetty RP, Keate T, Walker CS, Olivera BM. Gamma-Glutamyl carboxylation: an extracellular posttranslational modification that antedates the divergence of molluscs, arthropods, and chordates. Proc Natl Acad Sci U S A. 2002;99:1264–9. doi: 10.1073/pnas.022637099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling DJ, Berner RA. Feedbacks and the coevolution of plants and atmospheric CO2. Proc Natl Acad Sci U S A. 2005;102:1302–1305. doi: 10.1073/pnas.0408724102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner RA. The long-term carbon cycle, fossil fuels and atmospheric composition. Nature. 2003;426:323–326. doi: 10.1038/nature02131. [DOI] [PubMed] [Google Scholar]
- Berner RA, Kothavala Z. Geocarb III: A revised model of atmospheric CO2 over phanerozoic time. Am J Science. 2001;301:182–204. [Google Scholar]
- Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, Scholz CC, Slattery C, Leonard MO, McLoughlin P, Taylor CT. NF-κB links CO2 sensing to innate immunity and inflammation in mammalian cells. J Immunol. 2010;185:4439–4445. doi: 10.4049/jimmunol.1000701. [DOI] [PubMed] [Google Scholar]
- Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Crit Care. 2010;14:220. doi: 10.1186/cc8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angio CT, Finkelstein JN. Oxygen regulation of gene expression: a study in opposites. Mol Genet Metab. 2000;71:371–380. doi: 10.1006/mgme.2000.3074. [DOI] [PubMed] [Google Scholar]
- Dean JB. Hypercapnia causes cellular oxidation and nitrosation in addition to acidosis: implications for CO2 chemoreceptor function and dysfunction. J Appl Physiol. 2010;108:1786–1795. doi: 10.1152/japplphysiol.01337.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Ann NY Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- De Simone G, Supuran CT. Carbonic anhydrase IX: Biochemical and crystallographic characterization of a novel antitumor target. Biochim Biophys Acta. 2010;1804:404–409. doi: 10.1016/j.bbapap.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Frommer WB. Biochemistry. CO2mmon sense. Science. 2010;327:275–276. doi: 10.1126/science.1186022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, Kaufman F, Quayle K, Roback M, Malley R, Kuppermann N. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344:264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- Hanly EJ, Fuentes JM, Aurora AR, Bachman SL, De Maio A, Marohn MR, Talamini MA. Carbon dioxide pneumoperitoneum prevents mortality from sepsis. Surg Endosc. 2006;20:1482–1487. doi: 10.1007/s00464-005-0246-y. [DOI] [PubMed] [Google Scholar]
- Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, Johnson EA, Sporn PH, Sznajder JI, Beitel GJ. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc Natl Acad Sci U S A. 2009;106:18710–18715. doi: 10.1073/pnas.0905925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikechebelu JI, Obi RA, Udigwe GO, Joe-Ikechebelu NN. Comparison of carbon dioxide and room air pneumoperitoneum for day-case diagnostic laparoscopy. J Obstet Gynaecol. 2005;25:172–173. doi: 10.1080/01443610500051528. [DOI] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006;34:1–7. doi: 10.1097/01.ccm.0000194533.75481.03. [DOI] [PubMed] [Google Scholar]
- Lacis AA, Schmidt GA, Rind D, Ruedy RA. Atmospheric CO2: principal control knob governing Earth's temperature. Science. 2010;330:356–359. doi: 10.1126/science.1190653. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Engelberts D, Kavanagh BP. Buffering hypercapnic acidosis worsens acute lung injury. Am J Respir Crit Care Med. 2000;161:141–146. doi: 10.1164/ajrccm.161.1.9905080. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Honan D, Hopkins N, Hyvelin JM, Boylan JF, McLoughlin P. Hypercapnic acidosis attenuates endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 2004;169:46–56. doi: 10.1164/rccm.200205-394OC. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Jankov RP, Engelberts D, Tanswell AK, Post M, Lindsay T, Mullen JB, Romaschin A, Stephens D, McKerlie C, Kavanagh BP. Effects of therapeutic hypercapnia on mesenteric ischemia-reperfusion injury. Am J Respir Crit Care Med. 2003;168:1383–1390. doi: 10.1164/rccm.2108078. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- Lechner AJ. Respiratory adaptations in burrowing pocket gophers from sea level and high altitude. J Appl Physiol. 1976;41:168–173. doi: 10.1152/jappl.1976.41.2.168. [DOI] [PubMed] [Google Scholar]
