Abstract
The sense of orientation during locomotion is derived from our spatial relationship with the external environment, sensed predominantly by sight and sound, and from internal signals of motion, generated by the vestibular sense and the pattern of efferent and afferent signals to the muscles and joints. The sensory channels operate in different reference frames and have different time-dependent adaptive properties and yet the inputs are combined by the central nervous system to create an internal representation of self-motion. In normal circumstances vestibular, visual and proprioceptive cues provide congruent information on locomotor trajectory; however, in cases of sensory discord there must be a recalibration of sensory signals to provide a unitary representation. We develop a means of studying these fusion processes by perturbing each channel in isolation about a consistent behavioural axis. This review focuses on creating the vestibular perturbation of the orientation sense by transmastoidal galvanic stimulation, a technique generally used to evoke balance reflexes. Vector summation across the population of semicircular canal afferents creates a net signal that is interpreted by the brain as a vector of angular acceleration in a craniocentric reference frame. The signal feeds perceptual processes of orientation after transformation that resolves the 3-D signal onto the terrestrial or behavioural plane. Changing head posture changes the interpretation of the galvanic vestibular signal for balance and orientation responses. With appropriate head alignments during locomotion, the galvanic stimulus can be used to either steer trajectory over the terrestrial plane or perturb balance.

Rebecca St George (BSc, BA, PhD) has undergraduate degrees in Physics and Psychology from the University of Newcastle and a PhD in Physiology from the University of New South Wales. She has a broad interest in human sensorimotor control processes in health and disease with a focus on the neural processes that integrate sensory information from the vestibular, visual and proprioceptive systems. She is currently investigating the effect of deep brain stimulation on postural control in Parkinson's disease at the Oregon Health & Science University. Richard Fitzpatrick studied in Sydney, receiving his MD and PhD from the University of New South Wales. At Neuroscience Research Australia, his broad research interests include integrated aspects of human sensory, motor and cardiovascular function.
As we move about the world, a stream of sensory information informs the brain about our motion and orientation with respect to the local environment. During locomotion, our turn and trajectory is sensed by the visual system as patterns of optic flow, by somatosensors and proprioceptors as torsion of the body relative to the foot contact, by the vestibular organs as accelerations of the head, and by the auditory system as changes in the sound waves that arrive at the ears. In normal circumstances, neural processes of sensorimotor fusion unite these different sensory signals to create a single representation of self-motion and orientation in space that allows for functional motor actions.
Sensory systems encode information about the environment and our movement through it as patterns of activity across populations of neurons. A problem that the brain has to solve is that the different sensory systems transduce stimuli in different reference frames. Vision, hearing and olfaction operate in a reference frame tied to external landmarks. For our species, visual targets most commonly guide our behaviour and this might give vision a special role in relating the external world to the internal representation that is used to drive action. The vestibular system signals head movement in a gravito-inertial reference frame, which is not necessarily aligned with the visual reference frame. During locomotion, a motor command rotates the pelvis and trunk over the feet to steer progress in the desired direction and external forces act on the body. The proprioceptive system operates in internal body coordinates reporting on the relationship between body segments and the feet in contact with the ground. Each channel has specific sensitivities and optimal frequency responses with a high-pass vestibular system, low-pass visual system, and a relatively broadband proprioceptive system. How does the brain combine these different patterns of activity to create an internal representation with a common functional reference frame? To form this coherent internal map, the different sensory signals must be transformed in ways that allow flexibility for situations in which there is redundancy or ambiguity between inputs.
