Abstract
We perceive the world around us as stable. This is remarkable given that our body parts as well as we ourselves are constantly in motion. Humans and other primates move their eyes more often than their hearts beat. Such eye movements lead to coherent motion of the images of the outside world across the retina. Furthermore, during everyday life, we constantly approach targets, avoid obstacles or otherwise move in space. These movements induce motion across different sensory receptor epithels: optical flow across the retina, tactile flow across the body surface and even auditory flow as detected from the two ears. It is generally assumed that motion signals as induced by one's own movement have to be identified and differentiated from the real motion in the outside world. In a number of experimental studies we and others have functionally characterized the primate posterior parietal cortex (PPC) and its role in multisensory encoding of spatial and motion information. Extracellular recordings in the macaque monkey showed that during steady fixation the visual, auditory and tactile spatial representations in the ventral intraparietal area (VIP) are congruent. This finding was of major importance given that a functional MRI (fMRI) study determined the functional equivalent of macaque area VIP in humans. Further recordings in other areas of the dorsal stream of the visual cortical system of the macaque pointed towards the neural basis of perceptual phenomena (heading detection during eye movements, saccadic suppression, mislocalization of visual stimuli during eye movements) as determined in psychophysical studies in humans.

Frank Bremmer studied physics and physiology at Philipps University Marburg (PUM), Germany. He did his PhD in Neurobiology at Ruhr University Bochum (RUB), Germany, where he started to record from the visual cortical system of the awake non-human primate. After his postdoc at the College de France, Paris, France, he returned to RUB and started to combine neurophysiological experiments on the macaque with fMRI recordings and psychophysical studies in humans. In 2001, he became professor of Applied Physics and Neurophysics at PUM, where he combines neurophysiology, psychophysics and fMRI to study the representation of multisensory space and motion information in primates.
General introduction
During everyday life we are in constant motion. We move our eyes to redirect our gaze or track objects of interest. Simultaneously, we aim with our hands or our whole body for a target or we avoid an obstacle. In both cases, the action – eye movement or self-motion – has a large impact on our perception. Eye movements induce image shifts across the retina. Self-motion induces visual, tactile and auditory flow. In both cases, these motion signals occur in an otherwise stable and (in most cases) stationary world. Navigating and acting in space therefore is challenging. First, the brain needs to build an internal representation of the outside world. Second, the brain needs to differentiate self-induced from internally induced motion. Otherwise, goal-directed actions like a goal keeper's catching of a flying ball would be impossible.
In order to determine the neural basis of (i) perceptual stability and (ii) the representation of multisensory space and motion, we and others have concentrated in recent years on determining functional properties of neurons in the primate posterior parietal cortex. Neuropsychological studies in humans as well as lesion studies in macaques had pointed towards this part of the primate brain as being critically relevant for the processing of spatial and motion information. Together with neurophysiological studies in non-human primates, this has led to the view of two visual cortical pathways: a ventral pathway dedicated to the processing of object information and a more dorsal pathway dedicated to the processing of spatial and motion information. Sometimes, these processing streams are also called the what (ventral) and the how or where (dorsal) pathway (Ungerleider & Mishkin, 1982; Goodale & Milner, 1992). In our studies, we had concentrated on the investigation of two areas of the dorsal visual pathway. More specifically, we have recorded extracellularly the activity of neurons in the medial superior temporal area of the superior temporal sulcus (area MST) and of neurons in the ventral intraparietal area in the intraparietal sulcus (area VIP) (Fig. 1). In some cases, we tested cells from both areas in the same experimental protocol. This allowed us to determine how similar or how different space and motion are encoded along the dorsal visual pathway. In addition, we have performed fMRI recordings in human subjects to determine similarities in space and motion encoding between man and monkey.
Figure 1. Location of the ventral intraparietal area (area VIP) in the depth of the intraparietal sulcus.
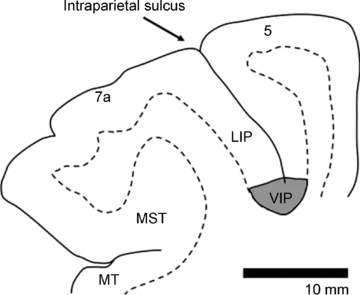
Borders between grey and white matter are based on histological sections. Neighbouring areas 7a, LIP (lateral intraparietal area) and 5 are indicated.