- Li AM, Quan Y, Guo YP, Li WZ, Cui XG. Effects of therapeutic hypercapnia on inflammation and apoptosis after hepatic ischemia-reperfusion injury in rats. Chin Med J (Engl) 2010;123:2254–2258. [PubMed] [Google Scholar]
- Li G, Zhou D, Vicencio AG, Ryu J, Xue J, Kanaan A, Gavrialov O, Haddad GG. Effect of carbon dioxide on neonatal mouse lung: a genomic approach. J Appl Physiol. 2006;101:1556–1564. doi: 10.1152/japplphysiol.01031.2005. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Ortega-Saenz P, Pardal R, Pascual A, Piruat JI, Duran R, Gomez-Diaz R. Oxygen sensing in the carotid body. Ann N Y Acad Sci. 2009;1177:119–131. doi: 10.1111/j.1749-6632.2009.05033.x. [DOI] [PubMed] [Google Scholar]
- Luo M, Sun L, Hu J. Neural detection of gases – carbon dioxide, oxygen – in vertebrates and invertebrates. Curr Opin Neurobiol. 2009;19:354–361. doi: 10.1016/j.conb.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Neumann JO, Chambers IR, Citerio G, Enblad P, Gregson BA, Howells T, Mattern J, Nilsson P, Piper I, Ragauskas A, Sahuquillo J, Yau YH, Kiening K. The use of hyperventilation therapy after traumatic brain injury in Europe: an analysis of the BrainIT database. Intensive Care Med. 2008;34:1676–1682. doi: 10.1007/s00134-008-1123-7. [DOI] [PubMed] [Google Scholar]
- Nichol AD, O’Cronin DF, Howell K, Naughton F, O’Brien S, Boylan J, O’Connor C, O’Toole D, Laffey JG, McLoughlin P. Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit Care Med. 2009;37:2953–2961. doi: 10.1097/CCM.0b013e3181b028ce. [DOI] [PubMed] [Google Scholar]
- O’Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O’Brien T, Laffey JG. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-κB dependent mechanism. Thorax. 2009;64:976–982. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- Royer DL, Berner RA, Park J. Climate sensitivity constrained by CO2 concentrations over the past 420 million years. Nature. 2007;446:530–532. doi: 10.1038/nature05699. [DOI] [PubMed] [Google Scholar]
- Schaefer KE, McCabe N, Withers J. Stress response in chronic hypercapnia. Am J Physiol. 1968;214:543–548. doi: 10.1152/ajplegacy.1968.214.3.543. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Sharabi K, Hurwitz A, Simon AJ, Beitel GJ, Morimoto RI, Rechavi G, Sznajder JI, Gruenbaum Y. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:4024–4029. doi: 10.1073/pnas.0900309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K, Suzuki Y, Nishio K, Takeuchi O, Toda K, Kudo H, Miyao N, Ishii M, Sato N, Naoki K, Aoki T, Suzuki K, Hiraoka R, Yamaguchi K. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-κB activation. Am J Respir Cell Mol Biol. 2003;29:124–132. doi: 10.1165/rcmb.2002-0126OC. [DOI] [PubMed] [Google Scholar]
- Tang XD, Santarelli LC, Heinemann SH, Hoshi T. Metabolic regulation of potassium channels. Annu Rev Physiol. 2004;66:131–159. doi: 10.1146/annurev.physiol.66.041002.142720. [DOI] [PubMed] [Google Scholar]
- Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke TR, Armstrong HA, Williams M, Paris F, Zalasiewicz JA, Sabbe K, Nolvak J, Challands TJ, Verniers J, Servais T. Polar front shift and atmospheric CO2 during the glacial maximum of the Early Paleozoic Icehouse. Proc Natl Acad Sci U S A. 2010;107:14983–14986. doi: 10.1073/pnas.1003220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Gates KL, Trejo H, Favoreto S, Jr, Schleimer RP, Sznajder JI, Beitel GJ, Sporn PH. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010;24:2178–2190. doi: 10.1096/fj.09-136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-α is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]