The processes of fusing different sensory signals for orientation can be explored in human subjects by examining locomotor trajectories during and after periods of sensory conflict. Experimental sensory conflict is created by injecting a false probe signal into a sensory channel. The visual signal of self-motion can be perturbed by whole-field optic flow, which evokes the well-known vection sensations of self-motion (Brandt et al. 1974) and after-rotation when walking (Gordon et al. 2003). Proprioceptive cues can likewise be perturbed in isolation by techniques such as muscle vibration (Bove et al. 2001, 2002) and podokinetic conditioning to misalign the locomotor somatosensory signal with the other sensory channels (Gordon et al. 1995; Rieser et al. 1995; Pick et al. 1999; Reynolds & Bronstein, 2004). Perturbing the vestibular system in isolation is more difficult as physically moving the head and body in the all-pervasive gravito-inertial field to activate the vestibular end-organs necessarily means changing the inflow into the other channels. In the section that follows, we consider how the technique of galvanic vestibular stimulation, which is known to evoke balance reflexes, can be used to evoke virtual, isolated signals of self-motion and altered orientation.
Balance reflexes evoked by galvanic vestibular stimulation
It has been known for more than a century that a transmastoidal current applied during standing disrupts balance and causes sway to the anodal side and, more recently, that this occurs mostly through an automatic stereotyped reflex response (Nashner & Wolfson, 1974; Britton et al. 1993; see review in: Fitzpatrick & Day, 2004). Although stereotyped, these responses are highly integrated with the sensorimotor postural control as their target muscles change in accord with the alignment of the head relative to the support base (Lund & Broberg, 1983; Britton et al. 1993; Pastor et al. 1993). For a long time this balance reaction was considered to result from a left–right imbalance of the discharge from vestibular otolithic afferents creating a signal of altered alignment relative to gravity. However, several lines of evidence indicated that an altered signal of motion rather than gravitational alignment generated the sway response (Day & Cole, 2002; Wardman et al. 2003a,b;). This implicated the semicircular canals as the basis of these responses. However, the galvanic stimulus is not selective for canal afferents over otolithic afferents. Instead, it appears that the alignment of hair cells over the macular surfaces is such that there is signal cancellation across the afferent population peculiar to the galvanic stimulus. We know from animal studies that anodal and cathodal currents modulate the ongoing discharge of individual irregularly firing canal afferents in the same way as ipsilateral and contralateral angular accelerations (Goldberg et al. 1982, 1984; Kim & Curthoys, 2004). Anodal currents decrease and cathodal currents increase firing rates. A reproducible galvanic-evoked signal of motion from the semicircular canals implies that the brain interprets the change in firing rates across the afferent population as equivalent to a real-world perturbation and generates an appropriate reflex response.
From the anatomical alignments of the canals in the skull (Blanks et al. 1975) and assuming that the spontaneous firing rates from all six semicircular canals are equally modulated, Fitzpatrick & Day (2004) calculated that by vector summation, the net rotational vector generated by transmastoidal galvanic stimulation (anode-right, cathode-left) would be directed posteriorly and inclined upward (Fig. 1A). Two balance studies have substantiated that prediction, showing that the reflex producing the response is not seen when the head is aligned so that predicted galvanic vector is aligned vertically with gravity so that the evoked vestibular signal has no relevance for balance (Cathers et al. 2005; Mian et al. 2010).
Figure 1. Rotational vectors evoked by galvanic stimulation.
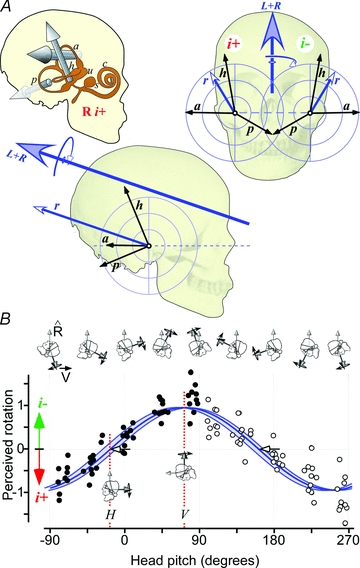
A, unit vectors for each canal (a, h, p) in head coordinates (relative to Reid's plane) are perpendicular to the plane of each canal with direction determined by the hair cell orientation and galvanic stimulus polarity (right i+, left i−). For each labyrinth, the vector sum (r) is directed posteriorly, laterally and upwards. The vector sum of both sides (L+R) is directed posteriorly and upwards, indicating a signal of approximate head roll towards the cathodal side. B, perceived rotation in normalised units for binaural bipolar galvanic stimuli (as in A) with the head at different angles of pitch with least-squares sinusoidal fit. Shaded, between the blue lines is the 95% confidence interval of the anatomical prediction. The head angle at which the vestibular stimulus produces no perception of rotation is shown (16.4 deg measured, 18.8 deg predicted).