I will start my review by considering eye movement-related responses in macaque areas MST and VIP. Then I will turn to the encoding of multisensory space and motion in both areas. Data from fMRI recordings in humans will complement this review.
Saccadic eye movements and spatial encoding
Primates move their eyes more often than their hearts beat. This is remarkable given that eye movements challenge visual perception (Bremmer & Krekelberg, 2003). Each eye movement induces a large, global shift of the image of the outside world across the retina. It is thus assumed that dedicated neural circuits in the sensorimotor system process not only the incoming sensory signals but also out-going commands controlling the oculomotor plant. These signals mirroring the eye movement commands usually are referred to as efference copy or corollary discharge (von Holst & Mittelstaedt, 1950; Sperry, 1950). Indeed, Sommer and Wurtz have provided clear evidence that neurons in the monkey medial dorsal nucleus of the thalamus carry such a corollary discharge signal. More specifically, the corollary signal is sent from the superior colliculus via the medio-dorsal thalamus to the frontal eye field as confirmed by antidromic and orthodromic stimulation experiments (Sommer & Wurtz, 2002, 2008; Wurtz & Sommer, 2004). Most importantly, inactivating this thalamic nucleus induced characteristic deficits in a classical double-step saccade task. Here, two saccade targets are briefly presented during steady fixation and are extinguished before onset of the first saccade. The subject's task is to make consecutive saccades to the first and to the second target. This task generates a sensorimotor conflict: after finishing the first saccade the correct vector for the second saccade does no longer correspond to the initial retinal vector between fovea and the second target. Instead, for correct performance, the vector of the first saccade has to be taken into account. Humans and non-human primates typically are able to perform this double-step saccade task quite well. After inactivating the medio-dorsal nucleus of the thalamus, however, the second saccade no longer is veridical. Instead, the second saccade is deflected in the direction of the retinal vector. Similar results have also been observed in patients suffering from a lesion in this thalamic nucleus (Bellebaum et al. 2005).
The lesion-induced error as observed in the study by Sommer and Wurtz amounts on average to about 20% of the maximum possible error (Sommer & Wurtz, 2002). Hence, it is generally agreed that other mechanisms must exist that keep track of the on-going eye movements. We and others have indeed found evidence for such additional signals. In areas MST and VIP, the activity of more than half of the cells is influenced by eye position (Bremmer et al. 1997, 1999a). In these studies, either spontaneous activity or visual-induced responses were tested with gaze directed at different locations. In many of these cells, spontaneous activity or response strength varied linearly with horizontal and vertical eye position. Interestingly, the average activity as obtained from a population of cells was no longer dependent on eye position. Rather, the average activity was balanced out. In two theoretical studies, we could show that such a response property (eye position effects at the single cell level, a balanced response at the population level) allows for head-centred encoding of visual signals (Bremmer et al. 1998; Boussaoud & Bremmer, 1999). This finding was in line with previous hypotheses on how space might be represented in the primate brain. We and others had argued that sensory–motor transformations as necessary for an interaction with the environment might be accomplished by a series of coordinate transformations of visual signals from eye-centred to head-centred, body-centred and eventually even world-centred coordinates (Andersen et al. 1993).
This view, however, was recently challenged by studies investigating the dynamics of the eye position signal. Goldberg and colleagues recorded activity in the somatosensory cortex S1 of macaques (Wang et al. 2007). The spatial tuning of these cells was similar to what had been described before for neurons in the visual cortex: eye position signals increased monotonically with increasing orbital eccentricity, with directional selectivity tuned in a Gaussian manner. All ON-directions of eye position were represented in a single hemisphere. Furthermore, the authors could show that the signal as recorded in S1 is proprioceptive, because it could be obliterated by anaesthetizing the contralateral orbit. Interestingly, this eye position signal lagged a change in eye position by at least 40 ms. More recently, our group has recorded eye position signals across saccades in areas MST and VIP (Morris et al. 2009). During fixation, a maximum likelihood operation could decode current eye position with high accuracy. Across saccades, however, the decoded signal was disturbed. Most remarkably, the decoded eye position signal anticipated the upcoming change in eye position and returned to veridical only comparably late after the saccade. Given the frequency of saccades in everyday life (two to three times per second), the time course of the eye position signal would be too slow to be used for the above-described coordinate transformations. This impressive result challenges the view of eye position signals as the building block for coordinate transformations in the sensorimotor system.