In contrast to vision or sound, the vestibular sense appears to be created by unifying signals from each side so that divergence carries no meaning (Day et al. 2010). Unlike real kinetic stimuli that produce consistent responses from the two labyrinths, the rotation signals evoked by the galvanic stimulus are bilaterally divergent (Fig. 1A) which would never be normally experienced, but bizarre, dysphoric or ‘head-splitting’ sensations are never reported. This vector summation process that averages and rejects oppositely directed signals operates at the first instance of a stimulus and does not require a period of adaptation, indicating that it is intrinsic to the processing of signals from the entire afferent population of the six canals.
Altered perceptions of orientation through vestibular stimulation
Vestibular afferents project through short-latency pathways to widespread areas of the cortex and are thought to underlie the processing of self-motion signals for different purposes (Lobel et al. 1999; de Waele et al. 2001). In accord with this, galvanic stimulation of vestibular afferents generates movement illusions, originally described as sway-like movements with the head upright and the body vertical (Fitzpatrick et al. 1994). In testing the prediction of the galvanic canal vector, Day & Fitzpatrick (2005) show that in seated subjects, the same stimulus generates illusions of whole-body rotation in the terrestrial plane that vary as a cyclic function of head pitch. As predicted by the theoretical direction of the galvanic vector, the stimulus creates a perception of whole-body yaw about the vertical with the head bent forward or backward to align the galvanic vector vertically but no yaw illusion with the head upright (Fig. 1B).
This cyclic relationship between the galvanic perceptual response and head pitch shows that the neural processes feeding orientation and self-motion perception perform the equivalent of calculating the dot product of the head rotation vector and the gravitational unit vector, in this situation of an earth-horizontal rotation. Whether the result applies for rotation about a horizontal axis in a vertical plane has yet to be determined.
Steering locomotion by galvanic stimulation
Vestibular pathology causes navigation errors unrelated to balance problems when walking without vision (Glasauer et al. 2002), indicating a specific central processing of information about movement in the terrestrial plane. Head-direction cells in forebrain and midbrain structures discharge according to specific head orientations in the earth-horizontal reference frame, regardless of whole-body orientation or other behaviours (Taube, 2007, 2010). When available, visual landmark cues dominate their firing patterns but ascending vestibular projections also influence this central head-direction signal (Stackman & Taube, 1997; Sharp et al. 2001). Place cells within the hippocampus code spatial location relative to environmental landmarks (rather than direction) and similarly depend on vestibular information (O’Keefe, 1976; Best et al. 2001; Stackman et al. 2002).
To update this complex neural substrate of the 2-D terrestrial map, the vestibular signal must be projected onto the map surface to isolate the orientation signal. Responses to galvanic stimulation during walking show that extracting the orientation signal relies on knowledge of the 3-D orientation of the head relative to the behavioural surface, which in this case is the horizontal terrestrial surface. When blindfolded subjects attempted to walk straight ahead, bipolar galvanic vestibular stimulation evoked balance responses when the head was upright (i.e. Reid's plane tilted 18 deg backwards; H in Fig. 1B) but, allowing for initial stumbling, subjects walked a straight trajectory (Fig. 2, left). In contrast, when the head was pitched forwards (72 deg; V in Fig. 1B) there were no balance reactions but walking trajectories curved in the direction of the anode as subjects cancelled the galvanic-evoked perception of turn towards the cathode (Fig. 2, right). By continuously modulating stimulus current, blindfolded subjects can be steered by ‘remote control’ along winding paths and avoid obstacles and people – although this also brings the skill of the driver into play. However, it is essential that they keep the head bent forward so that the canal signal represents turning. If for some reason they raise the head to vertical, as with startle or unexpected foot contact, they stumble sideways attempting to regain balance as the same vestibular signal in head coordinates becomes a roll or pitch signal in earth coordinates and drives automatic balance reflexes.