The question hence occurred how the visual system integrates visual and eye position information across eye movements and how it deals with the retinal blur induced by the saccades. Previous psychophysical studies had shown that visual processing is suppressed during saccades. Interestingly, this saccadic suppression affected almost exclusively stimuli driving the magnocellular rather than the parvocellular pathway (Ross et al. 1996), i.e. stimuli with low spatial frequency. It is generally assumed that the magnocellular stream provides the major input to the dorsal visual pathway. This in turn predicts a significantly reduced motion perception during saccades. Yet, we are not completely motion blind during saccades. Instead, contrast sensitivity is significantly increased making optimized high-contrast stimuli still visible (Ilg & Hoffmann, 1993; Castet & Masson, 2000). We were therefore interested to investigate visual processing in the dorsal pathway of the macaque. This part of the visual cortical system receives its major input from the magnocellular processing stream (Maunsell, 1992). We flashed large visual stimuli before, during and after saccades while recording activity in areas MST and VIP. Responses obtained from stimuli presented long before a saccade were considered as the reference signal. Compared to this reference, perisaccadic responses were clearly reduced (Bremmer et al. 2009). Response reduction was in the range of 15–20% (Fig. 2). We simulated such a response reduction by reducing the contrast of stimuli presented during steady fixation. Such stimuli were barely visible due to the reduced contrast. Accordingly, while the response strength was reduced by only 15–20% the perception of these stimuli is almost completely suppressed.
Figure 2. Comparison of visual responsiveness.
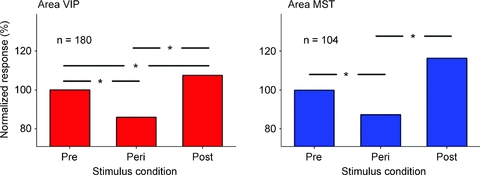
In areas MST and VIP, the median visual response during fixation long before a saccade was normalized to a value of 100%. The response was computed for a pre-selected response window (75–125 ms after stimulus onset). Responses in motion-sensitive areas MST and VIP were statistically different in the three stimulus periods (P < 0.001, repeated measures ANOVA on ranks). A pair-wise multiple comparison (Tukey test) revealed that the Peri-responses were significantly smaller than the responses in the Pre- and the Post-condition. In addition, in area VIP, the responses in the Post-condition were significantly stronger than in the Pre-condition (P < 0.05, Tukey test).
A further analysis of our data allowed us to determine the time course of this saccadic suppression (Fig. 3). It turned out that excitability of cells in areas MST and VIP was almost identical to the psychophysically found time course of contrast sensitivity. We hence assume that the functional properties of motion-sensitive areas MST and VIP can be considered as the neural basis of saccadic suppression.
Figure 3. Comparison of neural and behavioural data.
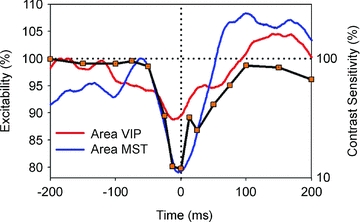
For the behavioural data, the horizontal axis shows time between stimulus presentation and saccade onset, the right vertical axis indicates normalized contrast sensitivity as taken from Diamond et al. (2000). Neuronal data were shifted along the time axis in order to correct for response and processing latencies and represent neuronal excitability (left vertical axis) of the MST population (blue curve) and the VIP population (red curve). The time course of neuronal excitability in both motion areas of the macaque shows a good qualitative match with the time course of perceptual loss of sensitivity around saccades in human subjects (modified from Bremmer et al. 2009).
Slow eye movements and spatial encoding
While saccades aim to bring targets of interest into the fovea, smooth pursuit eye movements aim to keep moving targets in the fovea (Carpenter, 1988). Different from saccades, smooth pursuit is under constant visual control. Untrained subjects are not able to perform smooth pursuit without a visual target. Different from introspection, the resultant eye movement is not smooth but rather an alternation of saccades and successive fixations (Carpenter, 1988).