Figure 2. Vestibular influences on balance and orientation during walking.
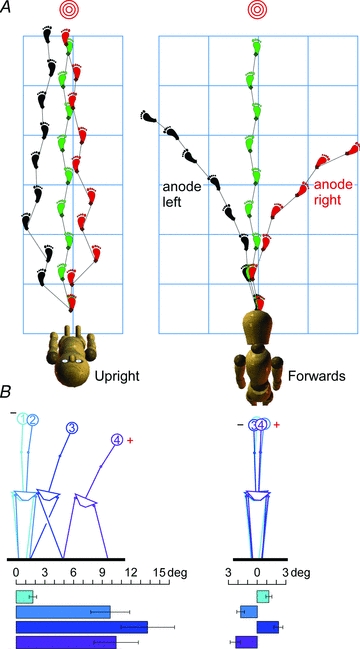
A, trajectories of a subject attempting to walk to the target with the eyes shut are shown as foot placements (adapted from:Fitzpatrick et al. 2006). On the left, with the head upright, the galvanic stimulus produces staggered foot placements to the side of the anode because of balance responses, not orientation responses. The balance responses, seen in B, show large trunk tilt during the first four steps. On the right, with the head tilted forward facing the floor, the galvanic stimulus causes smooth turning in the direction of the anodal electrode without balance responses.
The brain has available different sources of information about the orientation of the head relative to the behavioural surface to perform this transformation. The gravitational vector detected by the otolith organs is normal to a horizontal floor during walking and could provide an ‘in-head’ reference signal. However, head-direction cells maintain their firing pattern when an animal walks up an incline or wall, as if it is an extension of the floor, but lose directional sensitivity when the animal is upside down (Taube, 2010), and human subjects perceive rotations about a horizontal axis if they receive a galvanic stimulus when immobilized with the upright (Fitzpatrick et al. 1994). Proprioceptive signals of joint angles provide information about head alignment relative to the behavioural plane, in this instance ground contact with the feet during walking. As the direction of vestibular balance reflexes changes on turning the head horizontally in gravity, these transformations appear to involve proprioceptive signals. The directions of contact and thrust forces through the body provide information about the relationship between the behavioural plane and the gravitational vertical. Thus, the relationship between the behavioural surface, the gravitational vertical and the posture of the body all impact on the transformations that create the internal spatial map. Until the operational space of this internal map is understood, it is sensible that we keep in mind the distinction between the gravitational horizontal and the behavioural surface.
Adaptation of the vestibular orientation signal
Response adaptation to constant stimuli is common to neural processing of all sensory processes and is seen with sustained podokinetic and visual influences on self-motion perception. For the semicircular canals, rapid peripheral adaptation of constant angular-velocity stimuli by canal-cupula mechanics masks central adaptation processes. Galvanic stimulation acts at the hair-cell and afferent complex, bypassing the canal-cupula mechanics (Goldberg et al. 1982, 1984; Rabbitt et al. 2005). We used this galvanic activation property to examine the central adaptation of vestibular input to the self-motion spatial map (St George et al. 2010). When a kinetic rotation was delivered by real rotation, verbally reported perceptions of rotation rapidly declined to baseline (and transiently beyond) with a time constant (range of 10–20 s) reflecting the canal adaptation and its prolongation by the brainstem velocity-storage mechanism (Fig. 3). While stationary with the head pitched forward, the perception of rotation generated by galvanic stimulation declined to zero but this took almost 2 min. When the stimulus was turned off, an equivalent perception of rotation in the opposite direction indicates a central adaptation rather than habituation process.
Figure 3. Adaptation of vestibular signals for orientation and self-motion perception.