In a series of influential studies, Wurtz and colleagues had shown that neurons in area MT and MST are critically involved in the generation of smooth pursuit eye movements (Newsome et al. 1988; Komatsu & Wurtz, 1988a,b;), yet detailed differences of functional properties could be observed between the two areas. Most importantly, pursuit activity in area MT is critically dependent on the visual signal. A brief blanking of the visual stimulus evoking the pursuit, short enough to keep pursuit ongoing, diminishes activity in pursuit-related neurons in area MT. Differently to area MT, activity in many neurons in area MST stays up along with the eye movement. It is now generally accepted that pursuit activity in area MT reflects the driving visual signal while activity in area MST in many cases reflects extraretinal input. Follow-up studies have confirmed and even elaborated the functional role of both areas (Recanzone & Wurtz, 2000; Ilg, 2003; Ilg et al. 2004). Among other important findings, the authors of these studies showed that (i) a subset of neurons in area MST encodes visual information in world-centred coordinates during smooth pursuit, (ii) the onset of neural activity in area MST precedes the onset of smooth pursuit, and finally that (iii) inactivation of area MST disturbs smooth pursuit. Taken together, all studies emphasize a critical involvement of area MST in the generation of smooth pursuit eye movements.
Given these results for area MST, we aimed to determine the functional role of area VIP in smooth pursuit eye movements. We found that more than half of the neurons in area VIP have smooth pursuit-related activity (Schlack et al. 2003). Preferred directions for pursuit were uniformly distributed across neurons. Interestingly, most neurons were tuned for high pursuit velocities. This might be related to the fact that area VIP predominantly encodes motion in near-extra-personal space, i.e. the part of space clearly within reaching distance of the animal. Objects moving with a certain speed as measured in metres per second move at higher angular velocity (degrees per second) with decreasing distance to the observer. Hence, the encoding of near space requires a tuning for high velocities.
In a second set of recordings, the pursuit target was blinked off for 200 ms. This gap was short enough to allow monkeys to maintain their tracking eye movement. On the other hand, it was long enough to differentiate between retinal and extraretinal signals as the driving source for the observed activity. In none of the VIP neurons did we observe an MT-like response, i.e. in none of the neurons did the activity drop during the gap. Rather, neural activity stayed constant or even slightly increased. Hence, neurons in area VIP behave like those in neighbouring area MST: pursuit-related activity reflects extraretinal input. Accordingly, the efference copy of the oculomotor command, as discussed in the Introduction, would be available in areas MST and VIP in order to differentiate self-induced from externally induced motion. Indeed, some MST neurons seem to make use of this signal and encode object motion in world rather than in retinal coordinates (Erickson & Thier, 1991; Inaba et al. 2007).
Optokinetic nystagmus (OKN) is an alternation of slow and fast phases. Under certain circumstances, fast phases share functional properties with saccades (Kaminiarz et al. 2009). Depending on the experimental instructions, slow phases are characterized by long tracking phases with a low frequency of fast phases. This type of OKN is called look-nystagmus and is considered to be similar to smooth pursuit. Stare-nystagmus, on the other hand, is reflexively elicited by having a subject look more or less passively at the visual scene. Stare-nystagmus is characterized by smaller amplitude and a higher frequency of resetting eye movements as compared to look-nystagmus. We investigated OKN-related activity in humans (by means of fMRI) as well as in macaques (by means of extracellular recordings). In monkeys, we could show OKN-related discharges in area VIP (Bremmer et al. 2002b). A contribution of area MST had been known before (Ilg, 1997). In a concurrent fMRI study we showed activation of a complete cortical network during smooth pursuit and OKN (Konen et al. 2005). A direct comparison between activity during the execution of OKN and smooth pursuit yielded no oculomotor-related area exclusively dedicated to one or the other eye movement type. Remarkably, however, only look-nystagmus but not stare-nystagmus led to strong cortical activation.
One region of the OKN/pursuit network was the human MT motion complex. Among the activated areas was also a region in the depth of the human intraparietal sulcus (Fig. 4). Based on our own previous results this area most probably constitutes the functional equivalent of macaque area VIP (Bremmer et al. 2001). Accordingly, both approaches, fMRI in humans as well as single cell recordings in the macaque, point to an involvement of areas MST and VIP in the control of slow eye movements.
Figure 4. Activation pattern related to OKN relative to baseline as a result of the group analysis.
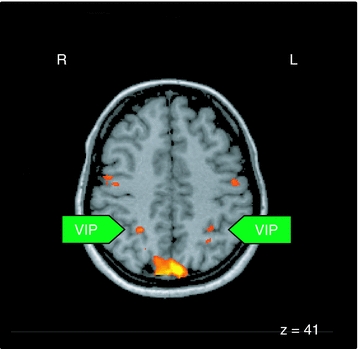
All areas were significant at P < 0.05 corrected. R, right hemisphere; L, left hemisphere; The z-value indicates the coordinate of the horizontal image, VIP, ventral intraparietal area.
As mentioned above, these signals might be used to compensate for self-induced motion signals. If this was always the case, the position of objects in the outside world should be encoded veridically during eye movements. Psychophysical data, however, clearly demonstrate that this is not the case. It has been shown that perceived location of targets in the outside world is shifted in the direction of smooth pursuit. This mislocalization, which is in the order of 1 to 2 deg for eye movements of 10 to 20 deg s−1, is stronger in the hemi-field the eye is heading towards (foveopetal) (van Beers et al. 2001; Koenigs & Bremmer, 2010). Recent studies showed that such mislocalization is not only found during visually guided smooth pursuit but also during the slow phase of OKN (Kaminiarz et al. 2007; Tozzi et al. 2007). The hemi-field asymmetry of localization as described during pursuit, however, was not found during OKN. Also the error pattern during optokinetic afternystagmus (OKAN) differed from the one found during smooth pursuit (Kaminiarz et al. 2008). This difference might be based on different cortical participation in the control of these eye movements. More psychophysical studies, however, are needed to understand the exact spatial perception during (slow) eye movements. Even more important, though, the neurophysiological basis of the observed misperceptions is as yet unclear and requires intense future research.
Self-motion and spatial encoding
So far we have considered the influence of eye movements on spatial encoding. Yet, as mentioned at the beginning, humans as well as non-human primates are constantly in motion. We and others have shown an involvement of areas MST and VIP in the encoding of self-motion by reporting vestibular-related activity in areas MST (Duffy, 1998; Bremmer et al. 1999b; Takahashi et al. 2007; Gu et al. 2007, 2008) and VIP (Bremmer et al. 2002b; Schlack et al. 2002). Yet, self-motion not only generates vestibular signals; it also induces retinal visual flow. Accordingly, the question arises as to how exactly visual stimuli look that arrive at the retina during self-motion and how they are processed at a cortical level.
It had been shown before that self-motion stimuli elicit reflexive eye movements that share properties with OKN (Lappe et al. 1998; Niemann et al. 1999). These eye movements distort the retinal flow pattern dramatically. Hence we were interested to see how neurons in area MST respond to such natural optical flow. In these experiments we employed a three-by-three experimental design. Three simulated self-motion directions were combined with three simulated eye-movement conditions. These nine conditions were presented interleaved to the fixating monkey. Indeed, about 45% of the MST cells encoded the simulated heading rather than the retinal flow (Fig. 5; Bremmer et al. 2010). In a second step, we analysed the position of the visual receptive field of the neurons as well as their preferred direction for visual motion in the frontoparallel plane. These functional properties were used to predict the preference for the visually simulated self-motion stimuli (left vs. straight ahead vs. right). Surprisingly, predictability of self-motion responses was at chance level. In other words, the preferences for visually simulated self-motion stimuli cannot be explained by knowledge of the location of the visual receptive field and the frontoparallel preference for visual motion. Preliminary data suggest that the same response behaviour can be observed in area VIP. These data clearly demonstrate that more research is needed to understand the neural basis of (self-)motion perception.
Figure 5. Retinal flow fields and related neuronal responses.
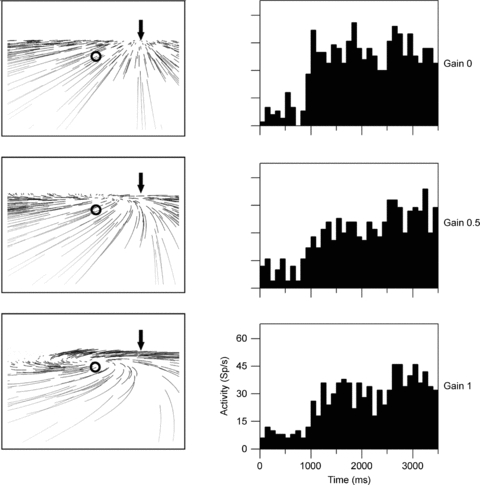
The left columns show the retinal flow fields seen by an observer moving in a rightward heading direction on top of a ground plane. All three panels display the same heading (arrow) but differ in terms of simulated eye movements. The pattern as shown in the top panel occurs in the absence of eye movements (Gain = 0.0). In the middle and bottom panel, eye movements which spontaneously occur in primates are included (Gain = 0.5 (middle) and Gain = 1.0 (bottom)). These eye movements track the motion in the direction of gaze (circle) and distort the structure of the flow on the retina, generating a motion pattern that resembles a spiral and in which the motion in gaze direction (circle) is minimized. Invariant responses to heading should be the same in all three cases, since heading is identical although flow structure is different. The right column shows responses from a neuron responding to rightward heading irrespective of eye movements. Histograms show the neuron's firing rate over time during presentation of optic flow stimuli.