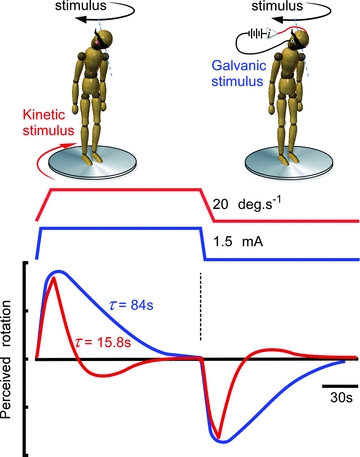
With the subject stationary on the platform, platform rotation produced a kinetic stimulus (real) with a ramp and hold velocity profile to evoke a vestibular signal of whole-body yaw. With the platform stationary and head alignment of Fig. 1B‘V’, a constant galvanic stimulus (virtual) also evoked a vestibular signal of whole-body yaw. Reports of self-motion during and after both stimuli showed signal adaptation to baseline then reversal of the perception when the rotation or current ceased. Adaptation to the kinetic stimulus is rapid through fast decay of canal-cupula mechanics (time constant 7.2 s) and an attempt to prolong the signal by the velocity storage mechanism (time constant 7.7 s). Adaptation to the galvanic stimulus, which bypasses the canal-cupula adaptation, reveals a neural adaptation with long time constant (76 s) likely to involve afferent and central processes.
Vestibular signals are frequency encoded around a central firing rate. How they maintain a stable sense over time is not understood, as the signals from the canals have no fixed reference. This central adaptive process identified by galvanic stimulation automatically zeros the vestibular signal to define the signal that represents no rotation. The central long-term adaptation of the vestibular signal may be a general property of the self-motion perception machinery, which receives information from multiple sensory sources and filters out unvarying signals regardless of their origin.
Galvanic stimulation ‘rules’ for behavioural responses
These results show that both balance reflexes and perceptions of motion and orientation evoked by galvanic stimulation follow the same rules of head orientation. Just as the three canal planes define the posture of the head for caloric testing and the direction of impulse stimuli for testing head-referenced vestibulo-ocular reflexes, Fig. 4 defines the head postures for bipolar galvanic stimuli that produce earth-referenced behavioural responses in subjects with normal function. Galvanic vestibular stimulation is being used more frequently in experimental and clinical situations and it is critical that head alignment is controlled and considered to interpret observed responses.
Figure 4. Galvanic stimulation to evoke responses in the three cardinal planes.
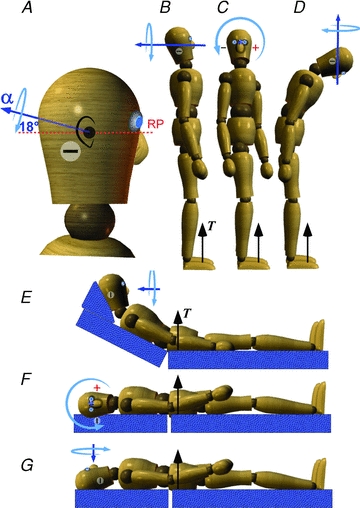
A, the net angular acceleration vector (α) evoked by bipolar binaural galvanic stimulation with the cathodal electrode on the right is directed posteriorly and inclined 18 deg upward from Reid's plane (RP). B, to generate a vestibular signal of roll (lateral) relative to the vertical gravitational thrust vector (T), the subject faces forward with the head tilted slightly backwards so that Reid's plane is inclined 18 deg down and back. C, to generate a signal of pitch (sagittal) relative to T, the same backward head tilt is maintained while turning the head 90 deg relative to the feet. D, to generate a signal of yaw (horizontal) the head is bent forward so that Reid's plane is inclined 72 deg down and forward. Thus, during standing, postures B and C will evoke lateral and sagittal sway, respectively, whereas D will not produce a balance response but will create a sense of horizontal rotation. E, F and G show the directions of perceptual and balance responses for the three head postures with the body supine.