Beyond vision: multisensory spatial encoding
Vision is the dominating sense in primates. Nevertheless, somatosensation, audition and also the vestibular sense contribute significantly to our internal representation of space. Accordingly, we and others have investigated whether or not cells in primate PPC would be activated by these non-visual stimuli. Colby and colleagues were the first to show tactile responses in area VIP (Colby et al. 1993). This finding was even more intriguing considering the fact that many neurons showed visual and tactile responses and that in such case the spatial locations of both receptive fields (visual and tactile) were spatially congruent. Such an encoding allows for perceptual spatial stability: a near visual stimulus might become a tactile stimulus if the monkey moves forward or the object (i.e. the visual stimulus) approaches the monkey. In both cases, the visual stimulus would turn into a tactile stimulus and could be encoded by the same neuron.
We extended this finding by showing that neurons in area VIP respond to moving tactile stimuli in an action-congruent manner (Bremmer et al. 2002a). In this study we tested neurons for their visual, vestibular and tactile responsiveness. Some of the VIP neurons responded to all three sensory stimulations. In such case the neurons’ preferred directions across the three sensory modalities were identical (Fig. 6). Such neurons would be suitable candidates for a supramodal encoding of motion in full 3-D space.
Figure 6. Action congruency of responses to visual, tactile and vestibular stimulation.
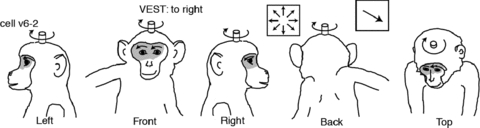
Shading and arrows on the animal drawings indicate, respectively, the extent of the somatosensory receptive field and the directional preference of the tactile stimulus. Insets on top of the drawings indicate the cell identification, whether the neuron was tested for vestibular responses, the optic flow response and the directional selectivity vector.
In a final experimental approach we investigated whether or not neurons in area VIP would respond also to auditory stimulation. Indeed, we found that about 80% of the cells also responded to auditory stimulation (Schlack et al. 2005). In a subset of neurons, we were able to map the location of the visual and the auditory receptive field. In almost 90% of these neurons the spatial locations of the receptive fields were spatially congruent (Fig. 7). It hence appears that neurons in area VIP encode space spatially congruent across different senses, i.e. vision, somatosensation, audition and vestibular. All these experiments, however, were performed with the fixating monkey. As shown in the first part of this review, fixation is rather an exception than a rule of oculomotor behaviour during everyday life. Hence, it remains to be determined how multisensory space is encoded across eye movements. First psychophysical results suggest that the spatial congruency as observed during fixation is challenged during saccades and slow eye movements (Koenigs et al. 2007; Klingenhoefer & Bremmer, 2009; Koenigs & Bremmer, 2010).
Figure 7. Spatially congruent visual and auditory RFs of an individual VIP neuron.
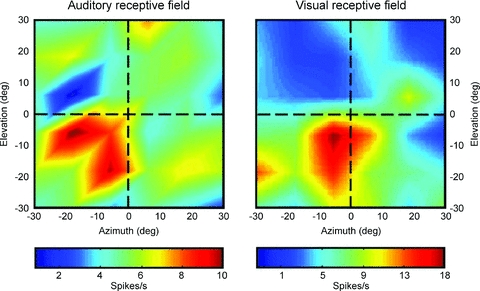
Neuronal responses are colour coded corresponding to high discharge values. The data have been recorded while the monkey fixated a central target. The two RFs largely overlapped, and the hotspots were almost identical.
In summary, the reviewed data clearly demonstrate that more research is needed to understand the neural basis of spatial perception during eye- and self-motion in primates.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Human Frontiers Science Program and European Union.
Glossary
Abbreviations
- MST
medial superior temporal
- OKAN
optokinetic afternystagmus
- OKN
optokinetic nystagmus
- PPC
posterior parietal cortex
- VIP
ventral intraparietal area
References
- Andersen RA, Snyder LH, Li C-S, Stricanne B. Coordinate transformations in the representation of spatial information. Curr Opin Neurobiol. 1993;3:171–176. doi: 10.1016/0959-4388(93)90206-e. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain. 2005;128:1139–1154. doi: 10.1093/brain/awh474. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Bremmer F. Gaze effects in the cerebral cortex: reference frames for space coding and action. Exp Brain Res. 1999;128:170–180. doi: 10.1007/s002210050832. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Duhamel J-R, Ben Hamed S, Graf W. Heading encoding in the macaque ventral intraparietal area (VIP) Eur J Neurosci. 2002a;16:1554–1568. doi: 10.1046/j.1460-9568.2002.02207.x. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Graf W, Ben Hamed S, Duhamel JR. Eye position encoding in the macaque ventral intraparietal area (VIP) Neuroreport. 1999a;10:873–878. doi: 10.1097/00001756-199903170-00037. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Ilg UJ, Thiele A, Distler C, Hoffmann KP. Eye position effects in monkey cortex. I. Visual and pursuit-related activity in extrastriate areas MT and MST. J Neurophysiol. 1997;77:944–961. doi: 10.1152/jn.1997.77.2.944. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Klam F, Duhamel J-R, Ben Hamed S, Graf W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP) Eur J Neurosci. 2002b doi: 10.1046/j.1460-9568.2002.02206.x. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Krekelberg B. Seeing and acting at the same time: challenges for brain (and) research. Neuron. 2003;38:367–370. doi: 10.1016/s0896-6273(03)00236-8. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Hoffmann KP, Krekelberg B. Neural dynamics of saccadic suppression. J Neurosci. 2009;29:12374–12383. doi: 10.1523/JNEUROSCI.2908-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Pekel M, Lappe M, Hoffmann KP. Linear vestibular self-motion signals in monkey medial superior temporal area. Ann N Y Acad Sci. 1999b;871:272–281. doi: 10.1111/j.1749-6632.1999.tb09191.x. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Pekel M, Hoffmann KP, Lappe M. Visual selectivity for heading in monkey area MST. Exp Brain Res. 2010;200:51–60. doi: 10.1007/s00221-009-1990-3. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Pouget A, Hoffmann KP. Eye position encoding in the macaque posterior parietal cortex. Eur J Neurosci. 1998;10:153–160. doi: 10.1046/j.1460-9568.1998.00010.x. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K-P, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. Movement of the Eyes. 2nd edn. London: Pion Ltd; 1988. [Google Scholar]
- Castet E, Masson GS. Motion perception during saccadic eye movements. Nat Neurosci. 2000;3:177–183. doi: 10.1038/72124. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. The analysis of visual space by the lateral intraparietal area of the monkey: the role of extraretinal signals. Prog Brain Res. 1993;95:307–316. doi: 10.1016/s0079-6123(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Diamond MR, Ross J, Morrone MC. Extraretinal control of saccadic suppression. J Neurosci. 2000;20:3449–3455. doi: 10.1523/JNEUROSCI.20-09-03449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CJ. MST neurons respond to optic flow and translational movement. J Neurophysiol. 1998;80:1816–1827. doi: 10.1152/jn.1998.80.4.1816. [DOI] [PubMed] [Google Scholar]
- Erickson RG, Thier P. A neuronal correlate of spatial stability during periods of self-induced visual motion. Exp Brain Res. 1991;86:608–616. doi: 10.1007/BF00230534. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gu Y, Angelaki DE, DeAngelis GC. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci. 2008;11:1201–1210. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10:1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg UJ. Slow eye movements. Prog Neurobiol. 1997;53:293–329. doi: 10.1016/s0301-0082(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Ilg UJ. Visual-tracking neurons in area MST are activated during anticipatory pursuit eye movements. Neuroreport. 2003;14:2219–2223. doi: 10.1097/00001756-200312020-00017. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Hoffmann K-P. Motion perception during saccades. Vision Res. 1993;33:211–220. doi: 10.1016/0042-6989(93)90159-t. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Schumann S, Thier P. Posterior parietal cortex neurons encode target motion in world-centered coordinates. Neuron. 2004;43:145–151. doi: 10.1016/j.neuron.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Inaba N, Shinomoto S, Yamane S, Takemura A, Kawano K. MST neurons code for visual motion in space independent of pursuit eye movements. J Neurophysiol. 2007;97:3473–3483. doi: 10.1152/jn.01054.2006. [DOI] [PubMed] [Google Scholar]