In addition to identifying the direction of the galvanic responses, the declining profile of the perceptual response to constant galvanic stimuli (St George et al. 2010) follows the profile of constant real acceleration stimulation (Guedry & Lauver, 1961). This agreement corroborates the view that the brain interprets the profile of a galvanic stimulus as if it was an angular acceleration.
Exploring multisensory fusion for the sense of orientation
The ability to produce a ‘pure’ vestibular stimulus of known direction and decay profile allows the interactions between sensory channels and their adaptations to be investigated. We have used the technique to study locomotor trajectories when a vestibular signal of rotation is accompanied by a visual or proprioceptive signal of no rotation, or rotations in the opposite or same direction about the same axis. This has allowed us to measure the adaptations of each channel and how they interact in different combinations.
Results of this research indicate that when the distribution of sensory inflow is such that an applied signal creates a sensation of self-motion, the reafference of all channels is recalibrated to the new weighted sensation so that a single internal representation is maintained (St George & Fitzpatrick, 2010).
Summary
Galvanic stimulation is a simple technique that can evoke controlled sensations of self-motion and altered orientation as well as balance and ocular responses when delivered appropriately. Independent of any real movement, they represent responses to a pure vestibular input that the brain interprets as an angular acceleration vector fixed in head coordinates. Whole-body sensations are created by transformations of vector summation and then projection on to the behavioural surface. The specific interpretation of the galvanic-evoked signal is determined by the orientation of the head relative to the rest of the body and its contact forces. The technique has many potential applications. Our focus in the work presented at The Journal of Physiology Symposium is to probe the neural processes of fusing the inputs from many sensory channels to construct a unified self-motion sense.
Acknowledgments
Supported by the National Health and Medical Research Council, Australia.
References
- Best PJ, White AM, Minai A. Spatial processing in the brain: the activity of hippocampal place cells. Annu Rev Neurosci. 2001;24:459–486. doi: 10.1146/annurev.neuro.24.1.459. [DOI] [PubMed] [Google Scholar]
- Blanks RH, Curthoys IS, Markham CH. Planar relationships of the semicircular canals in man. Acta Otolaryngol. 1975;80:185–196. doi: 10.3109/00016487509121318. [DOI] [PubMed] [Google Scholar]
- Bove M, Courtine G, Schieppati M. Neck muscle vibration and spatial orientation during stepping in place in humans. J Neurophysiol. 2002;88:2232–2241. doi: 10.1152/jn.00198.2002. [DOI] [PubMed] [Google Scholar]
- Bove M, Diverio M, Pozzo T, Schieppati M. Neck muscle vibration disrupts steering of locomotion. J Appl Physiol. 2001;91:581–588. doi: 10.1152/jappl.2001.91.2.581. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dichgans J, Buchle W. Motion habituation: inverted self-motion perception and optokinetic after-nystagmus. Exp Brain Res. 1974;21:337–352. doi: 10.1007/BF00237897. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005;563:229–234. doi: 10.1113/jphysiol.2004.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Cole J. Vestibular-evoked postural responses in the absence of somatosensory information. Brain. 2002;125:2081–2088. doi: 10.1093/brain/awf212. [DOI] [PubMed] [Google Scholar]
- Day BL, Fitzpatrick RC. Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol. 2005;567:591–597. doi: 10.1113/jphysiol.2005.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Marsden JF, Ramsay E, Mian OS, Fitzpatrick RC. Non-linear vector summation of left and right vestibular signals for human balance. J Physiol. 2010;588:671–682. doi: 10.1113/jphysiol.2009.181768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waele C, Baudonniere PM, Lepecq JC, Tran Ba Huy P, Vidal PP. Vestibular projections in the human cortex. Exp Brain Res. 2001;141:541–551. doi: 10.1007/s00221-001-0894-7. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Butler JE, Day BL. Resolving head rotation for human bipedalism. Curr Biol. 2006;16:1509–1514. doi: 10.1016/j.cub.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim MA, Viaud-Delmon I, Berthoz A. Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path. Exp Brain Res. 2002;145:489–497. doi: 10.1007/s00221-002-1146-1. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C, Smith CE. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res. 1982;252:156–160. doi: 10.1016/0006-8993(82)90990-8. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Gordon CR, Fletcher WA, Melvill Jones G, Block EW. Adaptive plasticity in the control of locomotor trajectory. Exp Brain Res. 1995;102:540–545. doi: 10.1007/BF00230658. [DOI] [PubMed] [Google Scholar]
- Gordon CR, Tal D, Gadoth N, Shupak A. Prolonged optokinetic stimulation generates podokinetic after rotation. Ann N Y Acad Sci. 2003;1004:297–302. doi: 10.1196/annals.1303.027. [DOI] [PubMed] [Google Scholar]
- Guedry F, Lauver L. Vestibular reactions during prolonged constant angular acceleration. J Appl Physiol. 1961;16:215–220. [Google Scholar]
- Kim J, Curthoys IS. Responses of primary vestibular neurons to galvanic vestibular stimulation (GVS) in the anaesthetised guinea pig. Brain Res Bull. 2004;64:265–271. doi: 10.1016/j.brainresbull.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lobel E, Kleine JF, Leroy-Willig A, Van de Moortele PF, Le Bihan D, Grusser OJ, Berthoz A. Cortical areas activated by bilateral galvanic vestibular stimulation. Ann N Y Acad Sci. 1999;871:313–323. doi: 10.1111/j.1749-6632.1999.tb09194.x. [DOI] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Mian OS, Dakin CJ, Blouin J-S, Fitzpatrick RC, Day BL. Lack of otolith involvement in balance responses evoked by mastoid electrical stimulation. J Physiol. 2010;588:4441–4451. doi: 10.1113/jphysiol.2010.195222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Res. 1974;67:255–268. doi: 10.1016/0006-8993(74)90276-5. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Day BL, Marsden CD. Vestibular induced postural responses in Parkinson's disease. Brain. 1993;116:1177–1190. doi: 10.1093/brain/116.5.1177. [DOI] [PubMed] [Google Scholar]
- Pick HL, Jr, Rieser JJ, Wagner D, Garing AE. The recalibration of rotational locomotion. J Exp Psych Hum Percept Perform. 1999;25:1179–1188. [Google Scholar]
- Reynolds RF, Bronstein AM. The moving platform aftereffect: limited generalization of a locomotor adaptation. J Neurophysiol. 2004;91:92–100. doi: 10.1152/jn.00495.2003. [DOI] [PubMed] [Google Scholar]
- Rieser JJ, Pick HL, Jr, Ashmead DH, Garing AE. Calibration of human locomotion and models of perceptual-motor organization. J Exp Psychol Hum Percept Perform. 1995;21:480–497. doi: 10.1037//0096-1523.21.3.480. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Holstein GR, Highstein SM. Hair-cell versus afferent adaptation in the semicircular canals. J Neurophysiol. 2005;93:424–436. doi: 10.1152/jn.00426.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Day BL, Fitzpatrick RC. Adaptation of vestibular signals for self-motion perception. J Physiol. 2011;589:843–853. doi: 10.1113/jphysiol.2010.197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Fitzpatrick RC. Fusion of sensory channels for the sense of orientation (at the Neural Processes of Orientation and Navigation Symposium) Proc Physiol Soc. 2010;19:SA71. [Google Scholar]
- Sharp PE, Blair HT, Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci. 2001;24:289–294. doi: 10.1016/s0166-2236(00)01797-5. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cell firing properties and behavioral performance in 3D space. J Physiol. 2011;589:835–841. doi: 10.1113/jphysiol.2010.194266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman DL, Day BL, Fitzpatrick RC. Position and velocity responses to galvanic vestibular stimulation in human subjects during standing. J Physiol. 2003a;547:293–299. doi: 10.1113/jphysiol.2002.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman DL, Taylor JL, Fitzpatrick RC. Effects of galvanic vestibular stimulation on human posture and perception while standing. J Physiol. 2003b;551:1033–1042. doi: 10.1113/jphysiol.2003.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]