- Kaminiarz A, Konigs K, Bremmer F. Task influences on the dynamic properties of fast eye movements. J Vis. 2009;9:1–11. doi: 10.1167/9.13.1. [DOI] [PubMed] [Google Scholar]
- Kaminiarz A, Krekelberg B, Bremmer F. Localization of visual targets during optokinetic eye movements. Vision Res. 2007;47:869–878. doi: 10.1016/j.visres.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Kaminiarz A, Krekelberg B, Bremmer F. Expansion of visual space during optokinetic afternystagmus (OKAN) J Neurophysiol. 2008;99:2470–2478. doi: 10.1152/jn.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenhoefer S, Bremmer F. Perisaccadic localization of auditory stimuli. Exp Brain Res. 2009;198:411–423. doi: 10.1007/s00221-009-1869-3. [DOI] [PubMed] [Google Scholar]
- Koenigs K, Bremmer F. Localization of visual and auditory stimuli during smooth pursuit eye movements. J Vis. 2010;10:8. doi: 10.1167/10.8.8. [DOI] [PubMed] [Google Scholar]
- Koenigs K, Knoell J, Bremmer F. Localization of auditory targets during optokinetic nystagmus. Perception. 2007;36:1507–1512. doi: 10.1068/p5849. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988a;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. III. Interaction with full-field visual stimulation. J Neurophysiol. 1988b;60:621–644. doi: 10.1152/jn.1988.60.2.621. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kleiser R, Seitz RJ, Bremmer F. An fMRI study of optokinetic nystagmus and smooth-pursuit eye movements in humans. Exp Brain Res. 2005;165:203–216. doi: 10.1007/s00221-005-2289-7. [DOI] [PubMed] [Google Scholar]
- Lappe M, Pekel M, Hoffmann K-P. Optokinetic eye movements elicited by radial optic flow in the macaque monkey. J Neurophysiol. 1998;79:1461–1480. doi: 10.1152/jn.1998.79.3.1461. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR. Functional visual streams. Curr Opin Neurobiol. 1992;2:506–510. doi: 10.1016/0959-4388(92)90188-q. [DOI] [PubMed] [Google Scholar]
- Morris AP, Kubischik M, Hoffmann K-P, Krekelberg B, Bremmer F. 2009 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2009. Decoding dynamic eye position from teh activity of macaque visual cortical neurons. Programme No. 455.2. [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Niemann T, Lappe M, Buscher A, Hoffmann KP. Ocular responses to radial optic flow and single accelerated targets in humans. Vision Res. 1999;39:1359–1371. doi: 10.1016/s0042-6989(98)00236-3. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J Neurophysiol. 2000;83:777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- Ross J, Burr D, Morrone C. Suppression of the magnocellular pathway during saccades. Behav Brain Res. 1996;80:1–8. doi: 10.1016/0166-4328(96)00012-5. [DOI] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Interaction of linear vestibular and visual stimulation in the macaque ventral intraparietal area (VIP) Eur J Neurosci. 2002;16:1877–1886. doi: 10.1046/j.1460-9568.2002.02251.x. [DOI] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Selectivity of macaque ventral intraparietal area (area VIP) for smooth pursuit eye movements. J Physiol. 2003;551:551–561. doi: 10.1113/jphysiol.2003.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlack A, Sterbing-D’Angelo SJ, Hartung K, Hoffmann KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J Neurosci. 2005;25:4616–4625. doi: 10.1523/JNEUROSCI.0455-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Gu Y, May PJ, Newlands SD, DeAngelis GC, Angelaki DE. Multimodal coding of three-dimensional rotation and translation in area MSTd: comparison of visual and vestibular selectivity. J Neurosci. 2007;27:9742–9756. doi: 10.1523/JNEUROSCI.0817-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, Morrone MC, Burr DC. The effect of optokinetic nystagmus on the perceived position of briefly flashed targets. Vision Res. 2007;47:861–868. doi: 10.1016/j.visres.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- van Beers RJ, Wolpert DM, Haggard P. Sensorimotor integration compensates for visual localization errors during smooth pursuit eye movements. J Neurophysiol. 2001;85:1914–1922. doi: 10.1152/jn.2001.85.5.1914. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das Reafferenzprinzip. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci. 2007;10:640–646. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA. Identifying corollary discharges for movement in the primate brain. Prog Brain Res. 2004;144:47–60. doi: 10.1016/S0079-6123(03)14403-2. [DOI] [PubMed] [Google Scholar]


